Copolymers from benzodithiophene and benzotriazole: synthesis and photovoltaic applications
Zhenhua
Zhang
a,
Bo
Peng
ab,
Bo
Liu
a,
Chunyue
Pan
a,
Yongfang
Li
*b,
Yuehui
He
c,
Kechao
Zhou
c and
Yingping
Zou
*a
aCollege of Chemistry and Chemical Engineering, Central South University, Changsha, 410083, China. E-mail: zyp2008@iccas.ac.cn
bBeijing National Laboratory for Molecular Sciences, Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, China
cState key Laboratory for Powder Metallurgy, Central South University, Changsha, 410083, China
First published on 7th July 2010
Abstract
Two new alternating low bandgap copolymers from benzodithiophene and benzotriazole units, namely poly{4,8-bis(2-ethylhexyloxy)benzo[1,2-b; 3,4-b]dithiophene-2,6-diyl-alt-2-octyl-4,7-di(thiophen-2-yl)-2H-benzo[d][1,2,3]triazole-5′,5′′-diyl} (PBDTDTBTz) and poly{4,8-bis(2-ethylhexyloxy)benzo[1,2-b;3,4-b]dithiophene-2,6-diyl-alt-2-dodecylbenzotriazole-4,7-diyl} (PBDTBTz), were designed and synthesized by a typical Stille coupling polymerization method. The copolymers were characterized by thermogravimetric analysis, UV-vis absorption and cyclic voltammetry. PBDTDTBTz and PBDTBTz possess moderate molecular weights and excellent thermal properties with a 5% weight loss temperatures (Td) around 300 °C. They exhibited good optical absorption, with peaks at 527 nm and 562 nm in the film state, respectively. Photovoltaic properties of the copolymers blended with [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) or [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) as electron acceptors, were investigated. The photovoltaic device with the PBDTDTBTz/PC71BM shows a power conversion efficiency of 1.7% with a short circuit current density of 4.5 mA cm−2 and a good fill factor of 0.62, while PBDTBTz demonstrated a moderate power conversion efficiency of up to 1.4%, under the illumination of AM 1.5, 100 mW cm−2 with a device structure of ITO/PEDOT: PSS/polymer: PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)/Ca/Al. All the above information highlighted that this kind of the copolymers is promising for the application of polymer solar cells.
4)/Ca/Al. All the above information highlighted that this kind of the copolymers is promising for the application of polymer solar cells.
Introduction
Bulk heterojunction (BHJ) polymer solar cells (PSCs) have stimulated broad interest due to their many advantages such as easy fabrication, low cost, light weight, and flexibility.1 BHJ PSCs involve a thin film blend of the conjugated polymer as the electron donor and a fullerene derivative as the electron acceptor. Among the various types of polymers designed for PSCs, the alternating electron rich and electron deficient units along the polymer backbone have been shown to achieve high power conversion efficiencies (PCEs) through optimizations of their absorption, open circuit voltage and relevant device parameters.2 During the past two years, benzodithiophene (BDT)-containing conjugated polymers have demonstrated exceptionally excellent photovoltaic properties as electron donors for PSCs. For instance, Yang and co-workers have synthesized a family of BDT based copolymers with optimized electronic and optical properties which led to PCEs as high as 7%.3 Recently, Leclerc and co-workers reported a new and interesting BDT-based polymer (PBDTTPD) with thienopyrrodione as the new accepting building block exhibiting high PCE up to 5.5% using a large active area of 1 cm2.4In order to modulate the bandgap and molecular energy levels, new electron accepting units play important role in developing the new D–A copolymers for photovoltaic applications. 2,1,3-Benzothiadiazole (BT) and 4,7-dithienyl-2,1,3-benzothiadiazole (DTBT) units are found to be widely used acceptors for the synthesis of low band gap copolymers.5 For instance, copolymers of DTBT with fluorene,5a silafluorene,5b carbazole,1j dithienylsilole,5c dithienylpyrrole,5d were synthesized and applied in PSCs, exhibiting some promising PCEs.
However, 1,2,3-benzotriazole (BTz) which is similar to 2,1,3-benzothidiazole is missing from the chemical inventory. In fact, BTz is a known heteroaromatic compound with a strong electron accepting feature because of two electron withdrawing imine (C ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) nitrogens, furthermore, easy modification of N–H bond of BTz unit can allow tuning of the structural and electronic properties for processable BTz-containing polymers. To the best of our knowledge, BTz based homopolymers and copolymers remain relatively unexplored. Very recently, the polymer from BTz and alkyl-thiophene synthesized using an electrochemical polymerization method has shown some photovoltaic properties for the first time.6
N) nitrogens, furthermore, easy modification of N–H bond of BTz unit can allow tuning of the structural and electronic properties for processable BTz-containing polymers. To the best of our knowledge, BTz based homopolymers and copolymers remain relatively unexplored. Very recently, the polymer from BTz and alkyl-thiophene synthesized using an electrochemical polymerization method has shown some photovoltaic properties for the first time.6
Taking all this into consideration, the combination of benzodithiophene and BTz should lead to some interesting features for photovoltaic applications. Herein, we firstly synthesized the copolymer (PBDTBTz) of BDT and BTz using a Stille coupling reaction. Furthermore we put thiophene units next to the BTz moiety to minimize the steric hindrance and to lower the band gaps.7 We synthesized another copolymer (PBDTDTBTz) of DBT and dithienyl benzotriazole (DTBTz). The two new conjugated polymers exhibit broad absorption, optimized HOMO–LUMO energy levels, efficient photovoltaic properties, together with good thermal stability. The PSC device based on PBDTDTBTz as the electron donor and PC71BM as acceptor demonstrated a PCE of 1.7% with a short circuit current density (Jsc) of 4.5 mA cm−2 and fill factor (FF) of 0.62 under the illumination of AM 1.5, 100 mW cm−2. The results indicate that the BTz unit is potentially an interesting building block for polymeric photovoltaic materials.
Experimental section
Materials
Pd(PPh3)4, thiophene-2-boronic acid were obtained from Alfa Asia Chemical Co, and they were used without further purification. Tetrahydrofuran (THF) was dried over Na/benzophenone ketyl and freshly distilled prior to use. Other reagents and solvents were purchased commercially as analytical-grade quality and used without further purification. 2-Octylbenzotriazole (1),8 4,7-Dibromo-2-octylbenzotriazole (2),8 2-dodecylbenzotriazole (5),8 4,7-dibromo-2-dodecylbenzotriazole (6),8 2,6-bis(trimethyltin)-4,8-bis(2-ethylhexoxy)benzo[1,2-b;3,4-b]dithiophene (7)9 were prepared according to the little modified method based on the reported literature. All the other compounds were synthesized following the procedures described here.Characterization
1H NMR and 13C NMR spectra were recorded using a Bruker AM-400 spectrometer, with tetramethylsilane (TMS) as the internal reference, chemical shifts were recorded in ppm. Molecular weight and polydispersity of the polymers were determined by gel permeation chromatography (GPC) analysis with polystyrene as standard (Waters 515 HPLC pump, a Waters 2414 differential refractometer, and three Waters Styragel columns (HT2, HT3, and HT4)) using THF (HPLC grade) as eluent at a flow rate of 1.0 mL min−1 at 35 °C. Thermogravimetric analysis (TGA) was conducted on a Shimadzu DTG-60 thermogravimetric analyzer with a heating rate of 10 K min−1 under a nitrogen atmosphere. The UV-vis absorption spectra were recorded on a JASCO V-570 spectrophotometer. For solid state measurements, polymer solution in chloroform was drop-cast on quartz plates. The optical bandgap was calculated from the onset of the absorption band. The cyclic voltammogram was recorded with a computer controlled Zahner IM6e electrochemical workstation (Germany) using polymer film on platinum disk as the working electrode, platinum wire as the counter electrode and Ag/Ag+ (0.1 M) as the reference electrode in an anhydrous and argon-saturated solution of 0.1 M of tetrabutylammonium hexafluorophosphate (Bu4NPF6) in acetonitrile. Electrochemical onsets were determined at the position where the current starts to differ from the baseline. The morphology of the polymer/PCBM blend films was investigated by a SPI 3800N atomic force microscope (AFM) in contacting mode with a 1 μm scanner.Fabrication and characterization of polymer solar cell
The PSCs were fabricated in the configuration of the traditional sandwich structure with an indium tin oxide (ITO) glass positive electrode and a metal negative electrode. Patterned ITO glass with a sheet resistance of 10 Ω/□ was purchased from CSG HOLDING Co., LTD. (China). The ITO glass was cleaned by sequential ultrasonic treatment in detergent, deionized water, acetone, and isopropanol, and then treated in an ultraviolet-ozone chamber (Ultraviolet Ozone Cleaner, Jelight Company, USA) for 20 min. Then PEDOT:PSS (poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate)) (Baytron P 4083, Germany) was filtered through a 0.45 μm filter and spin coated at 2000 rpm for 60 s on the ITO electrode. Subsequently, the PEDOT: PSS film was baked at 150 °C for 15 min in the air to give a thin film with a thickness of 40 nm. A blend of polymer and PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w, 10 mg mL−1 for polymers) or polymer and PC71BM (1
3 w/w, 10 mg mL−1 for polymers) or polymer and PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w, 10 mg mL−1 for polymers) was dissolved in ortho-dichlorobenzene (ODCB), and spin-cast at 3000 rpm for 45 s onto the PEDOT: PSS layer. The substrates were then dried at 70 °C for 15 min. The thickness of the photoactive layer is in the range 50–70 nm measured by Ambios Technology XP-2 profilometer. A bilayer cathode consisted of Ca (∼20 nm) capped with Al (∼60 nm) was thermal evaporated under a shadow mask in a base pressure of ca. 10−5 Pa. The device active area of the PSCs is 4 mm2. Device characterization was carried out under AM 1.5 G irradiation with the intensity of 100 mW cm−2 (Oriel 67005, 500 W) calibrated by a standard silicon cell. J–V curves were recorded with a Keithley 236 digital source meter. The action spectra of monochromatic incident photo-to-current conversion efficiencies (IPCEs) for the solar cells were also detected with a similar data acquisition system. The calibration of the incident light was performed with monocrystalline silicon diodes.
4 w/w, 10 mg mL−1 for polymers) was dissolved in ortho-dichlorobenzene (ODCB), and spin-cast at 3000 rpm for 45 s onto the PEDOT: PSS layer. The substrates were then dried at 70 °C for 15 min. The thickness of the photoactive layer is in the range 50–70 nm measured by Ambios Technology XP-2 profilometer. A bilayer cathode consisted of Ca (∼20 nm) capped with Al (∼60 nm) was thermal evaporated under a shadow mask in a base pressure of ca. 10−5 Pa. The device active area of the PSCs is 4 mm2. Device characterization was carried out under AM 1.5 G irradiation with the intensity of 100 mW cm−2 (Oriel 67005, 500 W) calibrated by a standard silicon cell. J–V curves were recorded with a Keithley 236 digital source meter. The action spectra of monochromatic incident photo-to-current conversion efficiencies (IPCEs) for the solar cells were also detected with a similar data acquisition system. The calibration of the incident light was performed with monocrystalline silicon diodes.
Synthesis
The synthetic routes for the monomers and polymers are shown in Scheme 1. The detailed synthetic processes are as follows.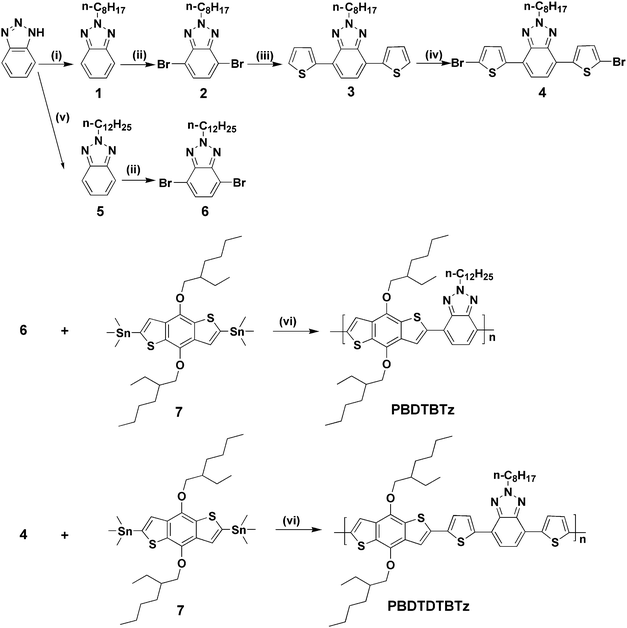 | ||
| Scheme 1 Synthetic routes of the monomers and polymers. Reagents and conditions: (i) C8H17Br, KOH, CH3OH, reflux for 24 h, 30% yield; (ii) Br2/HBr, 135 °C for 12 h, 75% yield; (iii) thiophene-2-boronic acid, Pd(PPh3)4, 1,2-dimethoxyethane (DME), 1 M NaHCO3, 90 °C for 12 h, 82% yield; (iv) NBS, CHCl3/HAc, 12 h at ambient temperature, 75% yield; (v) C12H25Br, KOH, CH3OH, reflux for 24 h, 30% yield; (vi) Pd(PPh3)4, toluene, 110 °C for 24 h. | ||
2-Octyl-4,7-di (thiophen-2-yl)-2H-benzo[d][1,2,3]triazole (DTBTz) (3)
Compound 2 (3.5 g, 9 mmol) and thiophene-2-boronic acid (3.0 g, 23.4 mmol), and Pd(PPh3)4 (208 mg, 0.18 mmol) were dissolved in 1,2-dimethoxyethane (80 mL), followed by the addition of aqueous sodium bicarbonate (1.0 M, 90 mL) with the reaction mixture vigorously stirred at 90 °C for 12 h under a nitrogen atmosphere. After cooling, the reaction solution was extracted with methylene chloride, the combined organic layers were washed with 1 M aqueous solution of NaOH and water, dried over anhydrous magnesium sulfate, and then concentrated under reduced pressure. Further purification was performed using silica gel column chromatography (petroleum ether/EtOAc = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent), compound 3 was obtained as yellow-green crystal. The yield was 82%.
1 as eluent), compound 3 was obtained as yellow-green crystal. The yield was 82%.
1H NMR (400 MHz, CDCl3, ppm): 8.01 (d, 2H), 7.52 (s, 2H), 7.28 (d, 2H), 7.09 (t, 2H), 4.60 (t, 2H), 2.10 (m, 2H), 1.35−1.17 (m, 10H), 0.80 (t, 3H).
Calculated for C22H25N3S2: C, 66.80; H, 6.37; N, 10.62; found: C, 66.78; H, 6.31; N, 10.58.
2-Octyl-4, 7-di(5-bromo-thiophen-2-yl)-2H-benzo[d] [1, 2, 3]triazole (4)
Under the darkness, compound 3 (2.37 g, 6 mmol) was dissolved in the mixture of CHCl3 (60 mL) and HOAc (60 mL), after cooling the mixture to 0 °C, NBS (2.35 g, 13.2 mmol) was added in portions. After 12 h, the reaction mixture was extracted with CH2Cl2 and washed by water twice, and then combined organic extractions were dried over anhydrous Na2SO4. The organic solvent was evaporated. The crude product was purified by recrystallization using dimethyl formide (DMF), yellow crystals were obtained with 75% yield.1H NMR (400 MHz, CDCl3, ppm): 7.79 (d, 2H), 7.51 (s, 2H), 7.13 (d, 2H), 4.80 (t, 2H), 2.18 (m, 2H), 1.41–1.27 (m, 10H), 0.87 (t, 3H).
13C NMR(100 MHz, CDCl3, ppm): 141.69, 141.23, 130.86, 126.93, 122.98, 122.16, 113.16, 59.91, 31.75, 30.03, 29.10, 28.97, 26.58, 22.62, 14.07.
Calculated for C22H23N3S2Br2: C, 47.75; H, 4.19; N, 7.59; found: C, 47.72; H, 4.23; N, 7.62.
Synthesis of PBDTBTz
2,6-Bis(trimethyltin)-4,8-bis-ethylhexyloxy-benzo[1,2-b:4,5-b′]dithiophene (231.6 mg, 0.3 mmol), 4,7-dibromo-2-dodecylbenzotriazole (133.5 mg, 0.3 mmol), and 10 mL of toluene were put into a two necked flask. The solution was flushed with argon for 20 min, and then 17 mg of Pd(PPh3)4 was added into the flask. The solution was flushed again for 10 min. The oil bath was heated to 100 °C carefully, and the reactant was stirred for 24 h at this temperature under an argon atmosphere. Then, the reactant was cooled to room temperature, and the polymer was precipitated by the addition of 50 mL of methanol and filtered through a Soxhlet thimble, which was then subjected to Soxhlet extraction with methanol, hexane, and chloroform. The polymer was recovered from the chloroform fraction by rotary evaporation. The red solid was dried under vacuum at 50 °C overnight to get the final product. (170 mg, 78%).1H NMR (400 MHz, CDCl3, ppm): 7.74 (br, 2H), 7.49 (br, 2H), 4.90 (br, 2H), 3.53 (br, 4H), 2.28 (br, 4H), 1.53–1.10 (br, 34H), 0.87 (br, 15H).
Synthesis of PBDTDTBTz
PBDTDTBTz was synthesized with a similar procedure to PBDTBTz from monomer 7 (231.6 mg, 0.3 mmol) and monomer 4 (165.9 mg 0.3mmol), giving a red dark solid of 34 mg. Yield: 14%.Results and discussion
Monomers and polymers synthesis
The synthetic routes of the monomers and polymers are shown in Scheme 1. The comonomer 4, was synthesized as the following process. Starting from 1,2,3-benzotriazole, an alkylation reaction was performed to get 2-octylbenzotriazole (compound 2) with a similar yield,8 by using KOH as base instead of t-BuOK reported in the literature. Because of the two possible alkylation sites of the benzotriazole, the desired isomer was isolated in 30% yield in the first step of the reaction sequence. However, this reaction stands as a practical step with relatively cheap starting materials and reagents; hence it can be performed in quite large scales. This step was then followed by a dibromination reaction using Br2/HBr system to yield compound 2 with 75% yield. Compound 2 took a Suzuki coupling reaction with 2-thiophene boronic acid to obtain the compound 3, this step revealed a quite satisfactory yield. A dibromination step of compound 3 using NBS as brominating agent to get comonomer 4 in 75% yield. The synthetic procedure for compound 6 was similar to that for compound 2. Compound 7 copolymerized with compound 4 or compound 6 through Stille coupling reactions to afford the target polymers (PBDTDTBTz and PBDTBTz), respectively,10 and the polymers were purified by sequential Soxhlet extraction with methanol, hexane and CHCl3. The CHCl3 fraction was then reduced in volume, precipitated into methanol, and collected by filtration yielding a dark red or red solid. The monomers and PBDTBTz were verified by 1H NMR spectra. However, we can't obtain the NMR of PBDTDTBTz because of its limited solubility. The copolymer PBDTBTz exhibits excellent solubility in common solvents such as THF, CHCl3, dichlorobenzene, and so forth, and PBDTDTBTz has limited solubility in the solvents. The number average molecular weight (Mn) of PBDTDTBTz and PBDTBTz is 9276 and 12387, with a PDI of 2.0 and 1.6, respectively.Thermal stability
The thermal stability of the two polymers was investigated with thermogravimetric analysis as shown in Fig. 1. The TGA analysis reveals that the 5% weight loss temperatures (Td) of the PBDTBTz and PBDTDTBTz are 310 and 324 °C, respectively, maybe due to the removal of the alkoxyl groups. The glass transition temperature (Tg) of the polymers was not observed from DSC measurements. The thermal stability of two polymers is favorable for their applications in PSCs and other optoelectronic devices.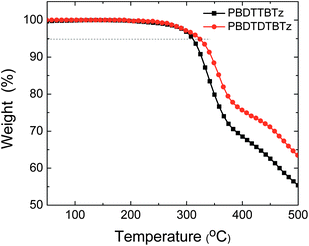 | ||
| Fig. 1 TGA plots of PBDTBTz and PBDTDTBTz with a heating rate of 10 °C min−1. | ||
Absorption spectra of the polymers
The photophysical characteristics of the copolymers were investigated by ultraviolet-visible (UV-vis) absorption spectra in diluted chloroform solutions and in solid films drop-cast on a quartz substrate. Fig. 2 depicts the absorption spectra of the polymer solutions and films, the optical data of the polymers are listed in Table 1. The absorption maxima peaks of PBDTDTBTz and PBDTBTz films are ca. 527 nm and 562 nm, respectively (see Fig. 2b), which is a little red-shifted in comparison with those of the polymer solutions (the absorption peaks of PBDTDTBTz and PBDTBTz solutions are located at 524 nm and 561 nm, respectively). This phenomena indicates that the conformation changed little from the solution to the film sate. The absorption of two polymer films exhibit well defined vibronic splitting peak at 524 nm and 566 nm for PBDTDTBTz and peaks at 517 nm and 561 nm for PBDTBTz, respectively, which reflects the high structural organization of the molecules in the films.11 The absorption edge of PBDTBTz and PBDTDTBTz are at 625 nm and 635 nm, corresponding to the energy gap of 2.0 eV and 1.95 eV, respectively. The absorption of the polymer and PC71BM (ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) blend films was observed. The blend films show similar broader absorption from 300–650 nm than those of pure polymer films due to the strong absorption of PC71BM between 400–530 nm.15
4) blend films was observed. The blend films show similar broader absorption from 300–650 nm than those of pure polymer films due to the strong absorption of PC71BM between 400–530 nm.15
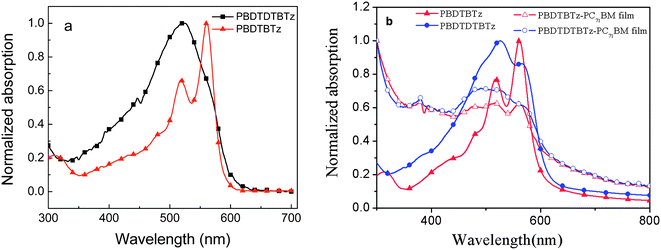 | ||
| Fig. 2 UV-vis absorption spectra of PBDTDTBTz and PBDTBTz: (a) solution in diluted CHCl3 at room temperature; (b) polymer films and polymer/PC71BM (1/4) blend on quartz. | ||
| Polymers | UV-vis-NIR absorption spectra | Cyclic voltammetry (vs Ag/Ag+) | ||||
|---|---|---|---|---|---|---|
| Solutiona | Filmb | p-doping | n-doping | |||
| λ max/nm | λ max/nm | λ onset/nm | E opt g /eV | E ox on (V)/HOMOd (eV) | LUMOe/eV | |
| a Measured in chloroform solution. b Cast from chloroform solution. c Bandgap estimated from the onset wavelength of the optical absorption.12 d HOMO= −e(Eoxon + 4.71) (eV).13 e LUMO = Eoptg + HOMO. | ||||||
| PBDTDTBTz | 524 | 527 | 635 | 1.95 | 0.35/−5.06 | −3.11 |
| PBDTBTz | 561 | 562 | 620 | 2.0 | 0.33/−5.04 | −3.04 |
Electrochemical properties
In order to investigate the electrochemical properties of PBDTBTz and PBDTDTBTz and estimate their the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels, cyclic voltammetry (CV) was carried out in a 0.1 M solution of Bu4NPF6 in acetonitrile at room temperature under argon with a scan rate of 50 mV s−1.13All potentials are reported vs. Ag/Ag+ with the ferrocene/ferrocenium couple used as an internal standard. CV curves of PBDTBTz and PBDTDTBTz are displayed in Fig. 3. The related electrochemical data are listed in Table 1. PBDTDTBTz exhibits one reversible oxidation peak while PBDTBTz shows a quasi-reversible oxidation peak. No reduction peak in the n-doping region is observed for these two polymers. In a positive potential region, the onset oxidation potential (Eoxon) is 0.33 V vs. Ag/Ag+ for PBDTBTz. For PBDTDTBTz, Eoxon is 0.35 V vs. Ag/Ag+.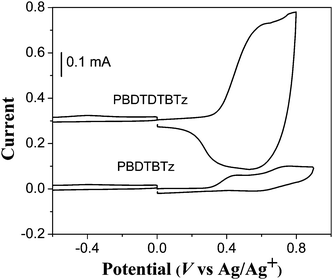 | ||
| Fig. 3 Cyclic voltammograms of PBDTBTz and PBDTDTBTz films on platinum electrode in 0.1 mol L−1 Bu4NPF6, CH3CN solution. | ||
From Eoxon of the polymers, we calculated the HOMO and LUMO energy levels of the polymer according to the equation:13
| EHOMO = −e(Eoxon + 4.71) (eV) |
We obtained ELUMO from the EoptEg and EHOMO. The ELUMO and EHOMO values of PBDTBTz are −3.04 eV and −5.04 eV, respectively. The ELUMO and EHOMO values of PBDTDTBTz are −3.11 eV and −5.06 eV, respectively, the HOMO level of PBDTBTz is very similar to that of PBDTDTBTz. The electrochemical results show that the two copolymers exhibit similar energy level.
Photovoltaic devices
To explore the potentials for solar cells, the photovoltaic properties of the copolymers were investigated in two composites (with either PC61BM or PC71BM) with the structure of ITO/PEDOT:PSS (40 nm)/polymer:fullerenes/Ca (20 nm)/Al (60 nm). Fig. 4a shows the J–V curves of the polymer solar cells with a polymer: PC61BM 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight ratio. The active layer thickness is about 50–60 nm. The relationship between the thickness of the active layer and PCE was investigated by controlling the spinning speed during the spin coating process. We found that the thickness is increased, the Jsc decreased slightly, while the Voc and FF seemed the same. This phenomenon indicates that the photovoltaic properties of the polymers may be limited by the poor charge transporting properties of the blend film.14 The optimized PBDTDTBTz device had a thickness of 60 nm, a Jsc value of 4.4 mA cm−2, and a relatively high FF of 0.55, combined with a Voc value of 0.61 V to give an efficiency of 1.5%. However for PBDTBTz, due to the relatively low FF and Jsc, the PCE of PBDTBTz was only 0.7%.
3 weight ratio. The active layer thickness is about 50–60 nm. The relationship between the thickness of the active layer and PCE was investigated by controlling the spinning speed during the spin coating process. We found that the thickness is increased, the Jsc decreased slightly, while the Voc and FF seemed the same. This phenomenon indicates that the photovoltaic properties of the polymers may be limited by the poor charge transporting properties of the blend film.14 The optimized PBDTDTBTz device had a thickness of 60 nm, a Jsc value of 4.4 mA cm−2, and a relatively high FF of 0.55, combined with a Voc value of 0.61 V to give an efficiency of 1.5%. However for PBDTBTz, due to the relatively low FF and Jsc, the PCE of PBDTBTz was only 0.7%.
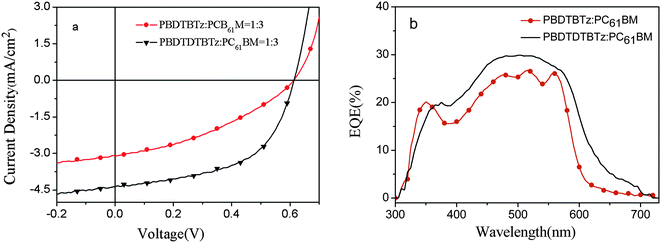 | ||
Fig. 4 (a) J–V curves of polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) based solar cell devices under illumination of AM 1.5G, 100 mW cm−2; (b) IPCE curves of the polymer 3) based solar cell devices under illumination of AM 1.5G, 100 mW cm−2; (b) IPCE curves of the polymer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) based device. 3) based device. | ||
The corresponding incident-photon-to current efficiency (IPCE) from polymer/PC61BM BHJ solar cells is shown in Fig. 4b. From their EQE curves of the polymers, it can be seen that the response range of PBDTDTBTz based device is relatively broader than that of PBDTBTz based device, in the range from 350 nm to 750 nm, PBDTDTBTz based devices also exhibit higher EQE values with a maximum plateau external quantum efficiency of 30% from 465 nm to 530 nm than those of PBDTBTz based devices, so Jsc of the PBDTDTBTz (4.4 mA cm−2) based devices is higher than that of PBDTBTz (3.1 mA cm−2).
It has been reported that PC71BM has similar electronic properties as PC61BM, but a higher absorption coefficient from 400–530 nm.15 The active layer was then further modified with the weight ratio of polymer: PC71BM at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and identical thickness of around 50– 60 nm. For the devices with PC71BM, the optimized weight ratio is 1
4 and identical thickness of around 50– 60 nm. For the devices with PC71BM, the optimized weight ratio is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. J-V curves and IPCE of the polymer/PC71BM (1
4. J-V curves and IPCE of the polymer/PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) based solar cell devices are shown in Fig. 5. The effect of the polymer: PCBM weight ratio on the photovoltaic performance can be explained as following. There is a balance between the absorbance and the charge transporting network of the active layer in the PSC devices. Too low content of PCBM will limit the electron transporting ability, while too high content of PCBM will decrease the absorbance and enhance the electron transporting ability of the active layer. The different optimized weight ratios for PC61BM and PC71BM could be due to their different electron transport and absorption properties.
4) based solar cell devices are shown in Fig. 5. The effect of the polymer: PCBM weight ratio on the photovoltaic performance can be explained as following. There is a balance between the absorbance and the charge transporting network of the active layer in the PSC devices. Too low content of PCBM will limit the electron transporting ability, while too high content of PCBM will decrease the absorbance and enhance the electron transporting ability of the active layer. The different optimized weight ratios for PC61BM and PC71BM could be due to their different electron transport and absorption properties.
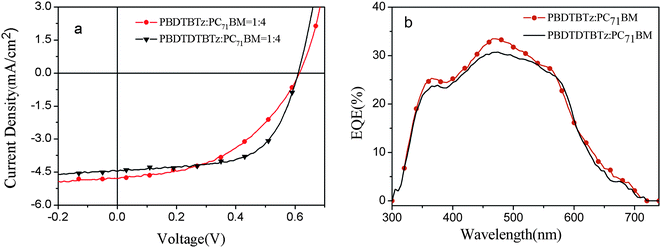 | ||
Fig. 5 (a) J–V curves of polymer/PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) based solar cell devices under illumination of AM 1.5G, 100 mW cm−2; (b) IPCE curves of the polymer/PC71BM (1 4) based solar cell devices under illumination of AM 1.5G, 100 mW cm−2; (b) IPCE curves of the polymer/PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) based device. 4) based device. | ||
From the data listed in Table 2, it can be seen the main difference between the photovoltaic performances of the polymers at different device conditions appeared in the Jsc and FF while the Voc remained constant. Compared to the polymer/PC61BM system, the FF improved which is indicative of better charge mobility balance in the polymer/PC71BM blend. Higher Jsc values were observed which may be the higher absorption coefficient of PC71BM in the visible region. For PBDTBTz, Jsc increased from 3.1 mA cm−2 to 4.8 mA cm−2, which is even higher than that of PBDTDTBTz, probably because the PC71BM can greatly compensate for the low absorption of PBDTBTz from 400– 530 nm. However, when PBDTDTBTz mixed with PC71BM, the device demonstrated a very high FF of 0.62, Jsc of 4.5 mA cm−2 with the device PCE of 1.7%. For PBDTBTz/PC71BM BHJ solar cells, with the increased Jsc and FF, the PCE of PBDTBTz increased from 0.7% to 1.4%. Compared to PBDTBTz-based photovoltaic cells, the PBDTDTBTz possesses higher FF, which is probably due to the smoother morphology and better interpenetrating network in the PBDTDTBTz/PCBM blend (the morphology of the blend is as shown in Fig. 6).
Polymers![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM ratio PCBM ratio |
Thickness | V oc/V | I sc/mA cm−2 | FF (%) | PCE (%) |
|---|---|---|---|---|---|
PBDTDTBTz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
60 nm | 0.61 | 4.4 | 55 | 1.5 |
PBDTDTBTz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
58 nm | 0.61 | 4.5 | 62 | 1.7 |
PBDTBTz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC61BM (1 PC61BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
56 nm | 0.61 | 3.1 | 37 | 0.7 |
PBDTBTz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
57 nm | 0.61 | 4.8 | 47 | 1.4 |
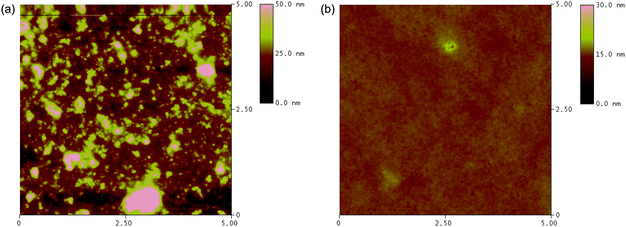 | ||
Fig. 6 AFM (5μm × μm) topography images of (a) PBDTBTz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); (b) PBDTDTBTz 4); (b) PBDTDTBTz![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC71BM (1 PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) blend films. 4) blend films. | ||
Conclusions
Two new benzotriazole-based polymers, PBDTDTBTz and PBDTBTz, were designed and synthesized through the typical Stille coupling polycondensation reactions. The polymers combine good thermal stability and broad absorption. The photovoltaic devices demonstrated a power conversion efficiency of up to 1.7% and 1.4%, respectively for PBDTDTBTz and PBDTBTz, under the illumination of AM 1.5, 100 mW cm−2 with a device structure of ITO/PEDOT:PSS/polymer:PC71BM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4)/Ca/Al. The results indicate that the BTz-based copolymers are promising for application in PSCs. We believe that with further structural and device optimizations, increased power efficiencies are possible in the future.
4)/Ca/Al. The results indicate that the BTz-based copolymers are promising for application in PSCs. We believe that with further structural and device optimizations, increased power efficiencies are possible in the future.
Acknowledgements
The authors acknowledge Miao Yang and Ping Ding for the synthesis of the intermediates. This work was supported by National Natural Science Foundation for Distinguished Young Scholar (No. 50825102), National Natural Science Foundation of China (Nos. 50803074, 50633050, 20976199), Lieying Project, the Fundamental Research Funds for the Central Universities, the Opening Fund of State Key Laboratory of Powder Metallurgy and Start-up fund of Central South University.References
- (a) Y. Li and Y. Zou, Adv. Mater., 2008, 20, 2952 CrossRef CAS; (b) P. Shen, B. Zhao, X. Huang, H. Huang and S. Tan, Eur. Polym. J., 2009, 45, 2726 CrossRef CAS.
- (a) J. Hou, M.-H. Park, S. Zhang, Y. Yao, L.-M. Chen, J.-H. Li and Y. Yang, Macromolecules, 2008, 41, 6012 CrossRef CAS; (b) Y. Zou, D. Gendron, R. Badrou-Aïch, A. Najari, Y. Tao and M. Leclerc, Macromolecules, 2009, 42, 2891 CrossRef CAS; (c) P. L. T. Boudreault, S. Beaupré and M. Leclerc, Polym. Chem., 2010, 1, 127 RSC; (d) N. Blouin, A. Michaud, D. Gendron, S. Wakim, E. Blair, R. N. Plesu, M. Belletête, G. Durocher, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2008, 130, 732 CrossRef CAS.
- H. Chen, J. Hou, S. Zhang, Y. Liang, G. Yang, Y. Yang, L. Yu, Y. Wu and G. Li, Nat. Photonics, 2009, 3, 649 Search PubMed.
- Y. Zou, A. Najari, P. Berrouard, S. Beaupré, R. B. Aich, Y. Tao and M. Leclerc, J. Am. Chem. Soc., 2010, 132, 5330 CrossRef CAS.
- (a) M. Svensson, F. Zhang, S. C. Veenstra, W. J. H. Verhees, J. C. Hummelen, J. M. Krron, O. Inganäs and M. R. Andersson, Adv. Mater., 2003, 15, 988 CrossRef CAS; (b) E. G. Wang, L. Wang, L. F. Lan, C. Luo, W. L. Zhuang, J. B. Peng and Y. Cao, Appl. Phys. Lett., 2008, 92, 033307 CrossRef; (c) L. J. Huo, H. Y. Chen, J. H. Hou, T. L. Chen and Y. Yang, Chem. Commun., 2009,(37), 5570 RSC; (d) E. J. Zhou, M. Nakamura, T. Nishizawa, Y. Zhang, Q. Wei and K. Tajima, Macromolecules, 2008, 41, 8302 CrossRef CAS.
- D. Baran, A. Balan, S. Celebi, B. M. Esteban, H. Neugebauer, N. S. Sariciftci and L. Toppare, Chem. Mater., 2010, 22, 2978 CrossRef CAS.
- Y. Zou, D. Gendron, R. Neagu-Plesu and M. Leclerc, Macromolecules, 2009, 42, 6361 CrossRef CAS.
- A. Balan, D. Baran, G. Gunbas, A. Durmus and L. Toppare, Chem. Mater., 2008, 20, 7510 CrossRef CAS.
- Y. Liang, Y. Wu, D. Feng, S. T. Tsai, H. J. Son, G. Li and L. Yu, J. Am. Chem. Soc., 2009, 131, 56 CrossRef CAS.
- Y. Zou, W. Wu, G. Sang, Y. Yang, Y. Liu and Y. Li, Macromolecules, 2007, 40, 7231 CrossRef CAS.
- (a) B. S. Ong, Y. Wu, P. Liu and S. Gardner, Adv. Mater., 2005, 17, 1141 CrossRef CAS; (b) M. Leclerc, Adv. Mater., 1999, 11, 1491 CrossRef.
- Y. Zou, G. Sang, W. Wu, Y. Liu and Y. Li, Synth. Met., 2009, 159, 182 CrossRef CAS.
- (a) Q. Sun, H. Wang, C. Yang and Y. Li, J. Mater. Chem., 2003, 13, 800 RSC; (b) Y. F. Li, Y. Cao, J. Gao, D. L. Wang, G. Yu and A. J. Heeger, Synth. Met., 1999, 99, 243 CrossRef CAS.
- Y. Yang, J. Zhang, Y. Zhou, G. Zhao, C. He, Y. Li, M. Andersson, O. Inganàs and F. Zhang, J. Phys. Chem. C, 2010, 114, 3701 CrossRef CAS.
- M. M. Wienk, J. M. Kroon, W. J. H. Verhees, J. Knol, J. C. Hummelen, P. A. val Hal and R. A. J. Janssen, Angew. Chem., Int. Ed., 2003, 42, 3371 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
