DOI:
10.1039/B923249B
(Paper)
Org. Biomol. Chem., 2010,
8, 1816-1820
Zn(OTf)2-catalyzed addition of amines to carbodiimides: efficient synthesis of guanidines and unpredicted formation of Zn–N amido species†
Received
5th November 2009
, Accepted 21st January 2010
First published on
8th February 2010
Abstract
Zn(OTf)2 acts as an excellent catalyst precursor for addition of various amine N–H bonds to carbodiimides under an atmosphere of air, offering a convenient synthesis of substituted guanidines with high functional-group tolerance. A Zn–N amido species is shown to act as the active species.
Introduction
The search for readily available and cheap catalyst system for more convenient and efficient chemical transformations is of great interest and importance for academic and industrial research. The general strategy is to explore the untouched elements with the expectation superior to the known catalysts. Recently, catalytic addition reaction of amine N–H bonds to carbodiimides (catalytic guanylation reaction of amines or hydroamination of carbodiimides) has received much current interest1,2 because it provides a highly efficient and straightforward preparation of multi-substituted guanidines serving as building blocks for many biologically relevant compounds.3 The titanium imido complexes were reported first in 2003 by Richeson and coworkers to effect the catalytic addition of only primary aromatic amines to carbodiimides because the catalytic process required the regeneration of a “Ti![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N” double bond moiety.4 In 2006, a rare earth metal–nitrogen single bond species generated in situ from a half-sandwich rare earth metal alkyl complex and amines was reported by Hou and coworkers to achieve the catalytic addition of both primary and secondary amines to carbodiimides through the nucleophilic addition mechanism of a metal–nitrogen single bond to a carbodiimide.2h,i Montilla and coworkers reported that the vanadium imido chloride Cl3V
N” double bond moiety.4 In 2006, a rare earth metal–nitrogen single bond species generated in situ from a half-sandwich rare earth metal alkyl complex and amines was reported by Hou and coworkers to achieve the catalytic addition of both primary and secondary amines to carbodiimides through the nucleophilic addition mechanism of a metal–nitrogen single bond to a carbodiimide.2h,i Montilla and coworkers reported that the vanadium imido chloride Cl3V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(C6H3iPr2-2,6) could also serve as a catalyst precursor for this addition, whose mechanism was confirmed via the pathway of carbodiimide insertion into a V–N amido bond combining the experimental and DFT calculation methods.5 Other catalyst systems bearing M–N (M = Li, Ti, Ca, Al, and lanthanides) amido moiety had been found to have high catalytic activity.2 These reported catalyst systems were generally sensitive to air and moisture and reactions were carried out under the inert atmosphere. In contrast, the use of late transition metal catalyst as well as the catalyst system open to air has not been reported previously. We report here readily available Zn(OTf)2 acts as an excellent catalyst precursor for the addition of amine N–H bonds to carbodiimides under a closed air atmosphere yielding efficiently substituted guanidines with high functional-group tolerance. The mechanistic studies show an unpredicted Zn–N amido species is produced by release of a guanidinium triflate, but acts as active intermediate in this process.
N(C6H3iPr2-2,6) could also serve as a catalyst precursor for this addition, whose mechanism was confirmed via the pathway of carbodiimide insertion into a V–N amido bond combining the experimental and DFT calculation methods.5 Other catalyst systems bearing M–N (M = Li, Ti, Ca, Al, and lanthanides) amido moiety had been found to have high catalytic activity.2 These reported catalyst systems were generally sensitive to air and moisture and reactions were carried out under the inert atmosphere. In contrast, the use of late transition metal catalyst as well as the catalyst system open to air has not been reported previously. We report here readily available Zn(OTf)2 acts as an excellent catalyst precursor for the addition of amine N–H bonds to carbodiimides under a closed air atmosphere yielding efficiently substituted guanidines with high functional-group tolerance. The mechanistic studies show an unpredicted Zn–N amido species is produced by release of a guanidinium triflate, but acts as active intermediate in this process.
Results and discussion
Catalytic addition of primary aromatic amines to carbodiimides
The neat aniline could not react with N,N′-diisopropylcarbodiimide iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr at room temperature or higher temperatures (Table 1, entry 1). Gratifyingly, addition of 5 mol% of AlCl3 at 80 °C led to rapid addition of aniline to iPrN
NiPr at room temperature or higher temperatures (Table 1, entry 1). Gratifyingly, addition of 5 mol% of AlCl3 at 80 °C led to rapid addition of aniline to iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr to give the N,N′,N′′-trisubstituted guanidine 1 in 59% yield (entry 2). This result encouraged us to screen various metal salts (entries 3–14). Finally, Zn(OTf)2 was discovered the best choice for the reaction, but the polarity of solvents did not show a significant influence on the catalytic activity (entries 11–14).
NiPr to give the N,N′,N′′-trisubstituted guanidine 1 in 59% yield (entry 2). This result encouraged us to screen various metal salts (entries 3–14). Finally, Zn(OTf)2 was discovered the best choice for the reaction, but the polarity of solvents did not show a significant influence on the catalytic activity (entries 11–14).
Table 1 Zn(OTf)2-catalyzed addition of aniline to iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPra
NiPra
|

|
| Entry |
cat. (mol%) |
Solvent |
T/°C |
Time/h |
Yieldb(%) |
|
Conditions: aniline, 0.52 mmol; N,N′-diisopropylcarbodiimide, 0.50 mmol.
Yields were determined by 1H NMR.
|
| 1 |
— |
C6D5Cl |
140 |
24 |
1 (0) |
| 2 |
AlCl3 (5) |
C6D6 |
80 |
3 |
1 (59) |
| 3 |
FeCl3 (5) |
C6D6 |
80 |
3 |
1 (0) |
| 4 |
ZnCl2 (5) |
C6D6 |
80 |
3 |
1 (0) |
| 5 |
CuCl2 (5) |
C6D6 |
80 |
3 |
1 (20) |
| 6 |
PdCl2 (5) |
C6D6 |
80 |
10 |
1 (>95) |
| 7 |
Pd(PPh3)4 (5) |
C6D6 |
80 |
24 |
1 (60) |
| 8 |
Pd(OAc)2 (5) |
C6D6 |
80 |
2 |
1 (95) |
| 9 |
Sc(OTf)3 (5) |
C6D6 |
80 |
3 |
1 (25) |
| 10 |
La(OTf)3 (5) |
C6D6 |
80 |
3 |
1 (30) |
| 11 |
Zn(OTf)2 (5) |
C6D6 |
80 |
3 |
1 (quant.) |
| 12 |
Zn(OTf)2 (3) |
C6D6 |
80 |
5 |
1 (quant.) |
| 13 |
Zn(OTf)2 (3) |
THF-d8 |
80 |
6 |
1 (quant.) |
| 14 |
Zn(OTf)2 (3) |
Toluene-d8 |
80 |
6 |
1 (quant.) |
A broad range of substituted aniline could be used for this catalytic addition reaction, which was not affected by either electron-withdrawing or -donating substituents or their positions at the phenyl ring with high functional-group tolerance (Table 2, entries 1–16). The aromatic C–X (X = F, Cl, Br, or I) bonds (entries 4–7), nitro NO2 (entry 12), and terminal alkyne unit (entry 9) can survive the reaction conditions. It is noted that the reaction of 4-acetylaniline and ethyl 4-aminobenzoate with iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr gave the corresponding products 15 and 16 in benzene at 80 °C, respectively (entries 14 and 15). This result is in striking contrast with what was observed previously for the reported catalysts such as a rare earth metal-alkyl or amido complexes1a,2h,i and AlMe3,2a which failed to produce the corresponding guanidines because of their sensitivity to carbonyl group and ester group. In addition, when a diamine was used to react with 2 eq. of iPrN
NiPr gave the corresponding products 15 and 16 in benzene at 80 °C, respectively (entries 14 and 15). This result is in striking contrast with what was observed previously for the reported catalysts such as a rare earth metal-alkyl or amido complexes1a,2h,i and AlMe3,2a which failed to produce the corresponding guanidines because of their sensitivity to carbonyl group and ester group. In addition, when a diamine was used to react with 2 eq. of iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
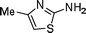
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr, the corresponding biguanidine 17 was produced in high yield (entry 16). Heteroatom-containing amines such as amino-substituted pyridine and thiazole were also applicable (entries 17 and 18).
NiPr, the corresponding biguanidine 17 was produced in high yield (entry 16). Heteroatom-containing amines such as amino-substituted pyridine and thiazole were also applicable (entries 17 and 18).
Table 2 Catalytic addition of various amines to carbodiimidesa
|

|
| Entry |
Ar–NH2 |
R, R′ |
Conditions |
Product (yield/%)b |
|
Conditions: amines, 2.06 mmol; carbodiimides, 2.00 mmol; Zn(OTf)2, 0.06 mmol; benzene, 5 mL.
Isolated yield.
Zn(OTf)2, 5 mol%.
The biguanidine was isolated when a carbodiimide (4.00 mmol) was used.
|
| 1 |
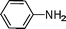
|
Cy |
C6H6, 80 °C, 5 h |
2, 96 |
| 2 |

|
t
Bu |
C6H6, 80 °C, 8 h |
3, 96 |
| 3 |
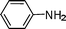
|
CH2Ph,Ph |
C6H6, 80 °C, 5 h |
4, 91 |
| 4 |

|
i
Pr |
C6H6, 80 °C, 3 h |
5, 97 |
| 5 |

|
i
Pr |
C6H6, 80 °C, 3 h |
6, 97 |
| 6 |
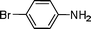
|
i
Pr |
C6H6, 80 °C, 3 h |
7, 97 |
| 7 |

|
i
Pr |
C6H6, 80 °C, 3 h |
8, 95 |
| 8 |

|
i
Pr |
C6H6, 80 °C, 3 h |
9, 96 |
| 9 |

|
i
Pr |
C6H6, 80 °C, 4 h |
10, 94 |
| 10 |
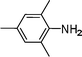
|
i
Pr |
C6H6, 80 °C, 3 h |
11, 97 |
| 11 |

|
i
Pr |
C6H6, 80 °C, 3 h |
12, 95c |
| 12 |

|
i
Pr |
CH2Cl2, reflux, 3 h |
13, 85c |
| 13 |

|
i
Pr |
C6H6, 80 °C, 3 h |
14, 92c |
| 14 |

|
i
Pr |
C6H6, 80 °C, 3 h |
15, 95c |
| 15 |

|
i
Pr |
C6H6, 80 °C, 3 h |
16, 95c |
| 16 |

|
i
Pr |
CH2Cl2, reflux, 3 h |
17, 94d |
| 17 |

|
i
Pr |
C6H6, 80 °C, 5 h |
18, 95 |
| 18 |
|
i
Pr |
THF, reflux, 5 h |
19, 93 |
Secondary amine, such as pyrrolidine in the presence of 3 mol% of Zn(OTf)2, could be added to iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr at 80 °C for 3 h, to give the corresponding N,N′,N′′,N′′-tetrasubstituted guanidines 19a in 94% yield (eqn (1)).
NiPr at 80 °C for 3 h, to give the corresponding N,N′,N′′,N′′-tetrasubstituted guanidines 19a in 94% yield (eqn (1)).
| |  | (1) |
Mechanistic studies
a) Reaction of 2- isopropylaniline, Zn(OTf)2 and carbodiimide
The reaction of Zn(OTf)2 with an excess amount of 2- isopropylaniline at elevated temperatures was attempted to obtain the active species in this catalytic process. Interestingly, the amine-coordinated zinc complex 20 was isolated quantitatively from a THF solution and confirmed by an X-ray diffraction analysis (Fig. 1).‡ There is a crystallographic inversion centre in 20. Complex 20 adopts a dimeric structure, in which the two Zn atoms are bridged by two triflate units through the oxygen atoms. Unexpectedly, a guanidinium triflate 22 was isolated from the reaction of 20 with iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr at room temperature. The structure of 22 was unambiguously characterized by NMR spectroscopy and an X-ray diffraction analysis (Fig. 2).‡ Further, we isolated the Zn–N amido species 21 from the residue after the guanidinium triflate 22 was isolated (Scheme 1). Although a single crystal of 21 suitable for X-ray crystallographic analysis was not obtained, its 1H and 13C NMR spectra was rather informative for the elucidation of the structure. The formation of 21 could be easily confirmed by the 1
NiPr at room temperature. The structure of 22 was unambiguously characterized by NMR spectroscopy and an X-ray diffraction analysis (Fig. 2).‡ Further, we isolated the Zn–N amido species 21 from the residue after the guanidinium triflate 22 was isolated (Scheme 1). Although a single crystal of 21 suitable for X-ray crystallographic analysis was not obtained, its 1H and 13C NMR spectra was rather informative for the elucidation of the structure. The formation of 21 could be easily confirmed by the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of Zn(OTf)2 with isopropylbenzenamido lithium which was in situ generated from the corresponding amine and n-BuLi. 21 could also serve as an excellent catalyst active species for the addition of 2-isopropylaniline to iPrN
1 reaction of Zn(OTf)2 with isopropylbenzenamido lithium which was in situ generated from the corresponding amine and n-BuLi. 21 could also serve as an excellent catalyst active species for the addition of 2-isopropylaniline to iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr to yield the guanidine 9 in almost quantitative yield (eqn (2)).
NiPr to yield the guanidine 9 in almost quantitative yield (eqn (2)).| |  | (2) |
 |
| | Fig. 1 ORTEP drawing of 20 with 30% thermal ellipsoids. Hydrogen atoms except those on the nitrogen atoms are omitted for clarity. Selected bond length (Å): Zn(1)–N(1) 2.090(3), Zn(1)–N(2) 2.106(3), Zn(1)–O(1) 2.115(3), Zn(1)–O(4) 2.194(3), Zn(1)–O(7) 2.036(3), S(2)–O(4) 1.455(3), S(2)–O(5) 1.442(3), S(2)–O(6) 1.441(3). ‘ = -x, -y + 1, -z + 2. | |
 |
| | Fig. 2 ORTEP drawing of 22 with 30% thermal ellipsoids. Triflate and hydrogen atoms except those on the nitrogen atoms are omitted for clarity. Selected bond length (Å) and angles (°): N(1)–C(7) 1.357(3), N(2)–C(7) 1.332(3), N(3)–C(7) 1.325(3), N(1)–C(7)–N(2) 119.94(19), N(1)–C(7)–N(3) 118.93(19), N(2)–C(7)–N(3) 121.11(19). | |
 |
| | Scheme 1 Formation of the Zn–N amido species 21 and guanidinium triflate 22. | |
b) Possible mechanism
Based on the above observations, a possible catalytic cycle for the addition reaction of primary aromatic amines to carbodiimides is proposed in Scheme 2.6 The reaction among primary amine, Zn(OTf)2 and carbodiimide should yield straightforwardly an amido species such as A by release of a guanidinium triflate. Nucleophilic addition of the amido species A to a carbodiimide would afford the guanidinate species B or C.7,8 Protonolysis of B or C by another molecule of primary amine would regenerate the amido A and release the more stable, final guanidine D.
 |
| | Scheme 2 A possible mechanism of catalytic addition of amines to carbodiimides. | |
Conclusions
We have demonstrated that Zn(OTf)2, under an atmosphere of air, serves as an excellent catalyst precursor for the addition of various primary and secondary amine N–H bonds to carbodiimides yielding substituted guanidines. This work has the following features: i) the use of cheap and readily available catalyst; ii) operational simplicity carried out for the first time under an atmosphere of air; iii) high functional-group tolerance, especially for carbonyl and ester group surviving the present conditions which are sensitive to previous catalyst systems. iv) an unpredicted Zn–N amido species, which is produced by release of a guanidinium triflate, acts as the active species in this catalytic process.
Experimental
General
Unless otherwise noted, all starting materials and solvents were commercially available and were used without further purification. C6D6, Toluene-d8, DMSO-d6 and THF-d8 (all 99+ atom% D) were obtained from Acros for NMR reactions. 1H and 13C NMR spectra were recorded on a JEOL-AL400 spectrometer (FT, 400 MHz for 1H; 100 MHz for 13C) or a JEOL JNM-AL300 spectrometer (FT, 300 MHz for 1H; 75 MHz for 13C) at room temperature.
Typical procedures for the catalytic reaction of primary aromatic amines to carbodiimides
i) NMR tube reaction.
Under an atmosphere of air, a J. Young valve NMR tube was charged with Zn(OTf)2 (6 mg, 0.015 mmol), C6D6 (0.5 mL), aniline (48 mg, 0.52 mmol), N,N′-diisopropylcarbodiimide (63 mg, 0.50 mmol). The tube was sealed and then heated at 80 °C. Formation of 1 was easily monitored by 1H NMR spectroscopy. The reaction was quantitative and finished within 5 h.
ii) Preparative scale reaction.
Under an atmosphere of air, a solution of aniline (192 mg, 2.06 mmol) in benzene (5 mL) was added to Zn(OTf)2 (22 mg, 0.06 mmol) in a Schlenk tube. N,N′-Diisopropylcarbodiimide (252 mg, 2.00 mmol) was then added to the above reaction mixture. The Schlenk tube was sealed, and the reaction was carried out at 80 °C for 5 h. After the solvent was removed under reduced pressure, the residue was extracted with ether and filtered to give a clean solution. After removing the solvent under vacuum, the residue was recrystallized in ether to provide a colorless solid 1.
14
.
Colorless solid, yield 92%. 1H NMR (300 MHz, C6D6): δ 0.87 (d, J = 6.3 Hz, 12H, CH3), 3.50–3.61 (m, 4H, CH and NH), 6.79–7.17 (m, 4H, C6H4). 13C NMR (75 MHz, C6D6): δ 23.0, 43.3, 103.4, 120.2, 123.8, 133.5, 150.0, 156.1. IR (neat): v (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) = 1574 cm−1; HRMS calcd. for C15H24N3O: 262.1914, found 262.1909.
O) = 1574 cm−1; HRMS calcd. for C15H24N3O: 262.1914, found 262.1909.
15
.
Colorless solid, yield 95%, m. p. 115.4∼115.9 °C. 1H NMR (300 MHz, C6D6): δ 1.01 (d, J = 6.3 Hz, 12H, CH3), 2.20 (s, 3H, CH3), 3.79–3.83 (m, 2H, CH), 4.26 (br, 2H, NH), 7.06–7.89 (m, 4H, C6H4). 13C NMR (75 MHz, C6D6): δ 23.1, 26.0, 43.4, 122.9, 129.9, 130.6, 151.0, 157.4, 196.3. IR (neat): v (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) = 1582 cm−1; HRMS calcd. for C16H26N3O2: 292.2019, found 292.2016.
O) = 1582 cm−1; HRMS calcd. for C16H26N3O2: 292.2019, found 292.2016.
Isolation and reaction of the zinc complex 20
To a solution of 2-isopropylaniline (608 mg, 4.5 mmol) in THF (1 mL) was added Zn(OTf)2 (727 mg, 2.0 mmol) in a Schlenk tube. The reaction mixture was stirred at 100 °C for 3 h. After cooling to room temperature, the reaction mixture was filtered and the solid product was washed with hexane until the filtrate became colorless. The filtrate was recrystallized in THF/Hexane to provide a colorless zinc complex 20. Then N,N′-Diisopropylcarbodiimide (252 mg, 2.0 mmol) was added to a solution of complex 20 (706 mg, 1.0 mmol) in THF (5 mL). The reaction was carried out at room temperature for 3 h. After the solvent was removed under reduced pressure, the residue was recrystallized in THF/Hexane to provide a colorless solid 22.
20
.
Single crystals suitable for X-ray analysis were grown in THF/Hexane at room temperature for 12 h. Colorless solid, yield 95%. 1H NMR (300 MHz, THF-d8): δ 1.20 (d, J = 6.9 Hz, 24H, CH3), 1.72–1.18 (br m, 8H, CH2), 2.90–3.04 (m, 4H, CH), 3.61–3.65 (br m, 8H, CH2), 4.45 (br, 8H, NH), 6.62–7.04 (m, 16H, C6H4). 13C NMR (75 MHz, THF-d8): δ 22.8, 28.1, 26.4, 68.4, 117.0, 119.2, 123.4, 125.8, 127.1, 133.5, 145.0.
22
.
Single crystals suitable for X-ray analysis were grown in THF/Hexane at room temperature for 12 h. Colorless solid, yield 92%. 1H NMR (300 MHz, THF-d8): δ 1.19–1.24 (m, 18H, CH3), 3.08–3.22 (m, 1H, CH), 3.92–4.02 (m, 2H, CH), 6.68 (br, 2H, NH), 7.18–7.43 (m, 4H, C6H4), 8.53 (br, 1H, NH). 13C NMR (75 MHz, THF-d8): δ 22.6, 23.7, 28.7, 45.9, 127.9, 128.2, 128.4, 129.5, 134.3, 146.4, 154.8.
Isolation of Zn–N amido species 21 from the reaction of the zinc complex 20 with N,N′-diisopropylcarbodiimide
N,N′-Diisopropylcarbodiimide (252 mg, 2.0 mmol) was added to a solution of complex 20 (706 mg, 1.0 mmol) in THF (5 mL). The reaction was carried out at room temperature for 3 h. After the solvent was removed under reduced pressure, the residue was recrystallized in THF/Hexane to provide a colorless solid 22 after filtering. Zn–N amido species 21 was obtained from the filtrate.
Preparation of the Zn–N amido species 21 by the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1 reaction of Zn(OTf)2 with isopropylbenzenamido lithium
1 reaction of Zn(OTf)2 with isopropylbenzenamido lithium
To a solution of 2-isopropylaniline (135 mg, 1.0 mmol) in THF was added n-BuLi (0.63 mL, 1.0 mmol) at −78 °C. After the mixture was stirred for 1 h at the same temperature, Zn(OTf)2 (364 mg, 1.0 mmol) was added and stirring was continued at −78 °C for 1 h. Then the reaction mixture was allowed to warm to room temperature for 1 h. After the solvent was removed under reduced pressure, the residue was washed with hexane. The residue was recrystallized in ether to provide a colorless solid 21.
21
.
Colorless solid, yield 90%. 1H NMR (300 MHz, DMSO-d6): δ 1.11 (d, J = 6.9 Hz, 6H, CH3), 1.72–1.76 (br m, 8H, CH2), 2.86–2.99 (m, 1H, CH), 3.56–3.60 (br m, 8H, CH2), 4.71 (br, 1H, NH), 6.48–7.34 (m, 4H, C6H4). 13C NMR (75 MHz, DMSO-d6): δ 22.6, 25.4, 26.7, 67.4, 115.3, 116.9, 119.0, 123.3, 125.2, 126.4, 131.7.
X-Ray crystallographic studies for 20 and 22
Crystals for X-ray analyses of 20 and 22 were obtained as described in the preparations. Data collections were performed at −100 °C on a Rigaku SATURN 724+ CCD diffractometer, using graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). The determination of crystal class and unit cell parameters was carried out by the CrystalClear (Rigaku Inc., 2008) program package. The raw frame data were processed using the CrystalClear (Rigaku Inc., 2008) to yield the reflection data file. The structure was solved by use of SHELXTL program.9 Refinement was performed on F2 anisotropically for all the non-hydrogen atoms by the full-matrix least-squares method. The hydrogen atoms were placed at the calculated positions and were included in the structure calculation without further refinement of the parameters. Crystal data, data collection and processing parameters for 20 and 22 were given only in Supporting Information.†
Acknowledgements
This work was supported by the Natural Science Foundation of China (Nos. 20702003, 20821062, and 20972006).
Notes and references
- Reviews:
(a) W.-X. Zhang and Z. Hou, Org. Biomol. Chem., 2008, 6, 1720–1730 RSC;
(b) H. Shen and Z. Xie, J. Organomet. Chem., 2009, 694, 1652–1657 CrossRef CAS.
- Selected examples:
(a) W.-X. Zhang, D. Li, Z. Wang and Z. Xi, Organometallics, 2009, 28, 882–887 CrossRef CAS;
(b) Y. Wu, S. Wang, L. Zhang, G. Yang, X. Zhu, C. Liu, C. Yin and J. Rong, Inorg. Chim. Acta, 2009, 362, 2814–2819 CrossRef CAS;
(c) X. Zhu, F. Xu and Q. Shen, Chin. J. Chem., 2009, 27, 19–22 CrossRef CAS;
(d) C. N. Rowley, T.-G. Ong, J. Priem, T. K. Woo and D. S. Richeson, Inorg. Chem., 2008, 47, 9660–9668 CrossRef CAS;
(e) C. N. Rowley, T.-G. Ong, J. Priem, D. S. Richeson and T. K. Woo, Inorg. Chem., 2008, 47, 12024–12031 CrossRef CAS;
(f) Z. Du, W. Li, X. Zhu, F. Xu and Q. Shen, J. Org. Chem., 2008, 73, 8966–8972 CrossRef CAS;
(g) J. R. Lachs, A. G. M. Barrett, M. R. Crimmin, G. Kociok-Köhn, M. S. Hill, M. F. Mahon and P. A. Procopiou, Eur. J. Inorg. Chem., 2008, 4173–4179 CrossRef;
(h) W.-X. Zhang, M. Nishiura and Z. Hou, Chem.–Eur. J., 2007, 13, 4037–4051 CrossRef CAS;
(i) W.-X. Zhang, M. Nishiura and Z. Hou, Synlett, 2006, 1213–1216 CAS;
(j) S. Zhou, S. Wang, G. Yang, Q. Li, L. Zhang, Z. Yao, Z. Zhou and H.-B. Song, Organometallics, 2007, 26, 3755–3761 CrossRef CAS;
(k) Q. Li, S. Wang, S. Zhou, G. Yang, X. Zhu and Y. Liu, J. Org. Chem., 2007, 72, 6763–6767 CrossRef CAS;
(l) T.-G. Ong, J. S. O'Brien, I. Korobkov and D. S. Richeson, Organometallics, 2006, 25, 4728–4730 CrossRef CAS;
(m) H. Shen, H.-S. Chan and Z. Xie, Organometallics, 2006, 25, 5515–5517 CrossRef CAS;
(n) X. Zhu, Z Du, F. Xu and Q. Shen, J. Org. Chem., 2009, 74, 6347–6349 CrossRef CAS;
(o) See also: M. R. Crimmin, A. G. M. Barrett, M. S. Hill, P. B. Hitchcock and P. A. Procopiou, Organometallics, 2008, 27, 497–499 Search PubMed;
(p) W.-X. Zhang, M. Nishiura, T. Mashiko and Z. Hou, Chem.–Eur. J., 2008, 14, 2167–2179 CrossRef;
(q) W.-X. Zhang, M. Nishiura and Z. Hou, Chem. Commun., 2006, 3812–3814 RSC.
-
(a)
Guanidines: Historical, Biological, Biochemical and Clinical Aspects of the Naturally Occurring Guanidino Compounds, A. Mori, B. D. Cohen and A. Lowenthal, ed., Plenum Press, New York, 1985 Search PubMed;
(b)
Guanidines 2: Further Explorations of the Biological and Clinical Significance of Guanidino Compounds, A. Mori, B. D. Cohen and H. Koide, ed., Plenum Press, New York, 1987 Search PubMed;
(c) R. G. S. Berlinck, A. C. B. Burtoloso and M. H. Kossuga, Nat. Prod. Rep., 2008, 25, 919–954 RSC;
(d) R. G. S. Berlinck and M. H. Kossuga, Nat. Prod. Rep., 2005, 22, 516–550 RSC;
(e) I. Bae, H. Han and S. Chang, J. Am. Chem. Soc., 2005, 127, 2038–2039 CrossRef CAS.
- T.-G. Ong, G. P. A. Yap and D. S. Richeson, J. Am. Chem. Soc., 2003, 125, 8100–8101 CrossRef CAS.
-
(a) F. Montilla, D. del Río, A. Pastor and A. Galindo, Organometallics, 2006, 25, 4996–5002 CrossRef CAS;
(b) F. Montilla, A. Pastor and A. Galindo, J. Organomet. Chem., 2004, 689, 993–996 CrossRef CAS.
- Though the proposed mechanism is most likely, other pathways can not be excluded. For example, Zn(OTf)2 acted as Lewis acid catalyst to activate a carbodiimide yielding the intermediate RN⊕
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-NR-Zn⊖(OTf)2 (E). Nucleophilic addition of an amine to E and proton transfer gave rise to the guanidine and regenerated Zn(OTf)2. See: N. Yamamoto and M. Isobe, Chem. Lett., 1994, 2299–2302 Search PubMed.
C-NR-Zn⊖(OTf)2 (E). Nucleophilic addition of an amine to E and proton transfer gave rise to the guanidine and regenerated Zn(OTf)2. See: N. Yamamoto and M. Isobe, Chem. Lett., 1994, 2299–2302 Search PubMed.
- For reviews of the chemistry of the guanidines and guanidinates, see:
(a) F. T. Edelmann, Adv. Organomet. Chem., 2008, 57, 183–352;
(b) M. P. Coles, Dalton Trans., 2006, 985–1001 RSC;
(c) P. J. Bailey and S. Pace, Coord. Chem. Rev., 2001, 214, 91–141 CrossRef CAS.
- Zinc guanidinates, see:
(a) S. Schmidt, S. Gondzik, S. Schulz, D. Bläser and R. Boese, Organometallics, 2009, 28, 4371–4376 CrossRef CAS;
(b) M. S. Khalaf, M. P. Coles and P. B. Hitchcock, Dalton Trans., 2008, 4288–4295 RSC;
(c) C. Jones, R. P. Rose and A. Stasch, Dalton Trans., 2007, 2997–2999 RSC;
(d) S. D. Bunge, J. M. Lance and J. A. Bertke, Organometallics, 2007, 26, 6320–6328 CrossRef CAS;
(e) S. J. Birch, S. R. Boss, S. C. Cole, M. P. Coles, R. Haigh, P. B. Hitchcock and A. E. H. Wheatley, Dalton Trans., 2004, 3568–3574 RSC;
(f) M. P. Coles and P. B. Hitchcock, Eur. J. Inorg. Chem., 2004, 2662–2672 CrossRef CAS;
(g) See also: J. Zhang, R. Cai, L. Weng and X. Zhou, Organometallics, 2004, 23, 3303–3308 Search PubMed.
-
G. M. Sheldrick, SHELXTL 5.10 for Windows NT: Structure Determination Software Programs, Bruker Analytical X-ray Systems, Inc., Madison, WI, 1997 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, X-ray data for 20 and 22, and scanned NMR spectra of all new products. CCDC reference numbers 741355 + 741356. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b923249b |
| ‡ Crystal data for 20: C48H68F12N4O14S4Zn2, Mw = 1412.04 g mol−1, T = 173(2) K, Tetragonal, space group I4(1)/a, a = 35.199(5), b = 35.199(5), c = 11.268(2) Å, β = 90°, V = 13960(4) Å3, Z = 8, ρc = 1.344 Mg m−3, μ = 0.894 mm−1, reflections collected: 62839, independent reflections: 6130 (Rint = 0.0623), Final R indices [I > 2σI]: R1 = 0.0644, wR2 = 0.1956, R indices (all data): R1 = 0.0681, wR2 = 0.1999. 22: C17H28F3N3O3S, Mw = 411.48 gmol−1, T = 173(2) K, Orthorhombic, space group Pna2(1)/a, a = 16.419(3), b = 12.458(3), c = 10.520(2) Å, β = 90°, V = 2151.8(7) Å3, Z = 4, ρc = 1.270 Mg m−3, μ = 0.196 mm−1, reflections collected: 16883, independent reflections: 4604 (Rint = 0.0369), Final R indices [I > 2σI]: R1 = 0.0441, wR2 = 0.0945, R indices (all data): R1 = 0.0482, wR2 = 0.0977. |
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N” double bond moiety.4 In 2006, a rare earth metal–nitrogen single bond species generated in situ from a half-sandwich rare earth metal alkyl complex and amines was reported by Hou and coworkers to achieve the catalytic addition of both primary and secondary amines to carbodiimides through the nucleophilic addition mechanism of a metal–nitrogen single bond to a carbodiimide.2h,i Montilla and coworkers reported that the vanadium imido chloride Cl3V
N” double bond moiety.4 In 2006, a rare earth metal–nitrogen single bond species generated in situ from a half-sandwich rare earth metal alkyl complex and amines was reported by Hou and coworkers to achieve the catalytic addition of both primary and secondary amines to carbodiimides through the nucleophilic addition mechanism of a metal–nitrogen single bond to a carbodiimide.2h,i Montilla and coworkers reported that the vanadium imido chloride Cl3V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(C6H3iPr2-2,6) could also serve as a catalyst precursor for this addition, whose mechanism was confirmed via the pathway of carbodiimide insertion into a V–N amido bond combining the experimental and DFT calculation methods.5 Other catalyst systems bearing M–N (M = Li, Ti, Ca, Al, and lanthanides) amido moiety had been found to have high catalytic activity.2 These reported catalyst systems were generally sensitive to air and moisture and reactions were carried out under the inert atmosphere. In contrast, the use of late transition metal catalyst as well as the catalyst system open to air has not been reported previously. We report here readily available Zn(OTf)2 acts as an excellent catalyst precursor for the addition of amine N–H bonds to carbodiimides under a closed air atmosphere yielding efficiently substituted guanidines with high functional-group tolerance. The mechanistic studies show an unpredicted Zn–N amido species is produced by release of a guanidinium triflate, but acts as active intermediate in this process.
N(C6H3iPr2-2,6) could also serve as a catalyst precursor for this addition, whose mechanism was confirmed via the pathway of carbodiimide insertion into a V–N amido bond combining the experimental and DFT calculation methods.5 Other catalyst systems bearing M–N (M = Li, Ti, Ca, Al, and lanthanides) amido moiety had been found to have high catalytic activity.2 These reported catalyst systems were generally sensitive to air and moisture and reactions were carried out under the inert atmosphere. In contrast, the use of late transition metal catalyst as well as the catalyst system open to air has not been reported previously. We report here readily available Zn(OTf)2 acts as an excellent catalyst precursor for the addition of amine N–H bonds to carbodiimides under a closed air atmosphere yielding efficiently substituted guanidines with high functional-group tolerance. The mechanistic studies show an unpredicted Zn–N amido species is produced by release of a guanidinium triflate, but acts as active intermediate in this process.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr at room temperature or higher temperatures (Table 1, entry 1). Gratifyingly, addition of 5 mol% of AlCl3 at 80 °C led to rapid addition of aniline to iPrN
NiPr at room temperature or higher temperatures (Table 1, entry 1). Gratifyingly, addition of 5 mol% of AlCl3 at 80 °C led to rapid addition of aniline to iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr to give the N,N′,N′′-trisubstituted guanidine 1 in 59% yield (entry 2). This result encouraged us to screen various metal salts (entries 3–14). Finally, Zn(OTf)2 was discovered the best choice for the reaction, but the polarity of solvents did not show a significant influence on the catalytic activity (entries 11–14).
NiPr to give the N,N′,N′′-trisubstituted guanidine 1 in 59% yield (entry 2). This result encouraged us to screen various metal salts (entries 3–14). Finally, Zn(OTf)2 was discovered the best choice for the reaction, but the polarity of solvents did not show a significant influence on the catalytic activity (entries 11–14).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPra
NiPra
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr gave the corresponding products 15 and 16 in benzene at 80 °C, respectively (entries 14 and 15). This result is in striking contrast with what was observed previously for the reported catalysts such as a rare earth metal-alkyl or amido complexes1a,2h,i and AlMe3,2a which failed to produce the corresponding guanidines because of their sensitivity to carbonyl group and ester group. In addition, when a diamine was used to react with 2 eq. of iPrN
NiPr gave the corresponding products 15 and 16 in benzene at 80 °C, respectively (entries 14 and 15). This result is in striking contrast with what was observed previously for the reported catalysts such as a rare earth metal-alkyl or amido complexes1a,2h,i and AlMe3,2a which failed to produce the corresponding guanidines because of their sensitivity to carbonyl group and ester group. In addition, when a diamine was used to react with 2 eq. of iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr, the corresponding biguanidine 17 was produced in high yield (entry 16). Heteroatom-containing amines such as amino-substituted pyridine and thiazole were also applicable (entries 17 and 18).
NiPr, the corresponding biguanidine 17 was produced in high yield (entry 16). Heteroatom-containing amines such as amino-substituted pyridine and thiazole were also applicable (entries 17 and 18).

















![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr at 80 °C for 3 h, to give the corresponding N,N′,N′′,N′′-tetrasubstituted guanidines 19a in 94% yield (eqn (1)).
NiPr at 80 °C for 3 h, to give the corresponding N,N′,N′′,N′′-tetrasubstituted guanidines 19a in 94% yield (eqn (1)).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr at room temperature. The structure of 22 was unambiguously characterized by NMR spectroscopy and an X-ray diffraction analysis (Fig. 2).‡ Further, we isolated the Zn–N amido species 21 from the residue after the guanidinium triflate 22 was isolated (Scheme 1). Although a single crystal of 21 suitable for X-ray crystallographic analysis was not obtained, its 1H and 13C NMR spectra was rather informative for the elucidation of the structure. The formation of 21 could be easily confirmed by the 1
NiPr at room temperature. The structure of 22 was unambiguously characterized by NMR spectroscopy and an X-ray diffraction analysis (Fig. 2).‡ Further, we isolated the Zn–N amido species 21 from the residue after the guanidinium triflate 22 was isolated (Scheme 1). Although a single crystal of 21 suitable for X-ray crystallographic analysis was not obtained, its 1H and 13C NMR spectra was rather informative for the elucidation of the structure. The formation of 21 could be easily confirmed by the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of Zn(OTf)2 with isopropylbenzenamido lithium which was in situ generated from the corresponding amine and n-BuLi. 21 could also serve as an excellent catalyst active species for the addition of 2-isopropylaniline to iPrN
1 reaction of Zn(OTf)2 with isopropylbenzenamido lithium which was in situ generated from the corresponding amine and n-BuLi. 21 could also serve as an excellent catalyst active species for the addition of 2-isopropylaniline to iPrN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NiPr to yield the guanidine 9 in almost quantitative yield (eqn (2)).
NiPr to yield the guanidine 9 in almost quantitative yield (eqn (2)).




![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) = 1574 cm−1; HRMS calcd. for C15H24N3O: 262.1914, found 262.1909.
O) = 1574 cm−1; HRMS calcd. for C15H24N3O: 262.1914, found 262.1909.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) = 1582 cm−1; HRMS calcd. for C16H26N3O2: 292.2019, found 292.2016.
O) = 1582 cm−1; HRMS calcd. for C16H26N3O2: 292.2019, found 292.2016.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1 reaction of Zn(OTf)2 with isopropylbenzenamido lithium
1 reaction of Zn(OTf)2 with isopropylbenzenamido lithium![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C-NR-Zn⊖(OTf)2 (E). Nucleophilic addition of an amine to E and proton transfer gave rise to the guanidine and regenerated Zn(OTf)2. See: N. Yamamoto and M. Isobe, Chem. Lett., 1994, 2299–2302 Search PubMed.
C-NR-Zn⊖(OTf)2 (E). Nucleophilic addition of an amine to E and proton transfer gave rise to the guanidine and regenerated Zn(OTf)2. See: N. Yamamoto and M. Isobe, Chem. Lett., 1994, 2299–2302 Search PubMed.

