Highly ordered microporous films containing a polyolefin segment fabricated by the breath-figure method using well-defined polymethylene-b-polystyrenecopolymers
Jian
Li
ab,
Qiao-Ling
Zhao
a,
Jian-Zhuang
Chen
a,
Lei
Li
*b,
Jin
Huang
a,
Zhi
Ma
*a and
Ya-Wen
Zhong
b
aShanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345#, Lingling Road, Shanghai, 200032, P. R. China. E-mail: mazhi728@mail.sioc.ac.cn; Fax: +86-21-54925395; Tel: +86-21-54925388
bCollege of Materials, Xiamen University, Xiamen, 361005, P. R. China. E-mail: lilei@xmu.edu.cn
First published on 11th December 2009
Abstract
Highly ordered microporous films containing polyolefin segment were successfully fabricated via the breath-figure (BF) method using well-defined polymethylene-b-polystyrene (PM-b-PS) diblock copolymers in CS2 under a static humid condition. The effects of molecular weight of diblock copolymers, relative humid, fabrication method and temperature on the morphology of film are investigated. The honeycomb porous films with different pore size (1.2–2.5 μm) were obtained by using PM-b-PS with different PM : PS ratio. The thick (7 μm) and thin (2 μm) films were fabricated via a different process. The film with a pothole-like structure was fabricated when using PM-b-PS with the shortest PS segment. Smaller satellite pores were found to surround the regular micropores of honeycomb films when the BF process was carried out at 28 °C.
Introduction
Porous materials based on polyolefins are widely used in various applications such as matrices for functional films,1 films for in-package processing of food,2 conical orbital implant,3 superhydrophobic film4 and so on. Until now, porous polyolefins have been fabricated by several strategies including a process based on melt extrusion with subsequent annealing and uniaxial extension,5gel -technology based on phase separation,6 technology using a polyolefin melt/channeling agent system3 and the controlled crystallization of polyolefins,4,7etc. However, to the best of our knowledge, there haven't yet been any reports on the fabrication of highly ordered porous polyolefins or their composites which are believed to have special surface properties themselves or after functionalization by physical or chemical methods.Recently, highly ordered honeycomb-structured microporous films fabricated by the so-called breath-figure (BF) method first described by François and co-workers8 have attracted a great deal of attention in the research area of functional materials.9 The formation of highly ordered honeycomb films in the BF procedure were influenced by various parameters such as relative humidity (RH), temperature, solvent, the architecture and component of polymers, polymer concentration etc.10 To fabricate such porous films targeting different application through the BF method, a variety of polymers with different architectures have been employed such as rod–coil block copolymers ,8,11block copolymers ,10,12 conjugated polymers,13 amphiphilic copolymers ,14dendronized polymers,15star polymers ,16 core-crosslinked star polymers ,17 just to name a few. However, to the best of our knowledge, there has been no study on the fabrication of highly ordered honeycomb film using polymers with non-polar polyolefin segments. Here we report the fabrication of honeycomb polyolefin composite films via the BF method using well-defined polymethylene-b-polystyrene (PM-b-PS) diblock copolymers.
Experimental
Synthesis of PM-b-PS
A series of narrow molecular weight distributed PM-b-PSdiblock copolymers with different molecular weight ratios of PM and PS segments were synthesized according to the procedure described in our previous work.18Preparation of honeycomb films
Two different methods were employed to fabricate the porous films. Method 1:14e one solution of PM-b-PS in CS2 (3.0 wt%) was cast onto the surface of glass slide with a microsyringe in a glass vessel with a cap at 20 °C or 28 °C until the solvent evaporated totally. Saturated relative humidity in the glass vessel was achieved by adding several droplets of distilled water beforehand. After complete solvent evaporation, a honeycomb thin film was formed on a glass slide. Method 2: the glass slide was dipped into the solution of PM-b-PS in CS2 (3.0 wt%), drawn out slowly and sustained by a bracket in the vessel describe above. When the solvent had evaporated completely, the white and opaque films on the both sides were formed.The honeycomb films were observed on a scanning electron microscope (JEOL 6390LV, Japan) operated at 10 kV. Atom force microscopic (AFM) images were obtained using a Veeco Nanoscope IVa Multimode system in tapping mode. A silicon cantilever with a bending spring constant of 20–60 N/m and a resonance frequency of about 300 kHz was used for imaging at a scan rate of 0.5–1.0 Hz.
Results and discussion
Among the various copolymers used in the BF method to fabricate the honeycomb films, diblock copolymers with non-polar polyolefin segments have not been employed yet. In our work, a series of PM-b-PSpolymers (PM2k-b-PS10k, PM2k-b-PS8k, PM2k-b-PS5k and PM2k-b-PS2k. Note: the subscript numbers mean the number molecular weight (g mol−1) of PM and PS segments; k = 103) with narrow molecular weight distributions (Mw/Mn < 1.1) were firstly prepared via a combination of living ylidespolymerization and atomic transfer radical polymerization according to our previous work18 (Scheme 1) and then employed in the BF procedure to fabricate polymer films.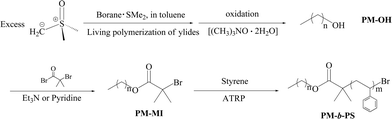 | ||
| Scheme 1 Synthesis of polymethylene-b-polystyrene (PM-b-PS) via a combination of living ylidespolymerization and ATRP of styrene.18 | ||
The fabrication of honeycomb structured polyolefin composite films was carried out at 20 °C in a static humid conditions of 50% relative humid (RH) via casting14e or dip coatingpolymer solution onto a clean glass substrate. In our cases, considering the solubility of PM-b-PS in CS2, the PM2k-b-PS10k with the highest molecular weight of PS which can be well-dissolved in CS2, was firstly used in the BF method. As observed by scanning electron microscopy (SEM) in Fig. 1(a), a highly ordered honeycomb structure film with average pore diameters of 2.5 μm was successfully fabricated via the BF method. Furthermore, the Mn of the PS segment was decreased gradually to 8000, 5000 and 2000 g mol−1, respectively, in order to know how the content of PS in PM-b-PS influences the morphology of the films. The results indicated that both PM2k-b-PS8k and PM2k-b-PS5k in CS2 solution can still generate the honeycomb films under the same casting condition (Fig. 1(b) and 1(c)). The average pore diameter of such films decreased to 1.50 μm and 1.40 μm, respectively. Although various mechanisms for the BF method have been proposed, the water templating mechanism8 is generally used to explain the formation of this ordered morphology, i.e., in our cases, firstly, water in the humid environment condensed onto the surface of the polymer solution because of its significantly decreased temperature resulting from rapid evaporation of solvent. Secondly, the water droplets arranged into a hexagonal array, sank into the polymer solution and were stabilized from coalescence by the precipitated PM-b-PS at the water–solvent interface. Finally, the highly ordered honeycomb film was generated after the evaporation of water droplets and solvent. According to the mechanism mentioned above , the faster evaporation of CS2, which results from the decreased viscosity of the solution when using polymers with lower molecular weight, probably leads to smaller pores.10b
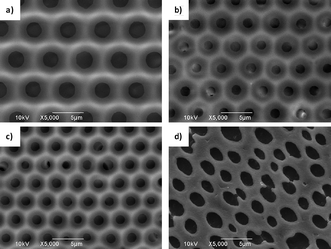 | ||
| Fig. 1 SEM images of porous PM-b-PS films fabricated via the casting/BF method at 20 °C in 50% RH. (a) PM2k-b-PS10k; (b) PM2k-b-PS8k; (c) PM2k-b-PS5k; (d) PM2k-b-PS2k. | ||
Interestingly, an irregular oval pothole-like structure was formed on the surface of film when using PM2k-b-PS2k with a shorter PS segment (Fig. 1(d)). This phenomenon will be discussed later.
The AFM images display the regular structure of the film (Fig. 2) with pore diameter of 1.2 μm (from point b and point c in the section line, Fig. 2(a)) and spacing between pores (from point a to point b in the section line, Fig. 2(a)) of 1 μm. The three-dimensional (3D) image of the honeycomb film is shown in Fig. 2(b). The inversion-cone indicates that the tip of AFM cantilever can't touch the bottom of the pore in the film.
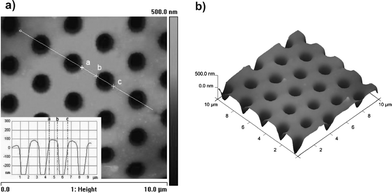 | ||
| Fig. 2 AFM images of PM2k-b-PS 5k honeycomb film. (a) height image (inset: section analysis of the white straight line); (b) 3D image of film. | ||
As reported the literature (ref. 10b and references therein), the relative humidity in the BF process has an important influence on the pore diameter and the regularity of films. The SEM images in Fig. 3 show the different morphology of the films (PM2k-b-PS8k) fabricated in two different relative humidities. Irregular pores were formed when the relative humidity increased to 70% (Fig. 3(a)) and 80% (Fig. 3(b)). Meanwhile, the average diameter of pores increased to 1.6 μm to 2.1 μm, respectively.
 | ||
| Fig. 3 SEM images of PM2k-b-PS8k honeycomb films fabricated in different relative humidities. (a) 70% RH; (b) 80% RH. | ||
The presence of the PS segment could benefit the solubility of PM-b-PS in CS2 by forming micelles similar to those in THF.18 So, the molar ratio of PS : PM might have profound influence on the formation of honeycomb or oval pothole-like structures. Moreover, the chain end bromine of the block copolymer , being a hydrophilic group, is also presumed to play an important role in the BF procedure. Further investigations on the mechanism of BF procedure in our case are currently underway.
Thick honeycomb films with one layer pores (∼2 μm depth) and dense polymer layer (∼5 μm thickness) (Fig. 4(b)) were generally fabricated by the casting method following a static BF procedure. In order to obtain thin films , the dipping procedure of polymer solution (Method 2) was employed and then the BF method was performed under a static humid environment. As shown in Fig. 4(a), we were delighted to find that the thickness of film fabricated by the dipping mode is sharply decreased and there is only a very thin layer of dense polymer. The SEM image in tilt angle of 40° (Fig. 4(c)) reveals that there are regular ‘pillars’ underneath the surface of the film and inter-connected holes. When the upper porous layer was peeled off, the pincushion structure could be observed (Fig. 4(d) and 4(f)). The diameter of the water droplet sunk in polymer solution is speculated to be about 2 μm from the SEM image in Fig. 4(d). The residual thin film walls connecting ‘pillars’ (the parts directed by arrows, Fig. 4(e) and 4(f)) are thought to be one evidence for the formation of precipitated polymer layers which surround the water droplets and prohibit their coalescence.
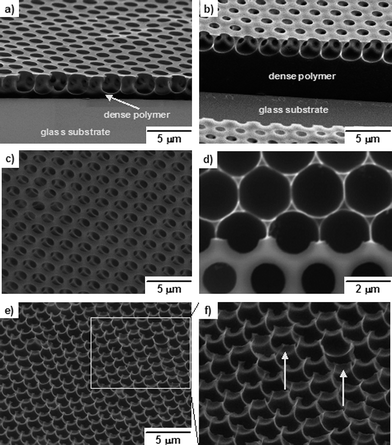 | ||
| Fig. 4 SEM images of honeycomb films of PM2k-b-PS10k. (a) and (c)–(f) PM2k-b-PS10k film fabricated by the dipping/BF method at 20 °C in 50% RH; (b) PM2k-b-PS10k film fabricated by the casting/BF method at 20 °C in 50% RH; (c) side view at tilt angle 40°; (d) top view; (e) pincushion structure (side view at tilt angle 40°); (f) magnified local view of (e). | ||
As mentioned earlier, the film with irregular morphology was obtained via the casting/BF method using PM2k-b-PS2k. Different from the morphology shown in Fig. 4(b), SEM image of the cross-sectioned film in Fig. 5(a) and the three-dimensional (3D) AFM image in Fig. 5(b) show irregular potholes separated by walls with thickness of 1–3 μm instead of partially penetrated holes. The factors which have influence on the stability and shape of water droplets, i.e., the aggregation state of PM2k-b-PS2k with lower PS : PM ratio in CS2, the decreased solubility, the concentration of Br, etc., might be possible explanations for the formation of this structure. Further research will be carried out to investigate the formation mechanism of such a morphology.
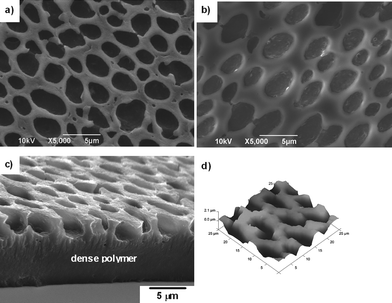 | ||
| Fig. 5 SEM and AFM images of PM2k-b-PS2k film. (a) and (b) top view; (c) side view; (d) 3D AFM image. | ||
A variety of parameters in the BF method such as temperature, relative humidity, polymer structure and polymer concentration, etc., should be well controlled to fabricate the porous film with regular pattern. In an experiment using PM2k-b-PS10k, when the system temperature was accidentally raised to 28 °C, some satellite pores were formed surrounding the regular micropores (Fig. 6). This structure was mentioned by Stenzel and co-workers12b and explained as formation of water-swollen inverse aggregates. While, the mechanism in our case seems to be very different because the PM-b-PS is mainly hydrophobic except for the bromine end group. So, a possible explanation will be given after further investigation.
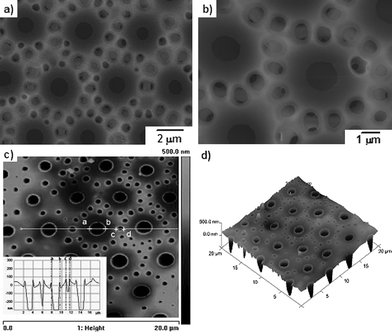 | ||
| Fig. 6 SEM and AFM images of PM2k-b-PS10k film fabricated at 28 °C in 50% RH (a) SEM top view of film; (b) magnified image of (a); (c) AFM height image (inset: section analysis of the white straight line); (d) 3D AFM image of film. | ||
Conclusions
In summary, we have successfully fabricated honeycomb polyolefin composite films via the BF method using well-defined PM-b-PSdiblock copolymers. The length of PS segment plays important role in the morphology of polymer film. The honeycomb films were obtained by using the solutions of PM2k-b-PS10k, PM2k-b-PS8k and PM2k-b-PS5k in CS2. Interestingly, the PM2k-b-PS2k with shorter PS segments can fabricate films with pothole-like structure. The structure of regular micropores surrounded by some satellites was formed when the system temperature increased to 28 °C. The possible formation mechanisms of different morphologies, as well as the relationship between film structure and various parameters in the BF procedure, are under investigation.Acknowledgements
Z. Ma thanks the National Natural Science Foundation of China (No. 20604032) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars (State Education Ministry, 2006) for financial supports. L. Li gratefully acknowledges the National Natural Science Foundation of China (No. 50703032) and the Program for New Century Excellent Talents of Ministry of Education of China. We acknowledge Prof. Y. Tang of Shanghai Institute of Organic Chemistry (Chinese Academy of Science) for his great help and Dr F. D'Agosto of Chimie Catalyse Polymères et Procédés (UMR 5265 CNRS/ESCPE/UCB Lyon1) for advice on the synthesis of PM-b-PS.Notes and references
- (a) W. Lee, T. Oshikiri, K. Saito, K. Sugita and T. Sugo, Chem. Mater., 1996, 8, 2618 CrossRef CAS; (b) R. Silva, E. C. Muniz and A. F. Rubira, Polymer, 2008, 49, 4066 CrossRef CAS.
- J. M. Goddard, J. N. Talbert and J. H. Hotchkiss, J. Food Sci., 2007, 72, E036 CrossRef CAS.
- K. Pal, S. Bag and S. Pal, J. Porous Mater., 2008, 15, 53 CrossRef CAS.
- X.-Y. Lu, C.-C. Zhang and Y.-C. Han, Macromol. Rapid Commun., 2004, 25, 1606 CrossRef CAS.
- I. Novák, G. K. Elyashevich, I. Chodák, A. S. Olifirenko, M. Števiar, M. Špírková, N. Saprykina, E. Vlasova and A. Kleinová, Eur. Polym. J., 2008, 44, 2702 CrossRef CAS.
- G. P. Andrianova and S. I. Pakhomov, Polym. Eng. Sci., 1997, 37, 1367 CrossRef CAS.
- X.-Y. Lu, J.-L. Zhang, C.-C. Zhang and Y.-C. Han, Macromol. Rapid Commun., 2005, 26, 637 CrossRef CAS.
- G. Widawski, M. Rawiso and B. François, Nature, 1994, 369, 387 CrossRef CAS.
- (a) M. B. Shiflett and H. C. Foley, Science, 1999, 285, 1902 CrossRef CAS; (b) J. E. G. Wijnhoven and W. L. Vos, Science, 1998, 281, 802 CrossRef CAS; (c) M. Imada, S. Noda, A. Chutinan, T. Tokuda, M. Murata and G. Sasaki, Appl. Phys. Lett., 1999, 75, 316 CrossRef CAS; (d) A. Boker, Y. Lin, K. Chiapperini, R. Horowitz, M. Thompson, V. Carreon, T. Xu, C. Abetz, H. Skaff, A. D. Dinsmore, T. Emrick and T. P. Russell, Nat. Mater., 2004, 3, 302 CrossRef; (e) D. J. Harris and J. A. Lewis, Langmuir, 2008, 24, 3681 CrossRef CAS.
- (a) B. François, O. Pitois and J. François, Adv. Mater., 1995, 7, 1041 CAS; (b) U. H. F. Bunz, Adv. Mater., 2006, 18, 973 CrossRef CAS.
- S. A. Jenekhe and X. L. Chen, Science, 1999, 283, 372 CrossRef CAS.
- (a) B. de Boer, U. Stalmach, H. Nijland and G. Hadziioannou, Adv. Mater., 2000, 12, 1581 CrossRef CAS; (b) D. Beattie, K. H. Wong, C. Williams, L. A. Poole-Warren, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Biomacromolecules, 2006, 7, 1072 CrossRef CAS; (c) T. Hayakawa and S. Horiuchi, Angew. Chem., Int. Ed., 2003, 42, 2285 CrossRef CAS; (d) A. Bolognesi, P. DiGianvincenzo, U. Giovanella, R. Mendichi and A. G. Schieroni, Eur. Polym. J., 2008, 44, 793 CrossRef CAS.
- (a) L. L. Song, R. K. Bly, J. N. Wilson, S. Bakbak, J. O. Park, M. Srinivasarao and U. H. F. Bunz, Adv. Mater., 2004, 16, 115 CrossRef CAS; (b) B. Erdogan, L. L. Song, J. N. Wilson, J. O. Park, M. Srinivasarao and U. H. F. Bunz, J. Am. Chem. Soc., 2004, 126, 3678 CrossRef CAS; (c) K. H. Wong, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Aust. J. Chem., 2006, 59, 539 CrossRef CAS.
- (a) T. Nishikawa, J. Nishida, R. Ookura, S. I. Nishimura, S. Wada, T. Karino and M. Shimomura, Mater. Sci. Eng., C, 1999, 8–9, 495 CrossRef; (b) K. H. Wong, T. P. Davis, C. Barner-Kowollik and M. H. Stenzel, Polymer, 2007, 48, 4950 CrossRef CAS; (c) E. Min, K. H. Wong and M. H. Stenzel, Adv. Mater., 2008, 20, 3550 CrossRef CAS; (d) A. Bolognesi, F. Galeotti, U. Giovanella, F. Bertini and S. Yunus, Langmuir, 2009, 25, 5333 CrossRef CAS; (e) L. Li, C.-K. Chen, A.-J. Zhang, X.-Y. Liu, K. Cui, J. Huang, Z. Ma and Z.-H. Han, J. Colloid Interface Sci., 2009, 331, 446 CrossRef CAS; (f) L. Li, C.-K. Chen, J. Li, A.-J. Zhang, X.-Y. Liu, B. Xu, S.-B. Gao, G.-H. Jin and Z. Ma, J. Mater. Chem., 2009, 19, 2789 RSC.
- (a) C. X. Cheng, Y. Tian, Y. Q. Shi, R. P. Tang and F. Xi, Langmuir, 2005, 21, 6576 CrossRef CAS; (b) L. A. Connal, R. Vestberg, C. J. Hawker and G. G. Qiao, Adv. Funct. Mater., 2008, 18, 3706 CrossRef CAS; (c) W.-Y. Dong, Y.-F. Zhou, D.-Y. Yan, Y.-Y. Mai, L. He and C.-Y. Jin, Langmuir, 2009, 25, 173 CrossRef CAS.
- (a) B. Francois, Y. Ederle and C. Mathis, Synth. Met., 1999, 103, 2362–2363 CrossRef CAS; (b) M. H. Stenzel-Rosenbaum, T. P. Davis, A. G. Fane and V. Chen, Angew. Chem., Int. Ed., 2001, 40, 3428 CrossRef CAS; (c) H. T. Lord, J. F. Quinn, S. D. Angus, M. R. Whittaker, M. H. Stenzel and T. P. Davis, J. Mater. Chem., 2003, 13, 2819 RSC; (d) M. H. Stenzel, C. Barner-Kowollik and T. P. Davis, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 2363 CrossRef CAS.
- (a) L. A. Connal, P. A. Gurr, G. G. Qiao and D. H. Solomon, J. Mater. Chem., 2005, 15, 1286 RSC; (b) L. A. Connal and G. G. Qiao, Adv. Mater., 2006, 18, 3024 CrossRef CAS; (c) L. A. Connal and G. G. Qiao, Soft Matter, 2007, 3, 837 RSC; (d) L. A. Connal, R. Vestberg, P. A. Gurr, C. J. Hawker and G. G. Qiao, Langmuir, 2008, 24, 556 CrossRef CAS.
- J.-Z. Chen, K. Cui, S.-Y. Zhang, P. Xie, Q.-L. Zhao, J. Huang, L.-P. Shi, G.-Y. Li and Z. Ma, Macromol. Rapid Commun., 2009, 30, 532 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
