Fabrication of functional polypyrrole (PolyPyr)-nanotubes using anodized aluminium oxide (AAO) template membranes. Compromising between effectiveness and mildness of template dissolution conditions for a safe release of PolyPyr-nanotubes†
Natasha
Esman
and
Jean-Paul
Lellouche
*
Bar-Ilan University, Department of Chemistry, Nanomaterials Research Center, Institute of Nanotechnology & Advanced Materials, Ramat-Gan 52900, Israel. E-mail: lellouj@mail.biu.ac.il; Fax: +972-3-738 4053; Tel: +972-3-531 8324
First published on 11th December 2009
Abstract
Functional acidic (COOH) pyrrole (Pyr)-containing oxidizable monomers can be template-polymerized using hard nanoporous anodized aluminium oxide (AAO) membranes in liquid phase polymerization (LPP) conditions. Accordingly, the safe and effective release of resulting functional polyCOOH–polypyrrole (polyPyr)-nanotubes from template membranes must be operated in controlled acidic, instead of commonly used Martin-like basic conditions (membrane digestion).
Introduction
The oxidative templated fabrication of high aspect ratio one-dimensional (1D) polymeric nanorods, nanofibers, and/or nanotubes based on conducting polymers (CPs) is a quite effective and general nanofabrication method.1,2 For that purpose, liquid phase polymerization (LPP) methods employ hard templates such as nanoporous anodized aluminium oxide (AAO) or track-etched polyester membranes that may be readily solubilized for nanostructure release.3,4 Both types of hard template membranes contain a high density of diameter-size-defined, well-separated, discrete nanopores as shape-defining nanoreaction vessels. Various LPP parameters—the membrane and monomer heterocycle types, the monomer concentration, the oxidant nature and concentration, the polymerization time, temperature, and solvent—have been shown to be influential for process optimization leading to the commonly adopted Martin LPP protocol.5 This sequential protocol comprises the FeCl3-mediated oxidation of non-functionalPyr-monomers within AAO template nanopores, followed by the strongly basic (2M NaOH) AAO template dissolution for nanostructure release. Most of the reported works mainly concern the fabrication of non-functional CP/polyPyr-nanotubes or nanofibers,6–9 which is likely to be due to a limited synthetic access to structurally complex CP precursors/monomers.In this context, the use of an AAO template in acidicversus strongly basic dissolution conditions may strongly influence the safe, mild, and effective release of polyPyr-nanostructures which will be functional, e.g.polycarboxylated (polyCOOH). This specific parameter has never been examined in depth, although strongly basic dissolution conditions might give rise to (a) the partial or total water solubilization of polyanionic (polyCOO−Na+) polyPyr-nanostructures resulting in nanomaterial loss, (b) their chemical decomposition due to base-induced polymer network decarboxylation/C-N-Pyr β-elimination resulting in network bond breaking, and (c) the base-mediated complexation of nanostructureCOO− groups by oxidizing Fe3+ cations affording impure Fe-contaminated nanomaterials.
This Communication aims to unravel the critical importance of using controlled acidic instead of basic AAO template dissolution conditions when dealing with functionalPyr-monomers/polyPyr-nanostructures. Indeed, the AAO templated oxidative polymerization of the three functional monoacidic N-substituted Pyr-based monomers 1–3 afforded morphologically well-defined functional polyCOOH–polyPyr-nanotubes when using an acidic template digestion (Scheme 1). The resulting well-shaped nanomaterials disclosed much higher aspect ratios. In fact, compromising between (i) the effectiveness of the AAO template dissolution (basic or acidic digestion), and (ii) the safe, mild, and effective release of fabricated base-sensitive polyPyr-nanotubes is a key factor of this overall nanofabrication process. Interestingly, this factor has been largely overlooked until the completion of the present studies.
 | ||
| Scheme 1 Chemical structures of monoacidic Pyr-containing oxidizable monomers 1–3 | ||
Accordingly, and dealing with Pyr-monomers 1–3 (Scheme 1), both acidic and basic template digestion protocols were tested and compared for the optimization of corresponding LPP Martin-like two-step nanofabrication sequences in the following way.
Experimental
AAO-templated LPP experiments
Monomers 1–3, prepared by a modified Clauson–Kaas reaction10 from corresponding amino acids,11 were chemically polymerized within 100 and 200 nm nanopores of corresponding nanoporous AAO templating membranes. Typically, an AAO filtration membrane (Whatman International Ltd, Anodisc 25; ø = 21 mm, 60 µm thickness, 100 nm or 200 nm average pore size, 1 × 109 pores/cm2 pore density) was extensively wetted with a 25% w/v aqueous solution of FeCl3 (oxidant loading, 1 h). Following the drying of the resulting FeCl3-loaded membrane (40 °C, 1 h), the excess FeCl3 oxidant was rubbed off with sand paper (Waterproof Silicon Carbide Paper FEPA #1200) from both top and bottom membrane surfaces. The clean, dry FeCl3-loaded AAO template membrane was then dipped in a 0.02 M CH2Cl2 solution of monomers 1–3 for polymerization (24 h, room temperature—the membrane turned black with the polymerization progress). At the completion of polymerization, the membrane was washed with CH2Cl2 (4 × 10 mL) in order to eliminate the excess monomer, dried (30 min, room temperature), and gently rubbed off (both top and bottom surfaces, with sand paper) before template digestion.Dissolution of the AAO templating membrane
The clean processed membrane was then dissolved according to both indicated basic and acidic dissolution protocols operated at room temperature for the safe and mild release of polymeric polyPyr-nanostructures. Following a 1st round wide screening of membrane dissolution experiments (various acid/base types and concentrations, dissolution times, results not shown), two particular sets of basic (aqueous 2M NaOH, 2 h) and acidic (aqueous 5M HCl, 24 h) conditions were selected for deeper examination due to their favorable features (membrane disappearance using visual testing and medium to good 1.0–2.0 mg/membrane yields of fabricated nanostructures). The resulting brown-black polyCOOH–polyPyr-based nanostructures precipitated and were isolated by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 10 min, room temperature), washed with EtOH (3 × 30 mL), and finally with H2O (2 × 30 mL) until pH neutrality. Purified polyPyr-nanostructures were then suspended in neutral H2O (1% w/v) for storage and characterization needs.
000 rpm, 10 min, room temperature), washed with EtOH (3 × 30 mL), and finally with H2O (2 × 30 mL) until pH neutrality. Purified polyPyr-nanostructures were then suspended in neutral H2O (1% w/v) for storage and characterization needs.
Characterization of fabricated polyCOOH–polyPyr-nanostructures
Both polycationic doped acid- and base-generated polyPyr-nanostructures disclosed identical FT-IR spectra (Bomem MB 100 FT-IR spectrometer, KBr pellet, ESI, Fig. S5† ). Characteristic π-conjugated aromatic/indolic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond stretching vibration peaks appeared at 1616.0 cm−1, while conjugated C–N stretching and C–H bending ones could be detected in the 1604.6–1388.9 cm−1 zone. In addition, C–H stretching peaks could be seen at 2890.0 cm−1. The polyCOOH functionality showed O–H (COOH function) stretching peaks at 3458.0 cm−1 (strong and weak absorption signals for acidic and basic digestions respectively).
C double bond stretching vibration peaks appeared at 1616.0 cm−1, while conjugated C–N stretching and C–H bending ones could be detected in the 1604.6–1388.9 cm−1 zone. In addition, C–H stretching peaks could be seen at 2890.0 cm−1. The polyCOOH functionality showed O–H (COOH function) stretching peaks at 3458.0 cm−1 (strong and weak absorption signals for acidic and basic digestions respectively).
In contrast, and depending on the membrane digestion system, combined SEM and TEM analyses performed on illustrative poly(DPL1)-based nanostructures disclosed huge differences in polyPyr-nanostructure morphologies (Fig. 1 and 2). First, SEM analysis indicated that the basic AAO membrane digestion always afforded 4.3 and 5.4 µm long end-truncated poly(DPL1)-nanorods for both 100 and 200 nm AAO membranes respectively (Fig. 1a and b). They were much shorter than the 12.0 and 18.7 µm long end-closed ones obtained using the acidic digestion protocol (Fig. 1a and bversusFig. 1c and d, 50 counted objects/SEM analysis for averaged measurements/minimization of data dispersion). In both cases, diameters were observed in the 198.0/234.5 (basic digestion) versus the 227.5/343.8 nm (acidic digestion) range for 100/200 nm AAO membranes respectively, which was indicative of a significant polyPyr-polymer swelling in neutral water. Nanorod aspect ratios significantly increased from 21.6/23.1 (basic digestion) to 52.7/54.3 (acidic digestion) for 100/200 nm AAO membranes when comparing both digestion systems. Second, regarding TEM analysis, TEM microphotographs of formerly SEM-characterized poly(DPL1) nanorods enabled further morphology refinement (Fig. 2). Base-released nanorods showed electron-dense nanostructures (Fig. 2a) which are likely to result from the strong electron absorption of a contaminating entrapped Fe elemental phase (10.77%, checking by HR-SEM/compositional EDAX analysis). Entrapped/complexed Fe2+/3+-based cations originating from FeCl3 provide a reasonable explanation for this result.
 | ||
| Fig. 1 SEM microphotographs of polyPyr-based poly(DPL1) nanostructures released from AAO templating membranes: basic digestion, 100 and 200 nm membrane pores (a and b respectively); acidic digestion, 100 and 200 nm membrane pores (c and d respectively). | ||
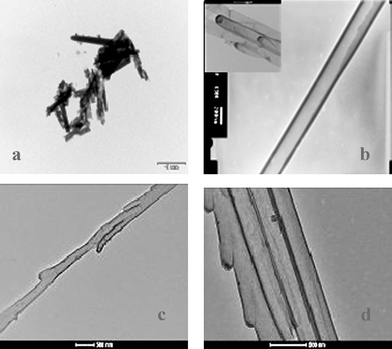 | ||
| Fig. 2 TEM microphotographs of polyPyr-based nano-structures released from AAO membranes: (a) poly(DPL1) nanorods, basic digestion, 100 nm membrane pores (scale bar: 1.0 µm); (b) poly(DPL1) nanotubes, acidic digestion, 200 nm membrane pores (scale bar: 200 nm), (inset: same nanomaterial for 100 nm membrane pores, scale bar: 500 nm); (c and d) poly(2) and poly(3) nanotubes respectively, acidic digestion, 100 nm membrane pores. | ||
In contrast, the same acid-released SEM-characterized poly(DPL1) nanorods appeared as well-shaped end-closed nanotubes that possess measurable average wall thicknesses of 30.1 and 39.6 nm for 100 and 200 nm AAO membranes respectively (Fig. 2b). The acidic template digestion system afforded a nanomaterial fully depleted in Fe (HR-SEM/compositional EDAX analysis, ESI, Fig. S4† ), showing a much higher level of chemical purity. Its effectiveness and mildness were also demonstrated by the following additional fact. Independent of pore sizes, some acid-released poly(DPL1), and poly(2–3) nanotubes presented smooth, angularly-grown protruding defects on their surface that delicately replicated inner structural defects present in pore walls of AAO membranes (Fig. 2b inset, c and d). To the best of our knowledge, this observation has no precedence in the field of CP-based nanostructures, but it found very recent confirmation during the AAO-templated fabrication of TNT-imprinted silica nanotubes for TNT recognition/sensing.12
The chemical purity of poly(DPL1) nanotubes has been readily examined and assessed from C, H, N elemental analysis data. These data showed the presence of C (48.73%), N (5.92%), and H (4.88%) for a measured C : N : H ratio of 6.7 : 8.0 : 0.7. This elemental ratio was found to be quite close to the calculated one, 7.0 : 8.0 : 1.0, arising from the molecular formula C14H16O2N2 of DPL1 monomer (H% as reference).
Moreover, it was also discovered that the basic digestion of AAO membranes afforded a 2nd contaminating phase, i.e. 40–50 nm spherical particles that are likely to have arisen from the corresponding polyPyr-phase during intra-pore monomer oxidation (Fig. 1a and b). This nanoparticle contaminating phase could not be separated from the corresponding poly(DPL1)-nanorods during base-mediated isolation/purification. Clearly, both features of elemental impurity (Fe contamination) and biphasic nanorod–nanoparticle composition preclude the further use of base-released polyCOOH–poly(DPL1)-nanorods in any potential end-application.
In a further extension of this study, similar morphology and chemical composition features were also observed for both acid-released polyCOOH–poly(2)/poly(3)-nanotubes using 100 and 200 nm AAO membranes (ESI, Fig. S1–S3,†Fig. 2c and d, and morphological data (ESI Table)† ). In addition, we also checked that all the acid-released poly(1–3)-nanotubes did not survive when contacted by the typical basic AAO membrane digestion system (aqueous 2M NaOH, 2 h, room temperature) used previously. This result is a clear demonstration of the high chemical sensitivity of functional polyCOOH–polyPyr-based nanomaterials to aggressive basic conditions as we suspected previously.
In conclusion, the safe and effective release of functional polyCOOH–polyPyr-nanotubes from nanoporous AAO templating membranes critically depended on finely tuned membrane digestion conditions. In our LPP studies, controlled mild acidic (aqueous 5M HCl) digestion conditions were found chemically compatible with released 1D-shaped polymeric nanostructures in contrast to commonly used basic ones (aqueous 2M NaOH). This previously underrated key parameter in LPP experiments should be systematically investigated on a case by case basis, notably when dealing with chemically sensitive and functional AAO membrane templated nanomaterials.
Notes and references
- M. Lu, X.-H. Li and H.-L. Li, Mater. Sci. Eng., A, 2002, 334, 291–297 CrossRef.
- A. Huczko, Appl. Phys. A: Mater. Sci. Process., 2000, 70, 365–376 CrossRef CAS.
- V. M. Cepak and C. R. Martin, Chem. Mater., 1999, 11, 1363–1367 CrossRef CAS.
- M. Acik and G. Sonmez, Polym. Adv. Technol., 2006, 17, 697–699 CrossRef CAS.
- J. C. Hulteen and C. R. Martin, J. Mater. Chem., 1997, 7, 1075–1087 RSC.
- S. M. Yang, K. H. Chen and Y. F. Yang, Synth. Met., 2005, 152, 65–68 CrossRef CAS.
- X. H. Li, W. M. Liu, C. W. Wang and H. L. Li, J. Mater. Sci. Lett., 2003, 22, 1519–1521 CrossRef CAS.
- B. H. Kim, D. H. Park, J. Joo, S. G. Yu and S. H. Lee, Synth. Met., 2005, 150, 279–284 CrossRef CAS.
- F.-L. Cheng, M.-L. Zhang and H. Wang, Sensors, 2005, 5, 245–249 CrossRef CAS.
- N. Elming and N. Clauson-Kaas, Acta Chem. Scand., 1952, 6, 867–874 CAS.
- K. Perié, R. Marks, S. Szumerits, S. Cosnier and J.-P. Lellouche, Tetrahedron Lett., 2000, 41, 3725–3729 CrossRef CAS.
- C. Xie, B. Liu, Z. Wang, D. Gao, G. Guan and Z. Zhang, Anal. Chem., 2008, 80, 437–443 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM/TEM microphotographs of poly(2–3) nanotubes, HR-SEM/EDAX analysis of poly(DPL1) nanotubes, table of morphological features of poly(2–3) nanotubes, and illustrative FT-IR spectra of poly(1–2) nanotubes. See DOI: 10.1039/b9py00206e/ |
| This journal is © The Royal Society of Chemistry 2010 |
