Anupam Bandyopadhyay, Neha Agrawal, Sachitanand M. Mali, Sandip V. Jadhav and Hosahudya N. Gopi
Org. Biomol. Chem., 2010,8, 4855-4860
DOI:
10.1039/C0OB00199F,
Paper
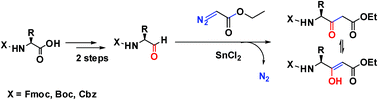
A facile synthetic route for the preparation of