Brønsted acid-catalyzed efficient Strecker reaction of ketones, amines and trimethylsilyl cyanide†
Abstract
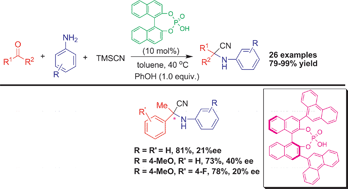
A general method for the one-pot, three-component Strecker reaction of ketones was developed using Brønsted acids as organocatalysts. A series of α-aminonitriles were obtained in good to excellent yields (79–99%). A preliminary extension to a catalytic enantioselective three-component Strecker reaction of ketones (up to 40% ee) is also described.

 Please wait while we load your content...
Please wait while we load your content...