The role of Nrf2 in ultraviolet A mediated heme oxygenase 1 induction in human skin fibroblasts
Julia L. Zhong†‡a, Gavin P. Edwards†a, Chintan Ravala, Haibin Lib and Rex M. Tyrrell*a
aDepartment of Pharmacy and Pharmacology, University of Bath, UK BA2 7AY. E-mail: prsrmt@bath.ac.uk; Fax: +44-1225-383408; Tel: +44-1225-386793
bCollege of Bioengineering, Chongqing University, Chongqing, China
First published on 28th October 2009
Abstract
Ultraviolet-A (UVA, 320–380 nm) radiation is an oxidative stress that strongly induces heme oxygenase 1 (HO-1) expression in cultured human primary skin fibroblasts (FEK4). In this study, we show that NF-E2-related factor 2 (Nrf2) protein accumulates and HO-1 is strongly induced following UVA irradiation of FEK4 cells. Down-regulation of Nrf2 with specific short interfering RNA (siRNA) against Nrf2 (siNrf2) largely abolished the induction of HO-1 following either UVA irradiation or hemin treatment, suggesting that Nrf2 activation mediated modulation of HO-1 by both these agents. Furthermore, a reduction of free heme levels led to a strong decrease in UVA-induced Nrf2 and HO-1 protein levels confirming a clear role for heme in the UV-mediated stress response. Knock-down of Nrf2 protein enhanced membrane damage induced by UVA irradiation, indicating that Nrf2 has a crucial protective role in these cells.
1. Introduction
Ultraviolet-A (UVA, 320–400 nm) radiation generates a significant oxidative stress to human skin1,2 and has been strongly implicated as a component of many of the deleterious effects of sunlight including erythema, immune suppression, photoaging, and skin cancer.3 Part of the cellular defence against such oxidative insult involves induction of several detoxifying enzymes, including HO-1,4 that contain at least one antioxidant response element (ARE) in the upstream region of their gene promoters. Under oxidative stress conditions, expression of ARE-driven genes is strongly enhanced through the activation of transcription factors, such as Nrf2 which bind to these cis-acting elements.4,5HO-1 is the first step and the rate limiting enzyme in heme degradation, catalysing the degradation of pro-oxidant heme to carbon monoxide, biliverdin and ferrous iron.6 It has anti-inflammatory, antiapoptotic and antioxidant activities as well as cell-signalling properties.7 HO-1 is highly inducible by a large number of chemical and physical factors, including UVA irradiation.2 This appears to be mediated by reactive oxygen species (ROS)8 and UVA-induced release of the heme from microsomal heme-containing proteins.9 However, the molecular mechanism underlying regulation of HO-1 by UVA irradiation remains poorly characterized. HO-1 gene expression is regulated by the transcriptional activator Nrf2 which competes with the repressor Bach 1 (BTB and CNC homology 1) to form heterodimers with three small Maf proteins (MafK, MafG and MafF) via the ARE or ARE-related Maf-associated recognition element (MARE).10,11 Under oxidative stress conditions, including hemin treatment, Nrf2 moves into the nucleus or further accumulates in the nucleus and replaces Bach1 to occupy the MARE site. This immediately initiates HO1 transcription followed by translation.11,12
The skin acts as a physiological barrier to protect the organism against pathogens and chemical or physical damage. The major environmental causes of skin damage are UV radiation and chemical pollutants. An understanding of the mechanism of up-regulation of HO-1 in skin cells may provide clues as to new modalities for skin protection against oxidative stress and future treatment of skin disorders. Previous studies have shown that UVA causes nuclear accumulation of Nrf2 in murine fibroblasts.13 However, in murine skin keratinocytes UVA has no effect, while electrophilic chemicals activate Nrf2 and induce Nrf2-mediated gene expression.14 Nrf2 was found to be involved in wound healing in mouse skin and Nrf2-deficient mice are impaired in their oxidative stress defence.15 Recently Nrf2-driven HO-1 induction was found to protect leukaemia cells against TNF-induced cell death.16
Using human primary skin fibroblasts (FEK4) as a model system, we observed activation of Nrf2 by UVA and heme, and examined the link between Nrf2, HO-1 and UVA-mediated membrane damage.
2. Experimental
2.1 Cell culture and reagents
Primary human skin fibroblasts FEK4 were maintained in Earle's modified Minimal Essential Medium (EMEM) supplemented with 15% fetal calf serum (FCS) with 2 mM L-glutamine and 50 U ml−1 penicillin/streptomycin. Cells were maintained at 37 °C in a 5% CO2/95% air humidified incubator. Cells were only employed for experimental procedures between passages 8 and 15. Anti-HO1 (OSA-110) and anti-HO2 (OSA-200) antibodies were from Assay Designs (USA). Nrf2 (H300, sc-13032), GAPDH (sc-20357) antibodies were obtained from Santa Cruz Biotechnology (USA). Actin (A3853) and secondary antibodies: anti-goat, anti-rabbit, anti-mouse/HRP were obtained from Sigma (UK) and immunostaining related secondary antibodies were from Invitrogen Life Technologies (UK).2.2 Treatments
Cells were irradiated using a broad-spectrum 4 kW UVA lamp (340–400 nm) (Sellas, Munich, Germany). The lamp exposure time was calculated using an IL1700 radiometer (International Light, Newbury, USA) with an SEE400 probe. UVA irradiation was performed as described previously17 and 1% FCS–EMEM medium was added back to cells after irradiation. Control cells underwent identical treatment, except that they were not irradiated (sham = 0 kJ m−2). Hemin (iron ferric protoporphyrin IX) was dissolved in DMSO and added with 1% FCS–EMEM to cells for up to 16 h. Succinyl acetone (SA) 1 M stock was made in sterilized water and added with 1% FCS–EMEM to cells for 16 h.2.3 RNA interference
All small interfering RNAs (siRNAs) are from Ambion and include scrambled silencer negative control siRNA (Sb, AM4611) and siβActin (AM4607). The sequences for two sets (s9491 and s9493) of siNrf2 reagents (NM006164) are shown in Table 1. Sub-confluent cells were detached and transfected with siRNAs use the siPORT™ NeoFX™ Transfection Agent (NeoFX AM4511, Ambion). For FEK4 cells, siRNA was diluted in 100 μl serum free medium (OPT-MEM) and NeoFX (4 μl) was also diluted in 100 μl OPT-MEM incubated for 10 min at room temperature (RT), then mixed well, and incubated at RT for 10 min to allow the formation of siRNA complexes. The siRNA complex was then placed in 6 cm plates and 2.5 × 105 cells in 2 ml of the normal cell growth medium were added to give a total volume of 2.2 ml. The following day, a half volume of fresh medium was added and cells were incubated for 48 h for further treatment.2.4 Protein extraction and Western blot
Following treatments, cells were collected and protein was extracted as previously described.17 Total protein lysate (20–40 μg according to the experiment) was separated on 4–15% gradient gels (Bio-Rad), transferred to PVDF (Millipore) membranes and probed with the antibody as indicated, following the standard protocol. Immuno-reactive proteins were visualized by the ECL Western blot detection system (Amersham Biosciences). Membranes were stripped and re-probed. If required, a second Western blot was carried out using the identical protein lysate. The intensity of bands was quantified by digital densitometry using the NIH Image J 1.33 software. Data were normalized to actin and expressed as the percentage or fold change compared with the corresponding control, which was set to 1.2.5 Immunocytochemistry
Cells were grown to 50–60% confluency on glass coverslips, collected and washed with PBS, fixed in 4% paraformaldehyde then incubated in 100% methanol at −20 °C. Cells were then blocked with Image-iT™ Fx signal enhancer, treated with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 Nrf2 antibody, washed with PBS then followed by Alexa-Fluor secondary antibody, washed again with PBS. Hoechst nuclear stain was applied and coverslips mounted using fluorescent mounting medium (Dako Cytomation, UK). The cells were analysed by oil immersion epifluorescence using a Nikon Eclipse TE2000-U microscope. Images were recorded using the software program UltraVIEW.
100 Nrf2 antibody, washed with PBS then followed by Alexa-Fluor secondary antibody, washed again with PBS. Hoechst nuclear stain was applied and coverslips mounted using fluorescent mounting medium (Dako Cytomation, UK). The cells were analysed by oil immersion epifluorescence using a Nikon Eclipse TE2000-U microscope. Images were recorded using the software program UltraVIEW.2.6 LDH levels
The levels of extracellular LDH were determined using the cytotoxicity detection kit for LDH (Cat. No. 11644793001, Roche Applied Science) following the instructions supplied. Five thousand cells treated with siNrf2 were seeded onto 96-well plates for 48 h, then UVA irradiated and re-incubated for 4 and 8 h. LDH release was then measured as described.18 The fraction of extracellular LDH was represented as the fold increase over vehicle/scrambled siRNA-treated control, which was set to 1.2.7 Statistical analysis
Statistical analysis was carried out using a two-tailed t-test and P values below 0.05 were considered to be statistically significant. The values in the graphs correspond to the mean, and the error bar indicates standard error (SE).3. Results and discussion
3.1 Nrf2 activation and HO-1 induction following UVA irradiation of primary human skin fibroblasts
The mechanism of Nrf2 activation was reviewed recently by Hayes and McMahon19 Both nuclear accumulation/translocation of Nrf2 and stablisation of the protein are considered to be important to the process. The latter is considered to be a major mechanism and occurs through inhibition of the ubiquitylation and subsequent degradation of Nrf2 via the 26S proteasome. Agents which induce Nrf2 often inactivate Keap 1 which is a redox-sensitive substrate adaptor for the ubiquitin ligase, Cullin-3/Rbx1. Hirota et al.13 found that Nrf2 accumulated in the nucleus following UVA irradiation in murine dermal fibroblasts, while no such induction was observed in keratinocytes14,20 Since the rodent and human HO-1 promoters have both different as well as common elements in enhancer regions, we investigated whether irradiation with an environmentally relevant dose of 250 kJ m−2 UVA up-regulated Nrf2 in FEK4 human skin fibroblasts, a treatment which induces high levels of HO-1. No significant enhancement of Nrf2 mRNA accumulation was observed following 250 kJ m−2 UVA irradiation (data not shown). However protein measurements showed that this UVA irradiation dose increases Nrf2 in both whole cells (Fig. 1A) and the nuclear fraction (data not shown) within 2 h and continues to accumulate for at least 8 h following UVA irradiation, after which there is a decline (data not shown). This finding is consistent with observations made using immunostaining with a Nrf2 antibody which showed that, following UVA treatment (Fig. 1B), Nrf2 accumulated in the nuclear region at 2 h, with further accumulation at 4 and 8 h. Nrf2 accumulation following UVA irradiation is therefore similar in murine13 and human fibroblasts in that it is mainly localized in the nucleus in the early hours following treatment. HO-1 induction was apparent at 4 h and further increased at 8 h (∼10 fold increase).20 These results are more consistent with the idea that Nrf2 activation following UVA irradiation occurs as a result of stabilization rather than re-distribution (nuclear translocation) of existing Nrf2 protein.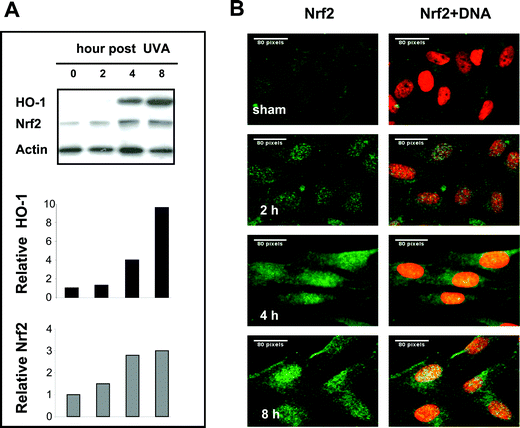 | ||
| Fig. 1 Nrf2 activation coordinates with HO-1 induction following UVA irradiation of human primary skin fibroblasts. FEK4 cells were collected at the times indicated following sham or 250 kJ m−2 UVA irradiation. A: Western blot analysis of 30 μg whole cell lysate with antibodies indicated. The intensity of bands was quantified by digital densitometry using the NIH Image J 1.33 software and were normalized to actin, as described under Experimental. The relative expression levels of HO-1 and Nrf2 are shown in the bar graph as the fold change compared with the sham irradiated control, which was set to 1. B: Immunostaining with Nrf2 (green) antibody showing Nrf2 accumulation in the nucleus following UVA irradiation at 2, 4, 8 h. Nuclei were visualised by Hoechst staining (red). The cells were analysed by oil immersion epifluorescence microscopy using the software package UltraVIEW. Scale bar: 80 pixels. One of the representatives of three separate experiments is shown. | ||
3.2 Nrf2 involvement in HO-1 induction following hemin treatment in FEK4 cells
HO-1 inducers, such as heme, cadmium and arsenite, can stabilize, induce accumulation of or even prevent degradation of Nrf2.4,5,11,23 The HO-1 substrate, heme, induces high expression of HO-1 in FEK4 cells22 as well as in many other cell lines.6 A previous study has shown that UVA releases heme from microsomal heme proteins and that the amount of heme released strongly correlates with the level of HO-1 activation.9 Although, the increase in Nrf2 levels in whole cells following hemin treatment is not always detectable,12 significant levels were observed 3–6 h after heme treatment of neuroblastoma cells whereas HO-1 protein induction peaked after 12 h.21 We therefore investigated both HO-1 and Nrf2 protein levels in FEK4 cells following 30 μM hemin treatment. An increase in HO-1 protein was observed within a few hours of hemin treatment (Fig. 2A) and was up to 12-fold higher at 12 h treatment, whereas a small increase in Nrf2 protein was observed 3–6 h after treatment (Fig. 2A). Similar results were obtained by immunostaining (data not shown). These findings are consistent with the observation in neuroblastoma cells21 that HO-1 protein induction is induced at later times (6–12 h) than Nrf2 protein induction (3–6 h) following hemin treatment.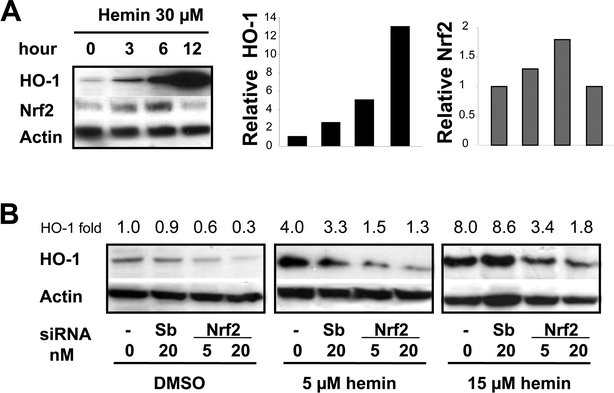 | ||
| Fig. 2 Nrf2 involvement in HO-1 induction by hemin-treatment in FEK4 cells. A: Western blotting was performed with 30 μg whole cell lysates obtained after 30 μM hemin treatment at the times indicated. B: FEK4 cells were transfected with negative scramble control siRNA (Sb), vehicle control (–), #1 siNrf2, using siPORT NeoFX transfection reagent (Ambion) as described under Experimental, and cultured for 48 h, then treated with hemin or vehicle DMSO for 12 h. Western blotting was performed with whole cell lysates (30 μg) obtained and probed with antibodies as indicated. Blot shows one representative example of three separate experiments. The relative HO-1 protein levels are shown in the bar graph (A) or values (B) as the fold change relative to the sham irradiated control, which was set to 1. | ||
Reduction of Nrf2 may be linked to reduced HO-1 induction in acute myeloid leukemia cells, KCL22 leukemia cells and murine embryonic fibroblasts, as well as other cell lines, in response to oxidative stress or HO-1 inducers.10,16,24,25 To clarify the involvement of Nrf2 in up-regulation of HO-1 by hemin treatment, we investigated the effect of silencing of Nrf2 on hemin-induced HO-1 levels in FEK4 cells. We first established that the optimal silencing time was between 48 h and 72 h following siRNA treatment, using siActin and siNrf2. The #1 siNrf2 treatment lowered endogenous (whole cell) Nrf2 levels and reduced HO-1 levels induced by 12 h hemin treatment when compared with scrambled control siRNA or vehicle control (Fig. 2B). Another siNrf2 that targeted different exons (Ambion, #2) showed similar results (data not shown), indicating that Nrf2 silencing was effective, specific and selective. Our findings support the notion that Nrf2 is involved in HO-1 induction in FEK4 cells following hemin treatment.
3.3 Heme depletion modulates UVA-induced HO-1 in FEK4 cells
We further investigated the role of hemin in induction of Nrf2 and HO-1 in FEK4 cells. Since hemin treatment followed by UVA irradiation will induce HO-1 refractoriness,22 we are not able to use this protocol and therefore an alternative approach was used. Succinyl acetone (SA) pre-treatment has been shown to inhibit heme synthesis and leads to a reduction in heme content.26,27 The effect of combining heme depletion and UVA-irradiation on HO-1 and Nrf2 protein levels in FEK4 cells was examined. SA pre-treatment did not noticeably reduce basal levels of HO-1, but reduced UVA-induced HO-1 levels (Fig. 3). The reduction in HO-1 was dependent on SA concentration. Evidently nuclear levels of Nrf2 may differ even when the total cell level remains the same.12 Heme synthesis inhibition led to the reduction of Nrf2 signal (Fig. 3) and a reduction in UVA-induced HO-1 levels consistent with the conclusion that the involvement of Nrf2 and heme in modulating induction of HO-1 by UVA is linked to heme-induced accumulation of Nrf2 in FEK4 cells. However it is important to note that under low heme conditions, the repressor protein, Bach1, will probably remain bound to the MARE sites in the HO-1 promoter11,12 and this will also contribute in a major way to the inhibition of HO-1 induction. Indeed, SA treatment has been shown to increase Bach1 protein levels in NIH 3T3 cells (rodent),27 although there are various mechanisms by which this may occur.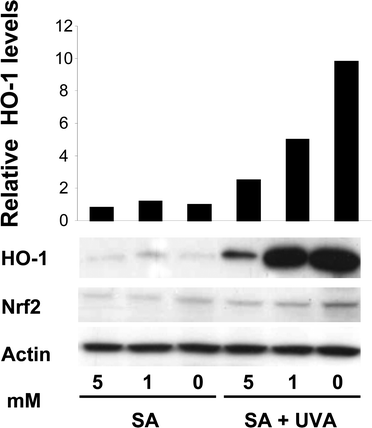 | ||
| Fig. 3 Heme depletion modulates UVA-induced HO-1 in FEK4 cells. Western blotting of whole cell lysates (30 μg) with anti-human HO-1, Nrf2 and actin antibodies shows that heme depletion by SA 16 h treatment reduced HO-1 induction at 8 h following UVA-irradiation. Blot shows one representative example of three separate experiments. The relative HO-1 protein levels are shown in the bar graph as the fold change relative to the sham irradiated control, which was set to 1. | ||
3.4 The effect of reduced Nrf2 on basal and UVA-induced HO-1 in FEK4 cells
Lower levels of Nrf2 in a HaCaT (keratinocyte-derived) cell line compared with the fibroblast FEK4 cell line may be linked to the lower inducibility of UVA-induced HO-1 protein expression levels observed in this cell line.20 Nevertheless Nrf2 has been shown to be involved in induction of various detoxifying enzymes by chemical inducers in keratinocytes.28 We examined the effect of silencing Nrf2 expression on the induction of HO-1 by UVA in FEK4 cells. Cells were treated with siNrf2 followed by UVA irradiation. Transfection of 5 and 20 nM siNrf2 led to 50% and 80% reduction in Nrf2 protein in FEK4 cells, respectively, and also decreased UVA-induced Nrf2 at 4 h and 8 h after UVA irradiation. There was a corresponding reduction in UVA-induced HO-1 levels (Fig. 4; see Fig. 2 also). Not surprisingly, levels of HO-2 were not affected by reducing Nrf2. Importantly, the basal HO-1 levels were lower in cells treated with siNrf2, p < 0.05, so that Nrf2 appears to be involved in controlling HO-1 levels in the absence of stress. While the effects of Nrf2 on basal HO-1 protein levels are well established,12,16,20 we provide evidence that reduction of Nrf2 protein levels leads to a reduction in basal and UVA-induced HO-1 proteins in FEK4 cells and suggest that Nrf2 regulates HO-1 levels under both normal physiological and stress conditions.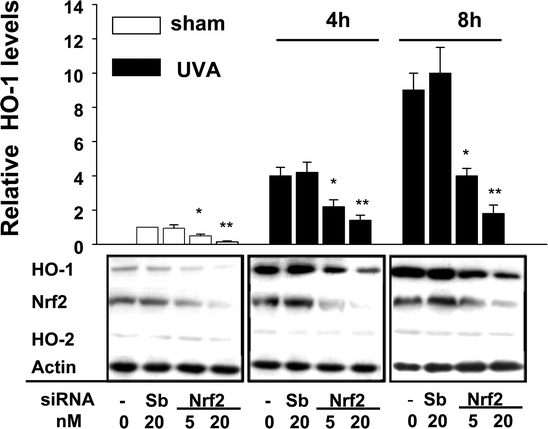 | ||
| Fig. 4 The effect of silencing Nrf2 on basal and UVA-mediated HO-1 induction. Whole cell lysate (40 μg) was subjected to Western blot analysis with anti-HO-1, HO-2, Nrf2 and actin antibodies. The intensity of the signal for HO-1 was normalized with respect to the intensity of the actin signal. Relative expression levels of HO-1 protein are shown in the bar graph as the ratio of the normalized value relative to the vehicle (non-siRNA), which was set to 1. Values are means ± SE (n = 4). * p < 0.05, ** p < 0.01 vs. scramble controls. | ||
These results also indicate that the up-regulation of Nrf2 by either heme or UVA radiation is at least partly responsible for HO-1 induction in human skin fibroblasts. The involvement of heme in UVA induction of HO-1 supports the original finding that heme stabilizes Nrf2 and leads to increased levels of the protein.23 However heme is also involved in the negative regulation of HO-1 by virtue of its binding to the repressor Bach1. This is known to release the protein from the promoter site and lead to de-repression by leaving the MARE site accessible to the Nrf2 complex.11 The degradation of Bach1 as a result of hemin treatment27 is an additional mechanism by which heme contributes to the maintenance of the de-repressed state of the HO-1 gene.
3.5 Nrf2 involvement in protection of FEK4 cells against UVA-mediated membrane damage
Braun et al.15 showed that Nrf2 was up-regulated in healing mouse skin and Nrf2 deficient mice have delayed wound repair and prolonged inflammation. Sensitization of cells to oxidative damage as a result of reduced Nrf2 was reported by Hirota et al.13 who found that mouse dermal fibroblasts deficient in Nrf2 show increased apoptosis following UVA irradiation. Induction of Nrf2-mediated gene expression protects cells from the toxicity of chemical reagents.14 Consistent with this, a study with Nrf2+/+ and Nrf2−/− mouse embryonic fibroblasts,24 revealed that Nrf2-dependent up-regulation of glutathione provides resistance to acrolein, CuOOH and chlorambucil, but not menadione. Recently Nrf2-driven HO-1 induction was found to be involved in protection of leukaemia cells against TNF-induced apoptosis.16 These results lead to the expectation that Nrf2 will be involved in protection of skin and skin cells against oxidative damage.UVA radiation is a membrane damaging agent in human skin fibroblasts.29 Since reduction of Nrf2 leads to a decrease in basal and UVA-induced HO-1 protein levels in FEK4 cells, and Nrf2-driven HO-1 induction may contribute to the resistance to oxidative stress, we determined whether silencing of Nrf2 would sensitize cells to membrane damage mediated by UVA irradiation. Loss of plasma membrane integrity, an important cause of cell death after UVA treatment, was quantified as the release of cytosolic LDH into the culture medium. As shown in Fig. 5, treatment with siNrf2 did not significantly increase LDH release in the absence of treatment but following UVA-irradiation led to increased LDH release at both 4 h and 8 h. This level increased further after pre-treatment with siNrf2, which increased LDH release by 2.5 to 3-fold compared with a 2-fold increase 4 h after UVA irradiation alone (p < 0.05). At 8 h post-UVA irradiation, LDH release was increased by 1.5-fold when compared with the sham control. These results indicate that the inhibition of Nrf2 protein levels increased UVA-induced damage as monitored by disruption of cell membranes. It is also consistent with our previous findings that UVA is a membrane damaging oxidative stress and that cells may recover to some extent 6 h after irradiation.17,18,29 We suggest that Nrf2 activation contributes to cell defence and plays a role in protection of human skin fibroblasts against oxidative damage. Although HO-1 is a candidate for the key enzyme involved in this protective response, other enzymes may also be involved or even be entirely responsible for the protection since Nrf2 is involved in regulation of several other enzymes involved in modulating oxidative stress, including glutathione biosynthetic enzymes, NAD(P)H:quinine oxidoredutase-1, aldo-keto reductase and thioredoxin reductase.5,19
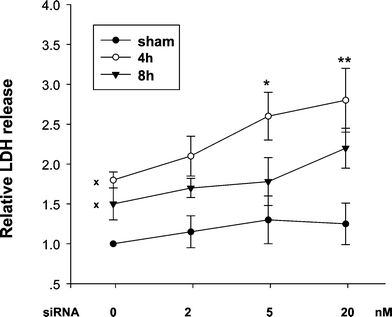 | ||
| Fig. 5 Reduced Nrf2 sensitizes cells to UVA-mediated membrane damage. Cells were treated with siNrf2 for 48 h, then sham and UVA-irradiated as in Fig. 4. At 4 and 8 h following UVA irradiation, LDH were detected using LDH assay kit (Roche) and LDH expressed as relative fold increase compared with the scrambled siRNA-treated sham-irradiated control, which was set to 1. Values are means ± SE (n = 4). * p < 0.05, ** p < 0.01 when compared with irradiated scrambled siRNA treated control. The X marked on the control (0 nM siRNA) values indicates that they are significantly (p < 0.05) different compared with the sham-irradiated control. | ||
In summary, we demonstrate that Nrf2 protein activation is critically involved in up-regulation of HO-1 expression by both UVA radiation and heme in human primary skin fibroblasts. Apparently this up-regulation protects FEK4 cells against UVA-mediated membrane damage so that Nrf2 is involved in pathways of human skin adaptation to this environmental stress and is therefore relevant to the current clinical interest in finding agents that enhance skin protection by modulating endogenous enzymes. This concept is supported by the recent finding of Kimura et al.30 who showed that Nrf2 is involved in the quercetin-mediated protection of HaCaT cells from oxidative damage induced by UVA radiation. It is important to note that modulation of the activity of the Bach1 repressor protein is also crucial to the UVA activation of HO-1 in FEK4 cells (unpublished data, this laboratory) consistent with the concept of a dynamic exchange of Bach 1 and NF-E2-related factors at the MARE region of the HO-1 promoter.10
Acknowledgements
This work was supported by a project grant (BB/D521530/1) from the Biotechnology and Biological Sciences Research Council (BBSRC), UK. The authors gratefully acknowledge the generous help of Professor Jawad Alam with preliminary studies.References
- R. M. Tyrrell, UVA (320–380 nm) radiation as an oxidative stress, in Oxidative Stress: Oxidants and antioxidants, ed. H. Sies, Academic Press, London, 1991, pp. 57–83 Search PubMed.
- S. M. Keyse and R. M. Tyrrell, Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 99–103 CrossRef CAS.
- Y. Matsumura and H. N. Ananthaswamy, Toxic effects of ultraviolet radiation on the skin, Toxicol. Appl. Pharmacol., 2004, 195, 298–308 CrossRef (review).
- J. Alam, K. Igarashi, K. S. Immenschuh, S. Shibahara and R. M. Tyrrell, Regulation of heme oxygenase-1 gene transcription: recent advances and highlights from the International Conference (Uppsala, 2003) on Heme Oxygenase, Antioxid. Redox Signal, 2004, 6, 924–933 CAS (review).
- K. Srisook, C. Kim and Y. N. Cha, Molecular mechanisms involved in enhancing HO-1 expression: de-repression by heme and activation by Nrf2, the “one-two” punch, Antioxid. Redox Signaling, 2005, 7, 1674–1687 Search PubMed (review)..
- N. G. Abraham and A. Kappas, Pharmacological and clinical aspects of heme oxygenase, Pharmacol. Rev., 2008, 60(1), 79–127 CrossRef CAS.
- L. E. Otterbein, M. P. Soares, K. Yamashita and F. H. Bach, Heme oxygenase-1: unleashing the protective properties of heme, Trends Immunol., 2003, 24, 449–455 CrossRef CAS.
- S. Basu-Modak and R. M. Tyrrell, Singlet oxygen: a primary effector in the ultraviolet A/near-visible light induction of the human heme oxygenase gene, Cancer Res., 1993, 53, 4505–4510 CAS.
- E. Kvam, A. Noel, S. Basu-Modak and R. M. Tyrrell, Cycloxygenase dependent release of heme from microsomal heme proteins correlates with induction of heme oxygenase 1 transcription in human fibroblasts, Free Radical Biol. Med., 1999, 26, 511–517 CrossRef CAS.
- K. Igarashi and J. Sun, The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation, Antioxid. Redox Signaling, 2006, 8, 107–118 Search PubMed (review).
- J. Sun, M. Brand, Y. Zenke, S. Tashiro, M. Groudine and K. Igarashi, Heme regulates the dynamic exchange of Bach1 and NF-E2-related factors in the Maf transcription factor network, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 1461–1466 CrossRef CAS.
- J. F. Reichard, G.T. Motz and A. Puga, Heme oxygenase-1 induction by NRF2 requires inactivation of the transcriptional repressor BACH1, Nucleic Acids Res., 2007, 35, 7074–7086 CrossRef CAS.
- A. Hirota, Y. Kawachi, K. Itoh, Y. Nakamura, X. Xu, T. Banno, T. Takahashi, M. Yamamoto and F. Otsuka, Ultraviolet A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: protective role in UVA-induced apoptosis, J. Invest. Dermatol., 2005, 124, 825–832 CrossRef CAS.
- M. Durchdewald, T. A. Beyer, D. A. Johnson, J. A. Johnson, S. Werner and U. auf dem Keller, Electrophilic chemicals but not UV irradiation or reactive oxygen species activate Nrf2 in keratinocytes in vitro and in vivo, J. Invest. Dermatol., 2007, 127, 646–653 CrossRef CAS.
- S. Braun, C. Hanselmann, M. G. Gassmann, U. auf dem Keller, C. Born-Berclaz, K. Chan, Y. W. Kan and S. Werner, Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound, Mol. Cell. Biol., 2002, 22, 5492–5505 CrossRef CAS.
- S. A. Rushworth and D. J. MacEwan, HO-1 underlies resistance of AML cells to TNF-induced apoptosis, Blood, 2008, 111, 3793–3801 CrossRef CAS.
- J. L. Zhong, A. Yiakouvaki, P. Holley, R. M. Tyrrell and C. Pourzand, Susceptibility of skin cells to UVA-induced necrotic cell death reflects the intracellular level of labile iron, J. Invest. Dermatol., 2004, 123, 771–780 CrossRef CAS.
- M. Salazar, A. I. Rojo, D. Velasco, R. M. de Sagarra and A. Cuadrado, Glycogen synthase kinase-3b inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2, J. Biol. Chem., 2006, 281, 14841–14851 CrossRef CAS.
- J. D. Hayes and M. McMahon, NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer, Trends Biochem. Sci., 2009, 34(4), 176–188 CrossRef CAS.
- J. L. Zhong, C. Raval, G. P. Edwards and R. M. Tyrrell, A role for Bach1 and HO-2 in suppression of basal and UVA-induced HO-1 expression in human keratinocytes, Free Radic. Biol. Med. DOI:10.1016/freeradbiomed.2009.10.037.
- K. Nakaso, H. Yano, Y. Fukuhara, T. Takeshima, K. Wada-Isoe and K. Nakashima, PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells, FEBS Lett., 2003, 546, 181–184 CrossRef CAS.
- A. Noël and R. M. Tyrrell, Development of refractoriness of induced human heme oxygenase-1 gene expression to reinduction by UVA irradiation and hemin, Photochem. Photobiol., 1997, 66(4), 456–463 CrossRef CAS.
- J. Alam, E. Killeen, P. Gong, R. Naquin, B. Hu, D. Stewart, J. R. Ingelfinger and K. A. Nath, Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2, Am. J. Physiol.: Renal Physiol., 2003, 284, F743–F752 CAS.
- L.G. Higgins, M. O. Kelleher, I. M. Eggleston, K. Itoh, M. Yamamoto and J. D. Hayes, Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents, Toxicol. Appl. Pharmacol., 2009, 237(3), 267–280 CrossRef CAS.
- H. Harada, R. Sugimoto, A. Watanabe, S. Taketani, K. Okada, E. Warabi, R. Siow, K. Itoh, M. Yamamoto and T. Ishii, Differential roles for Nrf2 and AP-1 in upregulation of HO-1 expression by arsenite in murine embryonic fibroblasts, Free Radical Res., 2008, 42(4), 297–304 CrossRef CAS.
- Y. Ding, Y. Z. Zhang, K. Furuyama, K. Ogawa, K. Igarashi and S. Shibahara, Down-regulation of heme oxygenase-2 is associated with the increased expression of heme oxygenase-1 in human cell lines, FEBS J., 2006, 273, 5333–5346 CrossRef CAS.
- Y. Zenke-Kawasaki, Y. Dohi, Y. Katoh, T. Ikura, M. Ikura, T. Asahara, F. Tokunaga, K. Iwai and K. Igarashi, Heme induces ubiquitination and degradation of the transcription factor Bach1, Mol. Cell. Biol., 2007, 27, 6962–6971 CrossRef CAS.
- L. Marrot, C. Jones, P. Perez, et al and J. R. Meunier, The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis, Pigm. Cell Melanoma Res., 2008, 21(1), 79–88 Search PubMed.
- G. F. Vile, S. Basu-Modak, C. W. Waltner and R. M. Tyrrell, Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts, Proc. Natl. Acad. Sci. U. S. A., 1994, 91, 2607–2610 CrossRef CAS.
- S. Kimura, E. Warabi, T. Yanagawa, D. Ma, K. Itoh, Y. Ishii, Y. Kawachi and T. Ishii, Essential role of Nrf2 in keratinocyte protection from UVA by quercetin, Biochem. Biophys. Res. Commun., 2009, 387(1), 109–114 CrossRef CAS.
Footnotes |
| † Joint first author. |
| ‡ Present address: College of Bioengineering, Chongqing University, Chongqing, China. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2010 |
