Understanding the mechanism of non-polar Diels–Alder reactions. A comparative ELF analysis of concerted and stepwise diradical mechanisms†
Abstract
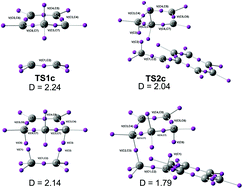
The electron-reorganization along the concerted and stepwise pathways associated with the non-polar Diels–Alder reaction between

 Please wait while we load your content...
Please wait while we load your content...