Facile one-pot approach for preparing fluorescent and biodegradable hyperbranched poly(amidoamine)s†
Guohua
Jiang
*ab,
Yin
Wang
b,
Xinke
Sun
b and
Jianjun
Shen
b
aKey Laboratory of Advanced Textile Materials and Manufacturing Technology, Zhejiang Sci-Tech University, Ministry of Education, Hangzhou, 310018, P. R. China. E-mail: polymer_jiang@hotmail.com; Tel: +86-571-86843527
bDepartment of Materials Engineering, College of Materials and Textile, Zhejiang Sci-Tech University, Hangzhou, 310018, P. R. China
First published on 19th March 2010
Abstract
Hyperbranched poly(amidoamine)s were synthesized by Michael addition of 1-(2-aminoethyl) piperazine (AEPZ) and methyl acrylate (MA). The resultant polymers can emit bright blue fluorescence intensity under excitation wavelength and their degradability depended on the environmental pH value.
During the past two decades, research on fluorescent polymers has become an interesting area because of their versatile application as luminescent probes in various fields.1–3 Among them, water-soluble fluorescent polymers are particularly useful tracers because they act as analogs in understanding the behavior of biological macromolecules.4,5 In general, there are two ways to synthesize such fluorescent polymers. One of them is the polymerization of polymerizable fluorescent monomers with some common monomers,6,7 the other is the chemical modification of polymers with fluorescent molecules or fluorescent oligomers.8,9 However, the synthesis of these fluorescent polymers requires multiple steps and it is cumbersome to synthesize and purify these polymers. Hyperbranched polymers are three-dimensional macromolecules with good solubility and high reactivity. They are usually prepared by one-pot synthesis from specific monomers with branching potential.10–12 Fortunately, polymer chemists found some dendrimers show an intrinsic fluorescent property under certain conditions. For example, poly(amidoamine) (PAMAM) is a commercialized dendrimer, and can emit a weak blue luminescence in aqueous solution, which becomes stronger after oxidation or acidication.13,14 As we all know, hyperbranched polymers have structures and functions similar to dendrimers, albeit with a low degree of branching, but their synthesis is much easier. If they could fluoresce strongly this would simplify the synthesis process and widen application areas for these polymers.
The aim of the present work was to develop a facile and one-pot method to prepare hyperbranched polymers with striking fluorescence properties. Scheme 1 shows the synthesis process for hyperbranched poly(amidoamine)s (HPAMAMs). For characterization of their structures, 1H NMR and 13C NMR spectra of the products, which were prepared respectively from the polymerization of AEPZ and MA at 40 and 80 °C, were measured. As shown in Fig. 1, the signals at δ = 181.2 and 174.6 ppm indicate the existence of carbonyl groups with different micro-environments. The carbon signals of carbonyl groups next to secondary amine appeared at δ = 181.2 ppm, and those next to tertiary amines appeared at δ = 174.6 ppm. More carbonyl groups next to a secondary amine are clearly seen for the polymers obtained higher temperature. When the polymerization was performed at a relatively high temperature, such as 80 °C, the 2° amines (formed) became reactive and were involved in the polymerization. The carbon signals of the methylene next to secondary amine appeared at δ = 36.2 ppm, and the carbon signals of three methylene adjacent tertiary amine groups at 43.8 ppm can be clearly seen for the polymers obtained from two samples. The signals at δ = 36.5 ppm indicate the existence of a terminal primary amine group in the polymers. Thus, two PAMAM samples with a different quantity of branching were successfully prepared. The relatively stronger peak at δ = 34.5 and 57.7 ppm can be observed indicate the existence of a higher branching structure in the polymer, resulting in the HPAMAM, which was also verified by their 1H NMR spectra, as shown in Fig. 2, the intensity of proton signals (e and f) of two methylene groups adjacent to tertiary amines at δ = 2.3 ppm increased. In general, the polymerization of AEPZ and MA at lower temperature (around 40 °C) produced low branching polymers, however, higher temperature (around >60 °C) yielded high branching polymers.
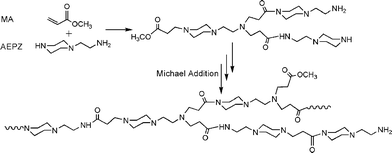 | ||
| Scheme 1 The reaction scheme for preparing fluorescent and biodegradable hyperbranched poly(amidoamine)s (HPAMAMs) from AEPZ and MA. | ||
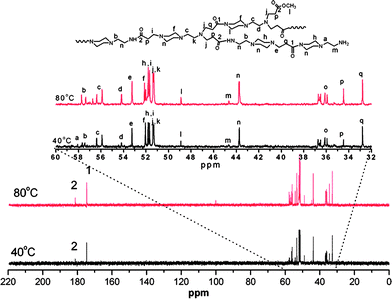 | ||
| Fig. 1 13C NMR spectra of polymers recorded in CDCl3 at 25 °C for the polymers obtained from the polymerization at 40 and 80 °C. | ||
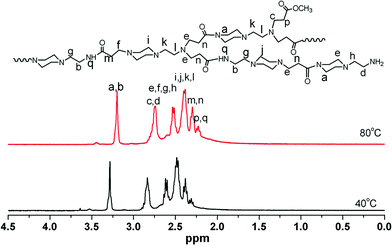 | ||
| Fig. 2 1 H NMR spectra of polymers recorded in CDCl3 at 25 °C for the polymers obtained from the polymerization at 40 and 80 °C. | ||
The low and high branching samples, which were obtained from the polymerization at 40 and 80 °C, were used to study the UV-visible properties. As shown in Fig. 3, both the samples in aqueous solution (1 wt%) showed a strong absorption band at 277 nm and weak shoulder band at 355 nm. However, the absorption intensity for the high branching sample is stronger than that of the low branching sample.
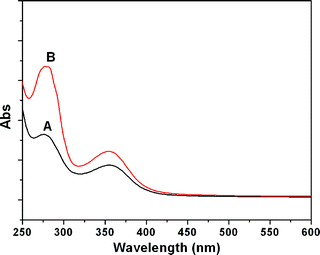 | ||
| Fig. 3 UV-visible spectra of HPAMAM aqueous solution samples with concentrations of 1 wt% (A and B are samples from the polymerization at 40 and 80 °C, respectively). | ||
Since the resultant polymers do not contain fluorescent functional groups from the classical viewpoint, exploring the source of the fluorescence should be interesting.15,16 As a matter of fact, we observed strong blue photoluminescence from the resultant polymers without any treatment or functionalization. Fig. 4 shows the fluorescence excitation and emission spectra of two samples. The sample obtained at 80 °C has excited bands at 260 and 325 nm. Under light irradiation at 260 nm, two emission bands at 320 and 420 nm can be observed. The intensity of the emission band at 320 nm is weaker than that at 420 nm. However, only an emission band at 410 nm is observed under light irradiation at 325 nm. For the sample obtained at 40 °C, similar phenomena are observed. The main difference present in this sample is the weaker excited band at 320 nm and emission bands at 320 and 420 nm. The weaker blue fluorescence intensity under an excitation wavelength of 365 nm can clearly seen from illumination photographs of aqueous solutions with concentration at 5 and 0.5 wt%. Similar interesting phenomena have already been observed by some groups. For example, Bard and co-workers13 reported that commercial poly(amidoamine) dendrimers (PAMAM) to show blue photoluminescence after simple oxidation treatment. Wang and Imae14 reported fluorescence emission from PAMAM upon adjusting the pH value. Very recently, Wang and Pan17 reported that PAMAM synthesized by Michael addition polymerization of N,N-cystaminebisacrylamide and 1-(2-aminoethyl) piperazine displayed bright fluorescence, and the emissions bands cover nearly the whole visible wavelength range. However, the fluorescence mechanism remains unresolved. We speculated that the fluorescence property of resultant polymers was in close relationship with their architecture. Since the low branching polymer with a lot of secondary amines and a few tertiary amines used in this study emitted only weak fluorescence, we presumed that the tertiary nitrogen with lone pair electrons inside the high branching structure played an important role in the photoluminescence, which is similar to other results reported.13–17
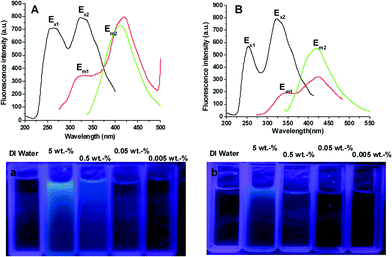 | ||
| Fig. 4 Emission (EM) and excitation (EX) spectra for two samples prepared respectively from the polymerization at 40 (A) and 80 °C (B) and illumination photographs (a and b) of their aqueous solutions with different concentrations under 365 nm irradiation. | ||
The high branching sample (80 °C) was used to investigate the degradation properties at room temperature. The molecular weight of degraded samples as a function of time is shown in Fig. 5. Under a basic environment, the polymer is easier to degrade, which is in agreement with the general principle of degradation for ester groups. In an acidic environment, degradation is faster, then slower as compared to that in a neutral solution. After 5 days, the molecular weight of the polymer decreases to about one-half of its initial value.
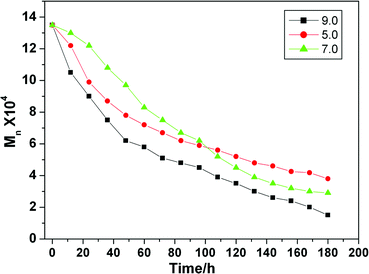 | ||
| Fig. 5 The molecular weight of degraded HPAMAM samples as a function of degradation time at different pH values. | ||
In conclusion, biodegradable hyperbranched poly(amidoamine) polymers have been successfully synthesized by Michael addition polymerization of AEPZ and MA. The resultant polymers can emit bright blue fluorescence intensity under excitation wavelength. The degradability of the polymers reported here depended on the environmental pH value. The simplicity of the synthesis process, low cost of raw materials, water-solubility, degradability, and branched backbone would make the polymers very attractive as good candidates for biological materials. Investigation of their potential applications in fluorescence imaging technology and biological science is in progress.
This work was financially supported by the National Natural Science Foundation of China (20604024), Natural Science Foundation of Zhejiang Province (Y406008), Scientific Research Foundation of Zhejiang Sci-Tech University (0901808-Y), Training Foundation for the Excellent Young Talents by the Key Laboratory of Advanced Textile Materials and Manufacturing Technology (ATMT), Ministry of Education (2008QN10) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT: 0654).
References
- S. A. Kushon, K. D. Ley, K. Bradford, R. M. Jones, D. McBranch and D. Whitten, Langmuir, 2002, 18, 7245 CrossRef CAS.
- H. Gao, C. Wang, W. Yang and S. Fu, J. Macromol. Sci., Part A: Pure Appl. Chem., 2004, 41, 357 Search PubMed.
- P. Wang, X. Wang, K. Meng, S. Hong, X. Liu, H. Cheng and C. C. Han, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3424 CrossRef CAS.
- N. J. Turro and K. S. Arora, Polymer, 1986, 27, 783 CrossRef CAS.
- C. Xue, S. P. Jog, P. Murthy and H. Liu, Biomacromolecules, 2006, 7, 2470 CrossRef CAS.
- L. F. Campo, F. S. Rodembusch and V. Stefani, J. Appl. Polym. Sci., 2006, 99, 2109 CrossRef CAS.
- Q. Xu, N. Li, F. Yan, X. Xia, J. Lu and X. Wen, Eur. Polym. J., 2008, 44, 1874 CrossRef CAS.
- S. Saffarian, Y. Li, E. L. Elson and J. Pike, Biophys. J., 2007, 93, 1021 CrossRef CAS.
- O. Malicka, I. Gryczynski, J. Fang and J. R. Lakowicz, Anal. Biochem., 2003, 317, 136 CrossRef.
- G. Jiang, L. Wang and W. Chen, Eur. Polym. J., 2006, 42, 3333 CrossRef CAS.
- G. Jiang, L. Wang, H. Yu, C. Chen, X. Dong, T. Chen and Q. Yang, Polymer, 2006, 47, 12 CrossRef CAS.
- D. Yan, Y. Zhou and J. Hou, Science, 2004, 303, 65 CrossRef CAS.
- W. I. Lee, Y. J. Bae and A. J. Bard, J. Am. Chem. Soc., 2004, 126, 8358 CrossRef CAS.
- D. Wang and T. Imae, J. Am. Chem. Soc., 2004, 126, 13204 CrossRef CAS.
- Y. Lin, J.-W. Gao, H.-W. Liu and Y.-S. Li, Macromolecules, 2009, 42, 3237 CrossRef CAS.
- D.-J. Wang, D.-D. Jia, Y.-G. Zhao, X.-H. Shen and Y.–D. Yang, Chin. Chem. Lett., 2006, 17, 502 CAS.
- W. Wang and C.-Y. Pan, Macromol. Rapid Commun., 2009, 30, 2096 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed synthesis procedures and characterization. See DOI: 10.1039/c0py00057d |
| This journal is © The Royal Society of Chemistry 2010 |
