Hydrophobic iron oxide and CdSe/ZnS nanocrystal loaded polyglutamate/polyelectrolyte micro- and nanocapsules
Xinrong
Teng
*a,
Hongting
Pu
a,
Helmuth
Möhwald
b and
Jinyu
Sui
a
aSchool of Materials Science and Engineering, Tongji University, 4800 Cao An Rd., Jiading District, 201804, Shanghai, China. E-mail: txr@tongji.edu.cn; Fax: +86-21-65982461
bMax Planck Institute of Colloids and Interfaces, D-14424, Potsdam, Germany
First published on 16th August 2010
Abstract
A novel, simple and generic method for the preparation of hydrophobic nanocrystal loaded composite capsules is introduced. Firstly, magnetic Fe3O4 nanocrystals prepared by pyrolysis of fatty acid iron salts in non-aqueous media were successfully incorporated into water-dispersible polyglutamate/polyelectrolyte capsules by combining an ultrasonic protocol and polyelectrolyte layer-by-layer (LBL) assembly. Then, inspired by the similar synthesis mechanism of oxide and semiconductor nanocrystals based on organometallic approaches in non-aqueous media, two kinds of fluorescent semiconductor quantum dots (zinc sulfide-capped cadmium selenide nanocrystals) were chosen as models to explore QD loaded composite capsules. With rhodamine B isothiocyanate (RBITC) tagging PEI as outer layers, fluorescence micrographs and confocal microscopy images indicate that CdSe/ZnS QDs were successfully incorporated into polyglutamate/polyelectrolyte capsules with almost unchanged optical properties and the color of RBITC tagging PEI shell can be changed under different excitation. Color transformation ascribed to spectral conversion of embedded QDs was also observed after the capsules were stored under day light for days. TEM, electron diffraction (ED), and ESEM revealed that the method leads to well-defined nanocrystal loaded composite nanocapsules and is simple and generic.
1. Introduction
Hollow micro- and nano-sized polyelectrolyte capsules have attracted much attention in view of their applications in drug delivery and biomedical contrast imaging etc. They can be fabricated by means of layer-by-layer adsorption of oppositely charged polyelectrolytes on the surface of colloidal template particles with sequential removal of the template core. The obtained hollow capsules can then be refilled with a wide range of molecules or particles, such as dyes, drugs, magnetic particles etc.1–5 However, such an encapsulation approach has some drawbacks: the colloidal templates (cores) should be removed first by acids or alkali, or by hazardous solvents, such as hydrofluoric acid;6 the subsequent encapsulation of substances and purification and isolation are tedious; the size of the resulting microcapsules is in the micron range if the template core is micron size.7,8 Recently we developed a new method to fabricate water-immiscible solvent loaded polyelectrolyte nanocapsules. The encapsulation of water-insoluble solvent and formation of capsules can be completed in a one-step process9via combining an ultrasonic protocol10–12 and polyelectrolyte LBL assembly technology. First, aqueous polyglutamate capsules filled with water-immiscible liquid were made by applying high intensity ultrasound to a two-phase system of aqueous polyglutamate solution and non-aqueous liquid. Then, the obtained polyglutamate nanocapsules were coated with polyelectrolytes using LBL deposition, which is based on the sequential adsorption of oppositely charged polyelectrolytes on the negatively charged polyglutamate capsules. The superior advantages of this combined method are that the outer coated polyelectrolyte layers can not only strengthen the stability of the proteinaceous capsules but can also be easily modified by coupling some functional groups, e.g. proteins, carbohydrates through the COOH or NH2 functional group of the polymer chain. Our previous study demonstrated that lipophilic dyes9 or drugs13 can be incorporated into polyglutamate/cationic polyelectrolyte nanocapsules by applying this combined technology. Herein, our interest is extended to fabricate magnetic proteinaceous nanocapsules by the combined method considering prospective applications of magnetic nanocapsules. In order to incorporate magnetic materials into polyglutamate capsules by ultrasonic irradiation, the magnetic materials should be first dispersible in water insoluble solvent. This inspired us to synthesize magnetic oxide nanocrystals in non-aqueous media, and then disperse them in water insoluble solvent for ultrasonic treatment. Oxide nanocrystals of the elements in the fourth row of the periodic table (Cr, Mn, Fe, Co, and Ni) are important magnetic materials with applications ranging from magnetic resonance imaging, drug delivery, battery materials, catalysts, biosensing, to nanoelectronic materials, etc.14–16 Peng and coworkers reported that size and shape controlled magnetic oxide nanocrystals can be synthesized by pyrolysis of fatty acid metal salts in non-aqueous media.17 The as-synthesized magnetic oxide nanocrystals are dispersible in typical non-polar solvents such as toluene as a stable colloidal solution. Enlightened by this idea, herein, we choose iron as an example of elements in the fourth row of the periodic table to illustrate the fabrication process (Fig. 1). First, magnetic iron oxide nanocrystals were synthesized by pyrolysis of iron stearate in octadecene. Then the precipitate was purified and re-dispersed in toluene for the following ultrasonic treatment and subsequent LBL deposition, thus Fe3O4 nanocrystal loaded polyglutamate/polyelectrolyte nanocapsules were successfully obtained. Inspired by the similar synthesis mechanism of oxide and semiconductor nanocrystals in non-aqueous media,17,18 we then used CdSe/ZnS nanocrystals as a model to explore the encapsulation of semiconductor nanocrystals into composite nanocapsules. Direct encapsulation of hydrophobic luminescent semiconductor QDs into nanocapsules has important significance in biological applications. As is known, for biological applications most nanocrystals are greatly restricted because of their poor solubility in aqueous solutions. By this novel method, surface modification to render hydrophobic QDs water soluble is not needed19,20 since the hydrophobic QDs can be directly encapsulated into aqueous suspended proteinaceous capsules. Moreover, the functional groups (COOH, NH2) of the polyelectrolyte chains are available for covalent coupling to various biomolecules (such as proteins, peptides and nucleic acid) to target specific organs or tumor types. The embedded QDs inside the capsules could also act as identification codes for rapid target identification. | ||
| Fig. 1 The formation of LBL-stabilized Fe3O4 nanocrystal-loaded polyglutamate capsules. Magnetic iron oxide nanocrystals were first synthesized by pyrolysis of iron stearate in non-aqueous octadecene (a). Then the precipitate (b) was purified and re-dispersed in toluene (c) for the following ultrasonic treatment (d) and subsequent layer by layer deposition (e). (PG: polyglutamate, PEI: polyethyleneimine, PAA: polyacrylic acid, LBL: layer-by-layer). | ||
2. Experimental
Materials
Poly-L-glutamic acid sodium salt (polyglutamate, Mw ∼50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–100
000–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), polyethyleneimine (PEI, Mw ∼25
000), polyethyleneimine (PEI, Mw ∼25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), polyacrylic acid (PAA, Mw ∼50
000), polyacrylic acid (PAA, Mw ∼50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and rhodamine B isothiocyanate mixed isomers (RBITC) were purchased from Sigma-Aldrich. PEI labelled with RBITC (PEI-RBITC) was synthesized as described elsewhere.21 1-Octadecene (tech.90%) was purchased from Lancaster (UK). Iron(II) stearate, dodecylamine, sodium dodecyl sulfate (SDS) and other reagents were purchased from Sinopharm Chemical Reagent Co. (China). CdSe/ZnS nanocrystals in hexane with concentration of 4 μM (emission maximum 575 nm and 560 nm, respectively) was provided by Wuhan Jiayuan Co., China. All these compounds were used without further purification.
000) and rhodamine B isothiocyanate mixed isomers (RBITC) were purchased from Sigma-Aldrich. PEI labelled with RBITC (PEI-RBITC) was synthesized as described elsewhere.21 1-Octadecene (tech.90%) was purchased from Lancaster (UK). Iron(II) stearate, dodecylamine, sodium dodecyl sulfate (SDS) and other reagents were purchased from Sinopharm Chemical Reagent Co. (China). CdSe/ZnS nanocrystals in hexane with concentration of 4 μM (emission maximum 575 nm and 560 nm, respectively) was provided by Wuhan Jiayuan Co., China. All these compounds were used without further purification.
Synthesis of magnetic Fe3O4 nanocrystals
0.622 g of iron stearate and 0.269 g of dodecylamine were mixed with 5 ml of 1-octadecene and heated to 300 °C under an argon atmosphere. The reaction was matured after 20 min of heating. Fe3O4 nanocrystals could be precipitated from the reaction mixture using a minimum amount of methanol/acetone and the black precipitate was collected after centrifugation with 5000 r/min. The precipitation/dispersion scheme was repeated 2-3 times to purify the nanocrystals. The purified Fe3O4 nanocrystals were re-dispersible in toluene.17Preparation of Fe3O4 nanocrystal and CdSe/ZnS nanocrystal loaded polyglutamate capsules
The purified Fe3O4 nanocrystals were dispersed in toluene yielding a stable colloidal solution at room temperature. Then 5–10% (mg ml−1) Fe3O4 nanocrystal toluene solution was layered over 5–10% (mg ml−1) polyglutamate aqueous solution with 1 × 10−6 to 1 × 10−5 M SDS as surfactant. A high-intensity ultrasonic horn was positioned at the aqueous-organic interface. The mixture was sonicated in an ice bath for 1–3 min employing an acoustic power of 150 W cm−2 at 20 kHz frequency. The unreacted toluene and polyglutamate solution were removed and the capsule suspension was re-suspended in a small volume of water. For fabrication of CdSe/ZnS nanocrystal loaded polyglutamate capsules, keeping other experimental conditions unchanged, 4 μM CdSe/ZnS hexane solution was firstly diluted in hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v), and then the diluted solution was layered for sonication.
10 v/v), and then the diluted solution was layered for sonication.
Assembly of polyelectrolyte multilayer on the surface of polyglutamate capsules
For fabrication of the LBL assembled polyelectrolyte shell, the polyglutamate suspension was diluted by a small amount of water and then PEI or PEI-RBITC and PAA solution (2 mg mL−1 in 0.5 M NaCl) were added alternately. Every drop of polyelectrolyte solution was fixed to 5–10 μl and the drops were added to the slightly stirred suspension. Usually 50–100 μl polyelectrolyte solution was added to 1–2 ml polyglutamate suspension. Then the mixture was gently shaken for 10–15 min. The next layers were deposited in the same manner. After 4–6 alternating deposition steps, the final (PEI/PAA)2–3 layered structure was formed on top of the polyglutamate surface. Due to a highly diluted polyglutamate suspension and the amount of the added polyelectrolyte was very low (0.05 ml for 1 ml suspension), the probability of forming free polyelectrolyte complexes in solution and capsules aggregation are considered low here.Characterization
The Quanta 200 FEG Environmental Scanning Electron Microscope (ESEM) (FEI Company, USA) was used for visualization of the size and morphology of nanocapsules in high-vacuum mode. Samples were prepared by applying a drop of the suspension onto glass wafers with sequential drying and gold sputtering.The morphology and electron diffraction (ED) of the nanocapsules were observed by using a Hitachi Model H-800 TEM, with a tungsten filament at an accelerating voltage of 200kV. Samples were prepared by placing a drop of the suspension on the surface of a copper grid.
X-Ray diffraction (XRD) patterns of Fe3O4 nanocrystals were obtained using a Rigaku D/max 2550 VB3+/PC with Cu Kα radiation. The operation voltage and current were kept at 40 kV and 100 mA, respectively. The 2θ range was scanned from 10° to 90° in steps of 0.02° with a count time of 0.4 s.
A fluorescence microscope (BX51, Olympus, Japan) equipped with a DP71 (Olympus) cooled digital color camera was used to obtain the true-color fluorescence micrographs. Excitation in the UV range (330–385 nm) and green range (530–550 nm) was provided by a 100 W mercury lamp. A high numerical aperture (NA = 1.4, 100×), oil-immersion objective was used, and the microscope is fitted with Image-Pro Plus for image analysis.
Confocal microscope images were taken with a confocal laser-scanning system attached to an upright microscope (Leica TCS SP2, Germany) that was equipped with a 100× oil immersion objective having a numerical aperture of 1.4. Excitations at 405 nm and 543 nm were for QDs and RBITC respectively. The fluorescence intensity profiles of the scanned capsules were quantified using the TCLS software.
A RF-5301PC spectrofluorometer and UV-vis 3150 spectrophotometer (Shimadzu; Kyoto, Japan) were used for photoluminescence (PL) and absorption spectra analysis.
The size of the capsules was measured by a Dynamic Light Scattering system (Autosizer 4700, Malvern, England).
3. Results and discussion
Synthesis of Fe3O4 nanocrystals
The synthesis of Fe3O4 nanocrystals was done according to Peng's approach.17 With commercially available Fe(II)-stearate as the precursor, 1-octadecene as the solvent and dodecylamine as activation reagent, black precipitates could be isolated using a minimum amount of methanol/acetone for purification. They remained dispersible in toluene after purification and were found to be stable as a colloidal solution at room temperature. The X-ray powder diffraction pattern (XRD) of the precipitates (Fig. 2(a)) obtained by thermal decomposition of Fe(II) stearate is similar to that of Fe3O4 crystals.17 Nearly monodispersed dot-shaped and some quasi-cube-shaped Fe3O4 nanocrystals were found in a size range below 10 nm (Fig. 2(b), arrows). Both dots and cubes were observed in the TEM image, which is probably due to the fact that the reaction temperature was not carefully controlled. However, size and shape controlled Fe3O4 nanocrystals which had already been investigated successfully by Peng's group are not our key interest in this paper. Our aim here is to utilize the as-prepared Fe3O4 nanocrystals dispersed in non-aqueous media for fabrication of polyglutamate nanocapsules.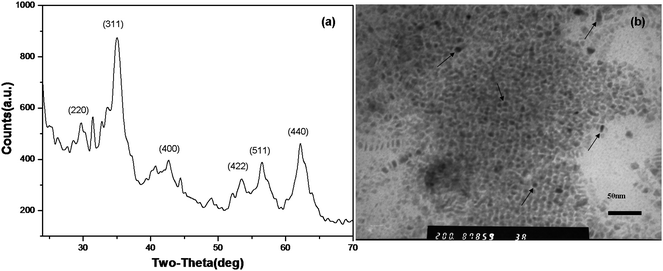 | ||
| Fig. 2 X-Ray power diffraction pattern (a) and TEM image (b) of Fe3O4 nanocrystals prepared by pyrolysis of Fe(II)-stearate in octadecene. | ||
Fabrication of Fe3O4 nanocrystal loaded polyglutamate/polyelectrolyte nanocapsules by ultrasonic irradiation and LBL assembly
The formation mechanism of oil filled polyglutamate capsules by ultrasonic irradiation had been discussed previously.9,11 For fabrication of Fe3O4 nanocrystal loaded polyglutamate capsules, 5–10% (mg ml−1) Fe3O4 nanocrystals were first dispersed in toluene and then the Fe3O4 toluene solution was layered on 5–10% aqueous polyglutamate solution for sonication. Since the initial polyglutamate capsules are not stable and most of them will disappear after several hours at room temperature, six layers of polyethyleneimine/polyacrylic acid (PEI/PAA) were deposited alternately on the surface of the initial polyglutamate capsules to obtain more stable ones. It was observed that the capsule suspension turned dilute gradually with increasing number of polyelectrolyte layers. The reason may be the increase in solubility of hydrophilic polyglutamate upon diluting and changing the pH of the media. Fig. 3(a) shows a TEM image of polyglutamate/(PEI/PAA)3 nanocapsules after 7 days storage at 4 °C. With polyelectrolytes as additional protective layers, spherical nanocapsules with a size of about 100–300 nm were observed. It is notable that such nanocapsules have dark-core/grey-shell structures and Fe3O4 crystalline electron microdiffraction patterns (Fig. 3(a) and 3(b)),which confirm that Fe3O4 nanocrystals were encapsulated into polyglutamate capsules. The formation of Fe3O4 nanocrystal loaded polyglutamate capsules may be explained as following: as sonochemistry arises from acoustic cavitation, upon ultrasonic irradiation, Fe3O4 nanocrystals dispersed in toluene accompanied by the volatile toluene are trapped inside the bubble, on which the hydrogen bonding networks of polyglutamate forms the outer shell. ESEM image (Fig. 3(c)) of 100–700 nm nanocapsules reveals the polydispersity of capsules, which is in accord with previous reports.11,22,23 The spherical shapes of nanocapsules (arrows) are consistent with the TEM image of Fig. 3(a). Different from those unstable polyglutamates which disappear quickly within several hours at room temperature, relatively stable capsules could be obtained after deposition of polyelectrolytes as outer layers. It indicates that the outer polyelectrolyte layers play an important role in stabilizing the capsules.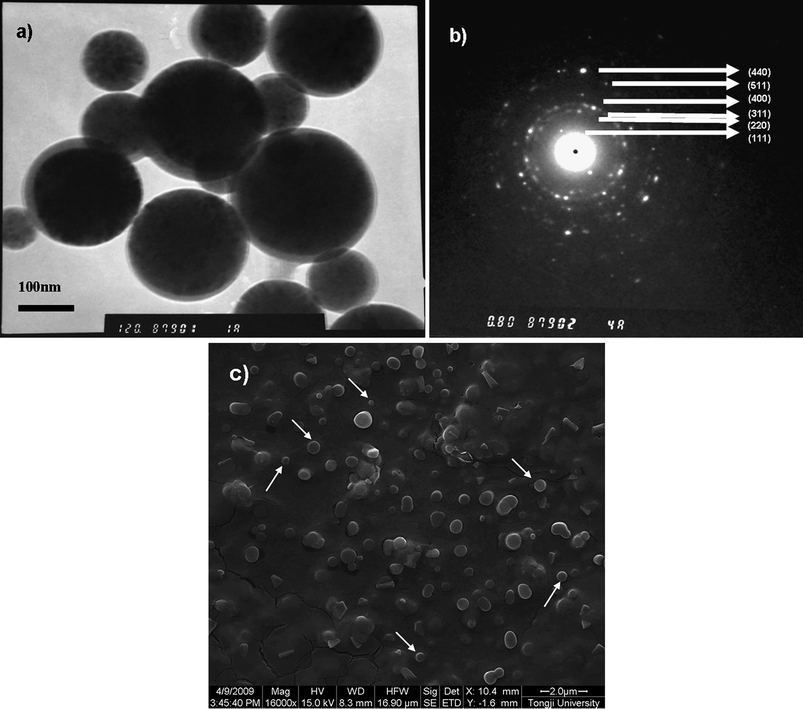 | ||
| Fig. 3 Fe3O4 nanocrystal-loaded polyglutamate nanocapsules with (PEI/PAA)3 multilayers. (a) TEM image of polyglutamate/(PEI/PAA)3 nanocapsules; (b) electron microdiffraction pattern of (a) measured by TEM; (c) ESEM image of polyglutamate/(PEI/PAA)3 nanocapsules. | ||
Up to now, Fe3O4 nanocrystals were only employed as a model for studying the fabrication of magnetic oxide nanocrystal loaded polyglutamate/polyelectrolyte nanocapsules, which can be replaced by a series of magnetic oxide nanocrystals in the fourth row of the periodic table. Moreover, inspired by the synthesis of oxide nanocrystals in non-aqueous approaches, we envision that they could also probably be replaced by II–VI semiconductors24,25 because the synthesis mechanism of these semiconductor nanocrystals and oxide nanocrystals based on organometallic approaches in non-aqueous solutions is similar.17 Both as-prepared nanocrystals can be dispersed in non-aqueous solvent such as toluene as a stable colloidal solution.17,18 If our concept is valid, the novel method will open a new door to explore fluorescent semiconductor nanocrystals as probes in biological fields.
Fabrication of CdSe/ZnS QD loaded polyglutamate/polyelectrolyte capsules
In order to verify our assumption above, CdSe/ZnS nanocrystals were chosen as models to explore whether semiconductor nanocrystals based on organometallic approaches in non-aqueous media could be encapsulated into composite capsules and whether these fluorescent QDs dispersed in water-insoluble solvent in capsules could keep their photostability. CdSe/ZnS QDs in hexane with emission maximum at 575 nm and 560 nm were used respectively in our experiments. First, CdSe/ZnS hexane solution was layered over aqueous polyglutamate solution for sonication, and then the initial polyglutamate bubbles were coated alternately with 4–6 layers of PEI and PAA.After sonication, a white milky emulsion was observed between the unreacted oil and the aqueous solution (Fig. 4(a)). It indicates that the bubbles tend to float up on the aqueous phase due to their lower densities compared to that of water. The emulsion of bubbles was coated with polyelectrolytes and the size distribution was measured. It shows that the freshly prepared sample has a broad size range from 30 nm to about 7 μm (with an average size of 751 nm, polydispersity index (PDI) 1.0, Fig. 4(b) solid line). However, bigger capsules usually can not be stored for a long time in contrast to smaller ones. Compared to the freshly prepared sample, the size distribution curve of the sample stored for 5 days (Fig. 4(b), dashed line) shows that the average size and PDI decreased to 218 nm and 0.678 respectively, which indicates that many larger capsules had disappeared due to their lower pressure difference according to Laplace's law. Smaller capsules can usually be stored at least for 3–4 weeks at 4 °C.
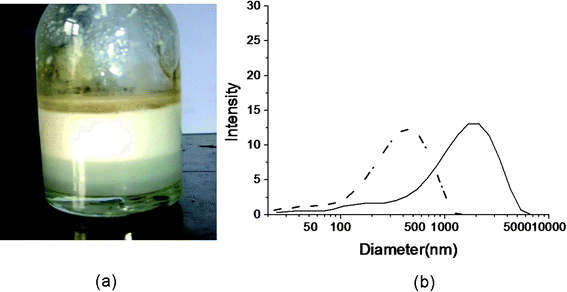 | ||
| Fig. 4 Polyglutamate capsules and their size distribution measured by dynamic light scattering system. (a) The emulsion of bubbles after sonication. (b) The size distribution curve of CdSe/ZnS QD loaded polyglutamate/(PEI/PAA)2 capsules before (solid line) and after aging for 5 days (dashed line). (QD max emission: 560 nm.) | ||
From the above analysis, one could see that the proteinaceous capsules prepared by sonication usually have a broad size distribution, from nano to micron scale. However, it should be pointed out that the polydispersed capsules in this paper appear inconsistent with our previous work,9 in which nearly monodispersed polyglutamate/polyelectrolyte nanocapsules were obtained. This can be explained by different experimental conditions used. In our previous work,9 the surfactant concentration (SDS) was very high (1 × 10−3 M), which resulted in a sharp increase of the amount of initial bubbles. Thus, strict filtration from a dense emulsion was used to obtain the monodispersed nanocapsules. In contrast, here, in order to simplify the experimental process, the SDS concentration was fixed to 1 × 10−6 to 1 × 10−5 M and filtration was not applied after sonication. In this paper, in order to observe the fluorescent signals of embedded QDs, we intentionally captured some larger capsules for fluorescence and confocal microscope observation due to the limited resolution of normal microscopes.
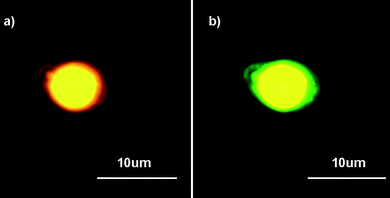
Firstly, the CdSe/ZnS QDs with maximum emission of 575 nm were used for fabrication and later observation. The true color fluorescence micrographs of composite capsules stored for about 24–36 h at room temperature (Fig. 5(a)) confirm that CdSe/ZnS QDs were incorporated successfully into composite capsules. The single yellow color indicates that the emission wavelength of embedded QDs is almost unchanged. Various shades of yellow observed in one capsule can be explained by heterogeneous QD dispersion in hexane instead of aggregation since the aggregation of QDs would cause spectral broadening, wavelength shifting and energy transfer.26,27 In order to distinguish the outer polyelectrolyte shell and embedded QDs, PEI labeled with rhodamine B isothiocyanate (RBITC) was used to replace unlabeled PEI for deposition on initial polyglutamate capsules. UV (335–380 nm) and green (530–550 nm) channels were used for excitation, respectively. Compared with those capsules without RBITC as labels, light green colors can be observed by UV irradiation of capsules with RBITC tagging shells (Fig. 5(b)). Moreover, these capsules turn to red fluorescence when switching to green excitation (Fig. 5(c)). Fig. 5(d) is the overlap of Fig. 5(b) and 5(c). The capsules are not overlapped completely because they floated in aqueous solution, which may be due to Brownian motion and electrostatic interaction. It indicates that the color of capsules can be changed under different excitation channels. The visual observation of green and red fluorescence is probably caused by the emission of RBITC. The fluorescence micrograph of the single capsule further confirms this point (Fig. 6). When using green excitation, the yellow core with the red circle of the capsule is assigned to the excitation of embedded QDs and RBITC on the shell respectively (Fig. 6(a)). In contrast, when switching to UV excitation, the inner yellow signals remain unchanged (even looks brighter) and the outer circle changes to green (Fig. 6(b)). The signals of QDs and RBITC can be simultaneously observed under both green and UV excitation is attributed to their intrinsic optical properties. Fig. 7(a) presents photoluminescence (PL) and UV absorption spectra of CdSe/ZnS QDs. It shows that CdSe/ZnS QDs can be excited at a nearly continuous excitation range from UV to 600 nm, but has a very narrow, symmetric emission band at 575 nm, which leads to their unchanged yellow signals upon both green and UV excitation.
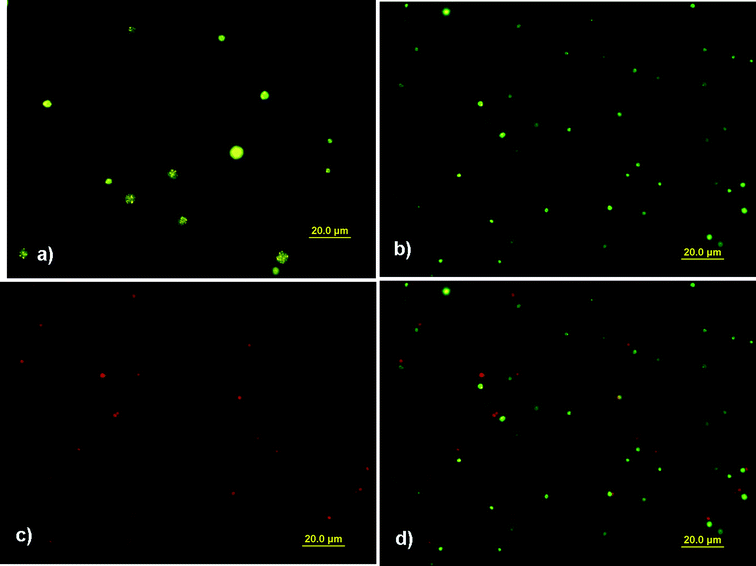 | ||
| Fig. 5 Fluorescence micrographs of CdSe/ZnS QD loaded polyglutamate/(PEI/PAA)3 capsules (a) without RBITC tagging PEI under UV excitation (335–380 nm); (b) with RBITC tagging PEI under UV excitation; (c) with RBITC tagging PEI under green excitation (530–550 nm); (d) the overlap of (b) and (c). (QD max emission: 575 nm.) | ||
 | ||
| Fig. 6 Fluorescence micrographs of CdSe/ZnS QD loaded polyglutamate/(PEI-RBITC/PAA)3 capsules (a) under green excitation (530–550 nm); (b) under UV excitation (335–380 nm). The channels were switched in a few seconds. (QD max emission: 575 nm.) | ||
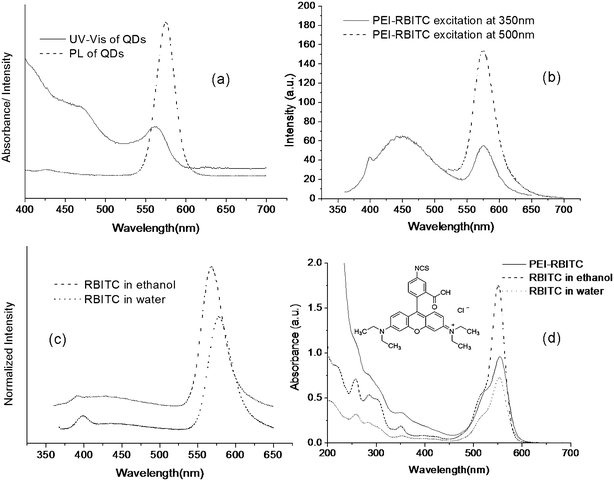 | ||
| Fig. 7 UV-vis and PL spectra of CdSe/ZnS QDs, RBITC and RBITC tagging PEI. *(a) UV-vis and PL spectra of CdSe/ZnS QDs with emission max at 575 nm; (b) PL spectra of PEI-RBITC excitation at 350 nm and 500 nm, respectively; (c) PL spectra of RBITC in different solvents excitation at 350 nm; (d) UV-vis spectra of RBITC in different solvents and of PEI-RBITC. The inset shows the molecular structure of RBITC. *For the PL spectra, the absorbance of all samples is lower than 0.1. | ||
As to RBITC, 0.1 mg ml−1 PEI-RBITC solution was excited at 350 nm (solid line) and 500 nm (dashed line) respectively (Fig. 7(b)). When exciting at 500 nm, there is only one emission peak at 576 nm. However, when exciting at 350 nm, there turns out another broad emission band with emission peak at 450 nm. The long-wavelength emission at 576 nm contributes to red fluorescence as we expected since it is widely being used. To confirm the existence of short-wavelength emission, the emission spectra of native RBITC in ethanol and water were measured at 350 nm excitation, respectively. It shows that both native RBITC in ethanol (dashed line) and in water (dot line) have broad, slight short-wavelength emissions (Fig. 7(c)), which turn to be obvious for the PEI-RBITC (Fig. 7(b)). It indicates that the short-wavelength emission is due to the intrinsic molecular structure of RBITC. For the lactone form of the amino derivatives of xanthene dyes, the fluorescence can be due not only to the xanthene chromophore, but also to an aminobenzoic fragment.28 The conjunction of an amino group of PEI with isothiocyanate group of benzoic fragment can decrease the energy level of benzoic fragment which contributes to short-wavelength emission. Consequently, more contributions of aminobenzoic fragments than the xanthane part in the photodissociation process28 result in the obviously increased fluorescence of short-wavelength emission and decreased fluorescence of long-wavelength emission (Fig. 7(b)) when comparing with native RBITC (Fig. 7(c)). The native RBITC and PEI-RBITC can be excited at 350 nm are also proved by their UV-vis absorption spectra (Fig. 7(d)). Thus it can conclude that PEI-RBITC has two evident emission bands. Under green excitation, the long-wavelength emission results in the red circle in Fig. 5(c) and Fig. 6(a). Under UV excitation, the overlapped emission spectra of long and short-wavelength lead to green fluorescence in Fig. 5(b) and Fig. 6(b).
In order to further confirm the above observation, another kind of CdSe/ZnS QDs with emission of 560 nm was incorporated into the capsules with PEI-RBITC shell. Fig. 8 is the true color fluorescence micrographs of freshly fabricated composite capsules. It shows that under green excitation, only red fluorescence of PEI-RBITC can be observed (Fig. 8(a)). However, when switching to UV excitation, there is no red fluorescence but only green and light brown colors as well as strong light which are attributed to the mixed fluorescence of QDs and PEI-RBITC. Green and light brown shells of capsules were observed instead of red shells (Fig. 8(b), arrows) which confirms our above analysis that the overlapped emission spectra of PEI-RBITC results in the green or light brown shell (Fig. 5(b), Fig. 6(b), Fig. 8(b)).
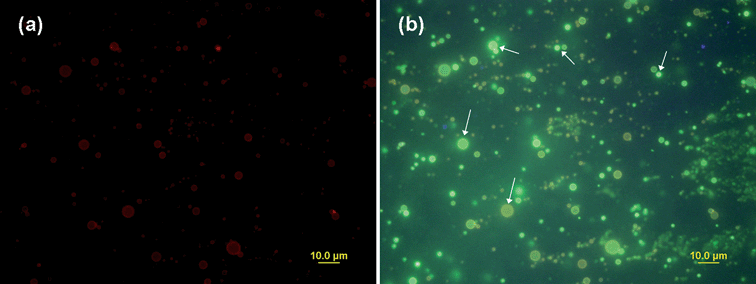 | ||
| Fig. 8 Fluorescence micrographs of CdSe/ZnS QD loaded polyglutamate/(PEI-RBITC/PAA)2 capsules (a) under green excitation (530–550 nm); (b) under UV excitation (335–380 nm). The channels were switched in a few seconds. (QD max emission: 560 nm.) | ||
Under green excitation, the fluorescence of QDs could not be observed in Fig. 5(c) and Fig. 8(a), which seems inconsistent with Fig. 6(a) in which the yellow QD signal was clearly observed. This is mainly due to green excitation for QDs is weak and the sizes of capsules are different. According to our visual observation, only very larger capsules have QD signals under green excitation since larger capsules with rich embedded QDs have stronger QD fluorescent signals. Under UV excitation, the fluorescent signals of QDs and PEI-RBITC were mixed together in Fig. 5(b) and Fig. 8(b). As a consequence, the yellow signals of QDs in Fig. 5(b) and the green signals of QDs in Fig. 8(b) were not as distinct as the yellow signals in Fig. 6(b). This could be ascribed not only to the size difference of capsules but also to other unspecific factors, such as the quality of different QDs and the amount of embedded QDs. Moreover, various brightness of capsules in Fig. 8(b) were partly due to they were on the different focal plane.
Confocal microscopy was also used to observe the same sample used in Fig. 8. 405 nm and 543 nm excitation channels were chosen for QDs and RBITC respectively. Fig. 9(a) is an overlapping of QD loaded capsules with PEI-RBITC shell. Weak green signals of QDs and red signals of PEI-RBITC were obtained by 405 nm and 543 nm excitation, respectively. The fluorescence profile (Fig. 9(b)) along the line indicated in the confocal image (Fig. 9(a)) clearly shows that QDs were successfully encapsulated into the PEI-RBITC shell. The green signals of QDs were weakly observed by confocal microscopy, which is not as strong as by normal fluorescence microscopy (Fig. 8(b)). This could be explained by the 405 nm excitation which was not stronger than UV excitation. The UV and PL spectra of QDs (560 nm emission) confirm this point (Fig. 9(c)).
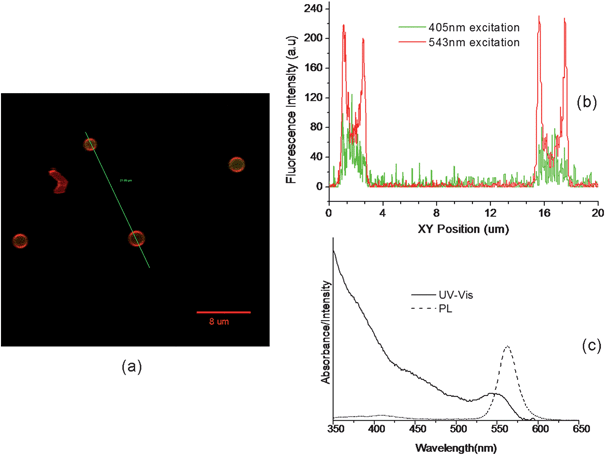 | ||
| Fig. 9 CdSe/ZnS QD loaded polyglutamate/(PEI-RBITC/PAA)2 capsules observed by confocal microscopy and the spectra of QDs used. (a) The overlapped confocal image of the capsules excited by 405 nm and 543 nm respectively. (b) Fluorescence profile along the line indicated in (a). (c) UV-vis and PL spectra of CdSe/ZnS QDs with emission maximum at 560 nm. | ||
Color transformation ascribed to the degradation of QDs resulting in spectral conversion was also observed after the capsules were stored under day light for days, starting from the original yellow emission and progressing to green, and blue, ultimately culminating in irreversible photobleaching. Fig. 10 is the true color fluorescence micrograph of CdSe/ZnS QD (emission 575 nm) loaded capsules stored under day light for 11 days. When using green excitation, only the red signals ascribed to PEI-RBITC could be observed (Fig. 10(a)). When switching to UV excitation, the red signals disappeared and blue fluorescence of QDs could be observed (Fig. 10(b)). The fact that the fluorescent signals of PEI-RBITC under UV excitation and QDs under green excitation could not be observed is probably due to their weak fluorescence intensities at both conditions. Fig. 10(c) is an overlap of Fig. 10(a) and Fig. 10(b), which clearly reveals that the hydrophobic QDs are inside the capsule while hydrophilic polyelectrolytes act as outer shell. It indicates that such QD loaded composite capsules could probably be utilized as probes in vivo cellular imaging due to the color change of embedded QDs if degradation can sustain a long time. For example, color change of QDs in cells can be tracked using real-time microscopy over extended observation periods to quantitate the kinetics of ligand binding, internalization, and processing. Besides, the color change of QDs can afford greater discrimination in protocols based on multiple probes (QDs and organic fluorophore) used simultaneously.
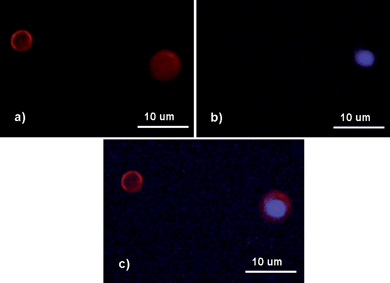 | ||
| Fig. 10 Fluorescence micrographs of CdSe/ZnS QD loaded polyglutamate/(PEI-RBITC/PAA)3 capsules stored under day light for 11 days. (a) Under green excitation (530–550 nm); (b) under UV excitation (335–380 nm); (c) the overlay of (a) and (b). (QD max emission: 575 nm.) | ||
Besides fluorescence observation, TEM and ESEM images also confirm that QD loaded capsules with polyelectrolytes as outer shells were successfully obtained. Similar to Fe3O4 nanocrystal loaded nanocapsules in Fig. 3, 40–200 nm, core-shell structures of CdSe/ZnS QD loaded nanocapsules stored for 7–8 days at 4 °C were also observed by TEM (Fig. 11(a)). The dark core can be assigned to CdSe/ZnS nanocrystals dispersed in hexane and the grey shell can be attributed to the polyglutamate/polyelectrolyte shell. The discrete spots of the crystalline electron microdiffraction pattern also confirm that CdSe/ZnS nanocrystals were encapsulated into the capsules (Fig. 11(b)). ESEM images (Fig. 11(c), 11(d)) show that about 100–800 nm nanocapsules were observed, which indicates the polydispersity of the obtained capsules. The well-dispersed QD loaded nanocapsules may be used as biological labeling carriers in multiplex detection schemes.26 By our simple nanocapsule fabrication approach, multicolor semiconductor QDs at multiple intensity levels may be embedded into the capsules by mixing different multicolor QD in organic solution at predetermined QD ratios for color multiplexing.
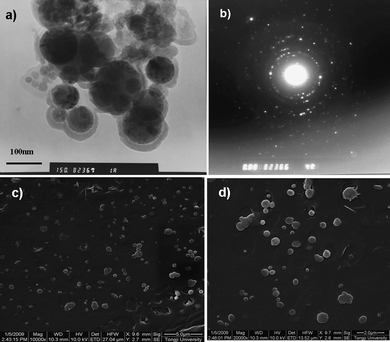 | ||
| Fig. 11 CdSe/ZnS QD loaded polyglutamate capsules with (PEI-RBITC/PAA)3 multilayers. (a) TEM image of polyglutamate/(PEI-RBITC/PAA)3 nanocapsules; (b) electron microdiffraction pattern of (a) measured by TEM; (c), (d) ESEM images of polyglutamate/(PEI-RBITC/PAA)3 nanocapsules at different magnification. The scale of (c) is 5.0 μm and the scale of (d) is 2.0 μm. (QD maximum emission: 575 nm.) | ||
The largest advantages of the method are that hydrophobic QDs do not need to be pre-modified to be hydrophilic,20 which often requires tedious steps and may cause a decrease of fluorescence efficiency;29 chemical modifications (e.g. to incorporate some biochemical functions, of proteins, receptors, or DNA) to target specific organs or tumor types can be introduced through the functional groups (COOH, NH2) of the polyelectrolyte shell. In addition, the dispersibility of hydrophobic QDs in organic solvent will avoid the aggregation of QDs and their fluorescence resonance energy transfer. The hydrophobic QD loaded nanocapsule with a hydrophilic multifunctional shell may have versatile biological applications. For example, the combinatorial labeling with QDs and traditional organic dyes may allow real-time observations of ligand-receptor interaction and of molecular trafficking in living cells30 if both the protein and polyelectrolyte shell are biocompatible; conjugation of fluorescent protein with embedded QDs via the outer polymer shell may be used as fluorescence resonance energy transfer probes.31
4. Conclusions
In this paper, magnetic Fe3O4 nanocrystals prepared by pyrolysis of fatty acid iron salts in non-aqueous media were firstly encapsulated into polyglutamate/polyelectrolyte capsules by combining an ultrasonic protocol and polyelectrolyte LBL assembly. Inspired by the similar synthesis mechanism of oxide and semiconductor nanocrystals in non-aqueous media, hydrophobic CdSe/ZnS nanocrystals were then successfully incorporated into composite capsules with almost unchanged optical properties. Such a hydrophobic QD loaded capsule with a hydrophilic multifunctional shell has important significance in biological applications. By this method, individually designed ligand molecules to render hydrophobic QDs water soluble are not needed. The hydrophobic QDs can be directly incorporated into the hydrophilic protein shell and the deposited polyelectrolyte layers can also be covalently coupled with functional biomolecules for target identification. In addition, using iron oxide and CdSe/ZnS nanocrystals as models, any other IV magnetic oxide and II–VI semiconductor nanocrystals based on organometallic approaches in non-aqueous media may be encapsulated into functional nanocapsules following the described procedure.Acknowledgements
This work was supported by the Shanghai Committee of Science and Technology, China (0852nm05400) and Scientific Research Foundation for Returned Scholars, Ministry of Education of China (20090315).References
- G. B. Sukhorukov, E. Donath, S. Davis, H. Lichtenfeld, F. Caruso, V. I. Popov and H. Möhwald, Polym. Adv. Technol., 1998, 9, 759 CrossRef CAS.
- L. Radtchenko, G. B. Sukhorukov, S. Leporatti, G. B. Khomutov, E. Donath and H. Möhwald, J. Colloid Interface Sci., 2000, 230, 272 CrossRef CAS.
- C. S. Peyratout and L. Dähne, Angew. Chem., Int. Ed., 2004, 43, 3762 CrossRef.
- E. Donath, G. B. Sukhorukov, F. Caruso, S. A. Davis and H. Möhwald, Angew. Chem., 1998, 110, 2323–2327 CrossRef; E. Donath, G. B. Sukhorukov, F. Caruso, S. A. Davis and H. Möhwald, Angew. Chem., Int. Ed., 1998, 37, 2201 CrossRef.
- E. Donath, S. Moya, B. Neu, G. B. Sukhorukov, R. Georgieva, A. Voigt, H. Baumler, H. Kiesewetter and H. Möhwald, Chem.–Eur. J., 2002, 8, 5481 CrossRef CAS.
- L. DPhne and B. Baude, Patent Application Az102004013637.8, 2004.
- C. SchQler and F. Caruso, Biomacromolecules, 2001, 2, 921 CrossRef CAS.
- O. Kreft, M. Prevot, H. Möhwald and G. B. Sukhorukov, Angew. Chem., Int. Ed., 2007, 46, 5605 CrossRef.
- X. Teng, D. G. Shchukin and H. Möhwald, Adv. Funct. Mater., 2007, 17, 1273 CrossRef CAS.
- K. S. Suslick and M. W. Grinstaff, J. Am. Chem. Soc., 1990, 112, 7807 CrossRef CAS.
- E. M. Dibbern, F. J. Toublan and K. S. Suslick, J. Am. Chem. Soc., 2006, 128, 6540 CrossRef CAS.
- S. Avivi, I. Felner, I. Novik and A. Gedanken, Biochim. Biophys. Acta, Gen. Subj., 2001, 1527, 123 Search PubMed.
- X. Teng, D. G. Shchukin and H. Möhwald, Langmuir, 2008, 24, 383 CrossRef CAS.
- O. Giraldo, S. L. Brock, W. S. Willis, M. Marquez, S. L. Suib and S. Ching, J. Am. Chem. Soc., 2000, 122, 9330 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS.
- F. Patolsky, Y. Weizmann, E. Katz and I. Willner, Angew. Chem., Int. Ed., 2003, 42, 2372 CrossRef CAS.
- N. R. Jana, Y. Chen and X. Peng, Chem. Mater., 2004, 16, 3931 CrossRef CAS.
- W. W. Yu, Y. A. Wang and X. Peng, Chem. Mater., 2003, 15, 4300 CrossRef CAS.
- M. Kim, Y. Chen, Y. Liu and X. Peng, Adv. Mater., 2005, 17, 1429 CrossRef CAS.
- W. C. W. Chan and S. Nie, Science, 1998, 281, 2016 CrossRef CAS.
- B. Richter and S. Kirstein, J. Chem. Phys., 1999, 111, 5191 CrossRef CAS.
- Y. S. Han, D. Radziuk, D. Shchukin and H. Möhwald, J. Mater. Chem., 2008, 18, 5162 RSC.
- US Pat. Appl., 20040258762 A1, 2004.
- C. B. Murray, D. J. Norris and M. G. Bawendi, J. Am. Chem. Soc., 1993, 115, 8706 CrossRef CAS.
- X. Peng, L. Manna, W. D. Yang, J. Wickham, E. Scher, A. Kadavanich and A. P. Alivisatos, Nature, 2000, 404, 59 CrossRef CAS.
- M. Han, X. Gao, J. Z. Su and S. Nie, Nat. Biotechnol., 2001, 19, 631 CrossRef CAS.
- W. C. W. Chan, D. J. Maxwell, X. Gao, R. E. Bailey, M. Han and S. Nie, Curr. Opin. Biotechnol., 2002, 13, 40 CrossRef CAS.
- N. V. Abrosimova and A. G. Stepanova, J. Appl. Spectrosc., 1986, 44, 39 CrossRef.
- Y. Liu, M. Kim, Y. Wang, Y. A. Wang and X. Peng, Langmuir, 2006, 22, 6341 CrossRef CAS.
- H. E. Grecco, K. A. Lidke, R. Heintzmann, D. S. Lidke, C. Spagnuolo, O. E. Martinez, E. A. Jares-Erijman and T. M. Jovin, Microsc. Res. Tech., 2004, 65, 169 CrossRef CAS.
- A. M. Dennis and G. Bao, Nano Lett., 2008, 8, 1439 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
