2D analogues of the inverted hexagonal phase self-assembled from 4,6-dialkoxylated isophthalic acids at solid–liquid interfaces†
Andrey S.
Klymchenko
*ab,
Shuhei
Furukawa‡
a,
Tanya
Balandina
a,
Klaus
Müllen
c,
Mark
Van der Auweraer
a and
Steven
De Feyter
*a
aDepartment of Chemistry, Laboratory of Photochemistry and Spectroscopy, and INPAC - Institute of Nanoscale Physics and Chemistry, Katholieke Universiteit Leuven, Celestijnenlaan 200-F, 3001, Leuven, Belgium. E-mail: Steven.DeFeyter@chem.kuleuven.be; Fax: +32 16 327990; Tel: +32 16 327921
bLaboratoire de Biophotonique et Pharmacologie, UMR 7213 CNRS, Université de Strasbourg, Faculté de Pharmacie, 74, Route du Rhin, 67401, ILLKIRCH Cedex, France. E-mail: aklymchenko@pharma.u-strasbg.fr; Tel: +33 368854255
cMax-Planck-Institut für Polymerforschung, Ackermannweg 10, D-55021, Mainz, Germany
First published on 13th July 2010
Abstract
Self-assembly of organic molecules at solid–liquid interfaces is a route for developing novel functional materials on surfaces and modeling assembly phenomena in 3D. 5-Alkoxylated isophthalic acids (ISA) are known to self-assemble into two-dimensional (2D) lamellae at the interface between a surface of Au(111) or HOPG (highly oriented pyrolytic graphite) and a solvent. Presently, the self-assembly of 4,6-dialkoxylated isophthalic acid derivatives with variable alkyl chain length is investigated at Au(111)–water, Au(111)–tetradecane and HOPG–tetradecane interfaces with a particular focus on the first one. The main aspect of this study is to evaluate the role of the molecular geometry and different interactions in the 2D assembly of amphiphilic molecules. In contrast to 5-alkoxylated ISA, 4,6-dialkoxylated ISA derivatives self-assemble preferentially into arrays of cyclic pentameric/hexameric structures, which appear as 2D analogues of the inverted hexagonal phase of lipids. As a general trend, the derivatives bearing shorter alkyl chains show a higher level of ordering at Au(111)–liquid interfaces. In particular, at the Au(111)–water interface, the 4,6-diheptyloxy ISA derivative forms exclusively pentamers, which are arranged in a quasi-hexagonal lattice. Moreover, the cyclic pentameric features are not empty but host a single isophthalic acid residue which is found to be dynamic. Finally, the packing of the diheptyloxy derivative shows a distinct potential dependence: while at more negative potentials the pentameric arrangement is converted into lamellae, at more positive potentials a loosely packed zig-zag pattern is formed. The present results show that at different solid–liquid interfaces 4,6-dialkoxylated ISA derivatives tend to form cyclic structures that are 2D analogues of an inverted hexagonal phase, akin to lipids having two hydrophobic alkyl chains and a small polar head group. Moreover, the substrate potential at the Au(111)–water interface can tune the 2D molecular arrangement.
Introduction
Assembly of molecules in a programmed manner is a highly attractive approach for the bottom-up manufacturing of nano-devices.1–3 In this respect, scanning tunneling microscopy (STM) is a very powerful tool to visualize molecular organization on atomically flat conductive surfaces, since it provides direct evidence of the assembly often with near atomic resolution.4 The solid–liquid interface is particularly suitable for studies of self-assembly by STM since it allows in situ imaging of the two-dimensional (2D) assembly from solution.5–7Molecules containing long alkyl chains are of particular interest for self-assembly studies.8 Alkyl chains favor the formation of highly ordered self-assembled structures in 3D, such as monolayer and bilayer membranes, nanotubes and liquid crystals, based on weak van der Waals interactions and/or the hydrophobic effect. Furthermore, molecules bearing long alkyl chains are suitable for self-assembly at solid–liquid interfaces too, especially but not exclusively on highly oriented pyrolytic graphite (HOPG).5–7 In this respect, isophthalic acid (ISA) derivatives bearing long alkyl chains and hydrogen bonding units are interesting building blocks for self-assembly into regular surface-constrained molecular patterns.9 The ISA residue allows for a number of different hydrogen bonding patterns, thus permitting the generation of different highly ordered nanostructures on surfaces.5,6 Most of the 5-alkoxy-isophthalic acid derivatives form lamellar structures at the HOPG–liquid interface. Within these lamellae the alkyl chains lie parallel to the graphite main axes, while the ISA residues form intermolecular H-bonds supporting the lamellar structure (Fig. 1). Only when the alkoxy chain is terminated by a bulky group, such as benzhydryl, do 5-alkoxy-ISA derivatives form cyclic hexameric structures, probably due to the large size of the aromatic residue interfering with the lamellar assembly.5,9 An attractive possibility to drive the assembly of ISA derivatives towards new non-lamellar structures on surfaces is to add a second alkyl chain, which in the case of 4,6-dialkoxy-ISA derivatives would change the molecular geometry from linear to triangular (Fig. 1). However, the only study of 4,6-dialkoxy-ISA molecules at the HOPG–1-octanol interface shows that they form a lamella-type structure, because one of the alkoxy chains is expelled from the graphite surface.9 As a result, the assembly of these molecules resembles to a large extent that of 5-alkoxy-ISAs. Thus, it is clear that to achieve control over the assembly of 4,6-dialkoxy-ISA molecules at the solid–liquid interface, one should modulate the solvent and substrate to optimize molecule–molecule, molecule–solvent and molecule–substrate interactions.
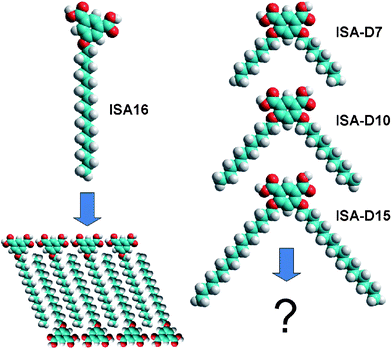 | ||
| Fig. 1 Left: Molecular model (Hyperchem) of 5-hexadecyloxy-isophthalic acid and its self-assembly pattern at the Au(111)–water interface under potential control. Right: Molecular models (Hyperchem) of the 4,6-dialkoxy-isophthalic acids under investigation. | ||
An alternative to HOPG is Au(111), which allows STM measurements with both organic and aqueous solutions, and shows high affinity to organic molecules.10,11 Particularly interesting in this respect is the Au(111)–water interface, because it should exhibit an increased affinity for poorly solvated alkyl chains. To observe assembly at the interface between a metallic solid and aqueous electrolyte, electrochemical STM (EC-STM) is frequently used.10,11 This technique allows control of the substrate potential, which is an attractive tool for modulating the molecule–substrate interaction and thus the self-assembly on surfaces.12–17 In our recent EC-STM studies, 5-alkoxylated ISA (ISA16, Fig. 1) has been shown to form a lamellar arrangement at the Au(111)–water interface.18–20 This arrangement was remarkably different from that at the HOPG–organic solvent interface: the alkyl chains did not follow the Au(111) main symmetry axes, while the ISA residues followed the next nearest neighbor (NNN) axes of the Au surface. Moreover, H-bonding and hydrophobic interactions play an important role in the assembly since individual lamellae could be observed at certain substrate potentials and in the presence of guest molecules.18,19
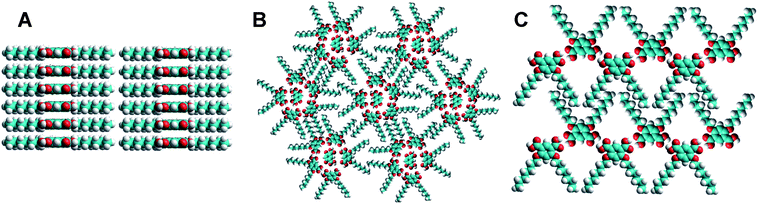
In the present work, we studied the self-assembly of 4,6-dialkoxy-ISA molecules of different chain lengths at the Au(111)–water interface at different substrate potentials. Variation of the length of alkyl chains was important to understand their role in the assembly. Moreover, the presence of the second alkyl chain as compared to 5-alkoxy-ISA should dramatically change the molecular geometry and thus, the self-assembly at the interface. We have also tested other interfaces, namely Au(111)–tetradecane and HOPG–tetradecane, and compared the results with already published data at the HOPG–1-octanol interface. According to the STM data, the 4,6-dialkoxy-ISA molecules form ordered nanostructures having both of their alkyl chains adsorbed to the surface at the Au(111)–water interface. Remarkably, close to the potential of zero charge (PZC), cyclic (mainly pentameric) structures were observed; the core of the cycles is formed by polar ISA residues, while the periphery is occupied by the alkyl chains. Similar structures were observed at the Au(111)–tetradecane interface and for some 4,6-dialkoxy-ISA derivatives at the HOPG–tetradecane interface. This preferential cyclic arrangement is attributed to the role played by the H-bonding interactions within the cycles as well as by the triangular shape of the molecules due to presence of two alkyl groups (Fig. 1).
Experimental section
4,6-Bis-heptyloxy-isophthalic acid (ISA-D7), 4,6-bis-decyloxy-isophthalic acid (ISA-D10) and 4,6-bis-pentadecyloxy-isophthalic acid (ISA-D15) were synthesized as described elsewhere.9EC-STM experiments have been performed using a home-built STM setup designed and constructed in the group of Prof. Klaus Wandelt (University of Bonn). The instrumental design allows us to perform cyclic voltammetry (CV) and STM measurements simultaneously.21 A platinum wire or a reversible hydrogen electrode were used as the reference electrode. All potentials are rescaled to the saturated calomel electrode (SCE). Taking into account the limited stability of Pt wire as reference electrode, the error of the substrate potential was estimated to be ±30 mV. Prior to each STM experiment, a Au(111) single crystal (MaTeck company, Julich, Germany) was electrochemically etched and flame annealed. For this purpose, the Au(111) crystal was immersed into 0.1 M sulfuric acid and an anodic potential of 10 V was applied between the crystal and a platinum foil for about 30 s. Then, after rinsing with Milli-Q water (Milli-Q purification system >18 MΩ cm) the sample was immersed into 0.1 M hydrochloric acid (ultra pure grade from Merck) in order to reduce the Au(111) surface. Again, the Au substrate was rinsed with Milli-Q water and flame annealed for another 2 min. To deposit ISA derivatives on the Au(111) substrate, a drop of an ethanol solution of the compound (1 mg ml−1) was placed on the Au(111) surface. After solvent evaporation the surface was thoroughly rinsed with ethanol and dried. The gold crystal with the ISA derivative deposit was then mounted into the EC-liquid cell and filled with the supporting electrolyte. The EC-STM measurements were performed in 0.1 M perchloric acid (HClO4 ultra pure grade from Merck) as supporting electrolyte, which was deoxygenated with argon gas one hour before use. EC-STM imaging was started after completion of the in situ CV test cycle (see ESI†). Therefore, the assemblies observed in this study were newly formed after the CV cycle. The STM tips were electrochemically etched from a 0.25 mm tungsten wire in 2 M KOH solution and subsequently isolated by passing the tip through a drop of hot-glue. Normally, this procedure isolates the entire tip except its apex. Conventional STM measurements were obtained by using a PicoSPM (Agilent) with a tip mechanically cut from a Pt/Ir wire (80/20, diameter 0.25 mm).
Results and discussion
Initially, we have studied ISA-D10 bearing alkyl chains of intermediate length. At the substrate potential of 350 mV vs. SCE, which is close to the PZC for Au(111) in perchloric acid,22,23 STM images show a quasi-ordered molecular organization (Fig. 2). The bright spots, corresponding to the aromatic ISA residues, form predominantly pentameric and hexameric cycles, while the alkyl chain groups are oriented away from these cycles. These structures appear like small 2D analogues of reverse micelles, where the core is formed by the polar (ISA) groups and the periphery is occupied by the apolar alkyl chains. These pentamers and hexamers sometimes organize into a hexagonal lattice, while in other cases the patterns are more disordered. Thus, from these observations, we can conclude that the present architectures at least in part do not respect the underlying substrate, so that the formed complexes are strongly controlled by the intermolecular interactions at the interface. Some dynamics are observed in the center of these complexes, which are usually occupied by a bright spot. According to our time-lapsed experiments, the exchange of the bright spots between the star-shaped structures occurs on a time scale of minutes (see video S1 in ESI†). However, neither the occupancy of these cyclic structures nor the dynamics of the exchange were dependent on the substrate potential (not shown). This indicates that the bright “guest” spots cannot be ions from solution. Finally, these bright spots displayed a rather similar contrast to the bright spots attributed to ISA residues. All these results suggest that these spots are also ISA residues of the ISA-D10 molecules which are not strongly adsorbed on the surface and therefore hop from one vacancy of the lattice to another. Remarkably, while pentamers systematically contain a single “guest” ISA residue, the hexamers contain exclusively two ISA residues. This phenomenon is clearly connected with the size of the void space of the cyclic structure. Indeed, the void space in pentamers matches perfectly a single ISA residue, while the void in a distorted hexamer fits two ISA residues (Fig. 3). The packing of the alkyl chains could also be resolved in the STM images. They usually appear as 3 to 4 parallel elongated structures that connect two neighboring cyclic structures (Fig. 2C). Remarkably, because these structures are not well organized, the alkyl chains also frequently do not follow a particular direction, confirming that the assembly does not respect the underlying substrate.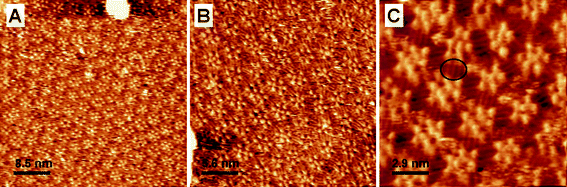 | ||
| Fig. 2 Representative STM images of ISA-D10 at the Au(111)–water (0.1 M HClO4) interface. Substrate potential, Ew = 350 mV vs. SCE; tunneling current, It = 1 nA; bias voltage, Ubias = −350 mV. The ellipse in C highlights the alkyl chains. | ||
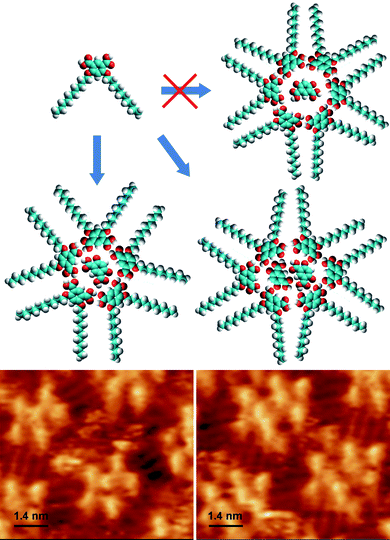 | ||
| Fig. 3 Top: Tentative molecular models of the supramolecular motifs formed by ISA-D10, in accordance with the STM data shown at the bottom; left: cyclic pentamer hosting one isophthalic acid molecule; right: cyclic hexamers hosting two isophthalic acid molecules. | ||
A similar pattern is observed for ISA-D10 at the Au(111)–tetradecane interface, while at the HOPG–tetradecane interface pentameric cyclic structures are mainly observed (see ESI, Fig. S2†). Thus, ISA-D10 reveals a tendency to form cyclic structures at different interfaces, in contrast to ISA16, which shows exclusively lamellar arrangements. The presence of the second chain probably breaks the lamellar arrangement of the ISA derivative. We explain this by the larger space taken by the hydrophobic residues in this derivative. Indeed in ISA16 lamellae the distance between two ISA residues corresponds to two times the distance between adjacent alkyl chains, i.e. two alkyl chains per ISA residue are allowed within the lamella (ca. 1.0 nm, see Fig. 1). However, a similar interdigitated arrangement for ISA-D10 would result in four alkyl chains per ISA residue within the lamella, which is impossible for steric reasons. Moreover, in ISA-D10, the alkyl chains are attached to the ISA residue in the meta-positions, which forces alkyl chains to attain a non-parallel orientation with respect to each other (Fig. 1). The triangular shape of ISA-D10 makes the lamellar arrangement sterically impossible but may favor the formation of cyclic structures (Fig. 3) as observed in the STM images (Fig. 2). Thus, ISA-D10 behaves similarly to detergents and lipids with two hydrophobic chains that tend to self-assemble into reverse micelles or an inverted hexagonal phase.24,25 The latter phases are usually formed due to the special geometrical shape of the detergent/lipid molecules, characterized by large hydrophobic (normally two) chains and a small polar group.24,25 Finally, we should mention the possibility of the hydrogen bonding between the carboxylic acid groups of the ISA residues, which may stabilize their cyclic arrangement.9
These results stimulated us to tune the molecular geometry of 4,6-dialkoxy-ISA by varying the length of its alkyl chains. In addition, the alkyl chain length could affect the dynamics of the molecules on the surface which may also control the assembly process. ISA-D15 forms more disordered patterns at the Au(111)–water interface, with some tendency to arrange into short lamellae with parallel interdigitated alkyl chains (Fig. 4A). ISA residues, which appear as bright spots, are commonly organized in tetramers. The alkyl chains of each molecule of the tetramer are oriented almost perpendicular to each other, being interdigitated with those of the neighboring tetrameter (or oligomer). At the Au(111)–tetradecane interface a very similar arrangement is observed (Fig. 4B). In contrast, at the HOPG–tetradecane interface ISA-D15 molecules self-assemble into either ordered pentamers (Fig. 4C) or lamellae (Fig. 4D). In the latter case, the alkyl chains arrange parallel to the HOPG main axes. The major difference between the ISA-D15 arrangement and that of ISA-D10 is the leading role of the long alkyl chains in the assembly that tend to form short lamellae of interdigitated alkyl chains. However, since the lamellar arrangement of ISA-D15 molecules at the Au(111)–liquid interface is geometrically unfavorable, they tend to form rectangular (tetramer, hexamer, octamer, etc.) structures, where alkyl chains are able to interdigitate with those of the neighboring structures. In addition, due to the very long alkyl chains, ISA-D15 molecules may interact “too” strongly with the Au(111) surface, which could freeze their dynamics on the surface, so that they cannot reach the most thermodynamically stable arrangement. At the HOPG–tetradecane interface, the dynamics of ISA-D15 is probably sufficiently fast to achieve highly organized arrangements: pentamers and lamellae based. Moreover, the latter arrangement is possible because as it appears from our model, only three out of four alkyl chains are adsorbed to the surface, in line with previous studies at the HOPG–1-octanol interface.9
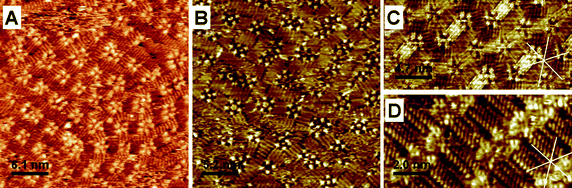 | ||
| Fig. 4 Representative STM images of ISA-D15 at the Au(111)–water (0.1 M HClO4) (A), Au(111)–tetradecane (B) and HOPG–tetradecane (C and D) interfaces. (A) Ew = 350 mV vs. SCE; It = 1 nA; Ubias = −350 mV; (B) It = 0.225 nA, Ubias = −128 mV; (C and D) It = 135 pA, Ubias = −800 mV. The full scale images are presented in ESI.† | ||
In the next step we studied ISA-D7, which bears much shorter alkyl chains. In contrast to ISA-D10 and ISA-D15, ISA-D7 forms a well organized and uniform arrangement at the Au(111)–water interface (substrate potential close to the PZC, 350 mV vs. SCE). It can be seen in the STM images that ISA-D7 molecules form pentameric cyclic structures, which appear similar to those observed for ISA-D10 (Fig. 5). However, unlike ISA-D10, the ISA-D7 molecules assemble exclusively into pentameric cycles, without any hexamers. Furthermore, the pentameric cycles are arranged in an apparent hexagonal lattice (Fig. 5A). The distance between the pentamers is 2.7–3.0 nm. This hexagonal arrangement is somewhat surprising taking into account the fact that the hexagonal arrangement does not fit to the symmetry of the supramolecular pentamer. However, the high resolution images reveal that the individual pentamers form rows, where the pentamers are of the same orientation (Fig. 5). The neighboring rows are antiparallel, which probably provides more dense packing of the pentamers. Between the two parallel edges of adjacent pentamers the alkyl chains are well resolved, while no alkyl chains could be resolved between the pentamers in the same row. Remarkably, the distance between the two parallel edges of the adjacent pentamers, d1 = 1.3 ± 0.1 nm (Fig. 5B), corresponds to the estimated length of ISA-D7 alkyl chains (0.9 nm), which suggests that the alkyl chains of ISA-D7 from the neighboring pentamers are interdigitated (Fig. 5B). The distance between the interdigitated chains is 0.4–0.5 nm, which corresponds well to the expected tight packing of alkyl chains. Thus, the packing of the supramolecular pentamers is quasi-hexagonal. Indeed, while the pentamers are arranged in a hexameric super-lattice, only one of the three main rows in this lattice presents pentamers of the same orientation (hence the real symmetry of the packing is p2, and the unit cell parameters are: a = 5.0 ± 0.1 nm, b = 2.8 ± 0.1 nm, α = 92 ± 5°, see Fig. 5A).
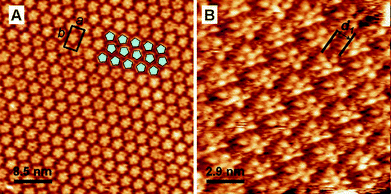 | ||
| Fig. 5 Representative STM images of ISA-D7 at the Au(111)–water (0.1 M HClO4) interface. Ew = 350 mV vs. SCE; It = 1 nA; Ubias = −300 mV. Image B is slightly distorted due to drift during the measurement. The ellipse in B highlights the alkyl chains. | ||
Similarly to ISA-D10, ISA-D7 shows a bright spot in the middle of the pentamers, which also seems to be a dynamic entity (see video S2 in ESI†). For instance, comparison of two consecutive STM images illustrates the hopping of bright spots between neighboring pentamers at four places simultaneously within the time required for recording these two images (ca. 1 min, see Fig. 6). This finding clearly indicates that these dynamics are not connected with a random adsorption–desorption of some species from solution, but with the migration of a molecule from one vacancy to the neighboring one. It confirms our conclusion that these bright spots in the center of the pentamers are ISA residues, which are weakly adsorbed within the 2D supramolecular lattice. However, because we have never observed domains of pentagons where the bright spot in the middle is absent, we can conclude that these weakly adsorbed ISA-D7 guests are essential to maintain the cyclic structures. Thus, the molecular arrangement of ISA-D10 and ISA-D7 in the pentameric structures is probably very similar (Fig. 3 and 7). The models highlight the fact that the cyclic structures can also be stabilized by intermolecular hydrogen bonding between carboxylic acid groups of the adjacent ISA residues. It has been previously documented that these types of H-bonding play an important role in controlling the 2D assembly of ISA derivatives.9,18 Remarkably, at the Au(111)–tetradecane interface, we also observed a mainly pentameric arrangement of ISA molecules (see ESI†). Moreover, most of the pentamers were filled with a bright spot in the middle, thus confirming that it is an ISA residue. Thus, as expected, at the Au(111)–liquid interface, ISA-D7, presenting sufficient level of dynamics, develops highly organized self-assembled structures, corresponding to ordered pentamers. This implies that pentameric organization is probably energetically more favorable than the hexameric one.
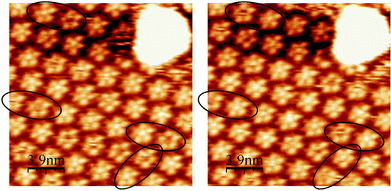 | ||
| Fig. 6 Two consecutive STM images with a 1 min delay showing the mobility of the bright spots in the middle of pentamers (highlighted by ellipses). Ew = 350 mV vs. SCE; It = 1 nA; Ubias = −300 mV. See also video S2 in ESI.† | ||
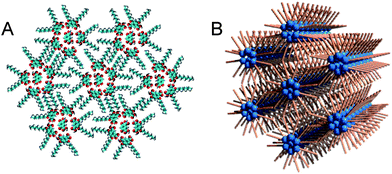 | ||
| Fig. 7 Proposed 2D assembly of ISA-D7 at the Au(111)–water interface based on the STM data (A) and its comparison with a well-established 3D model of the inverted hexagonal phase, observed for some lipids in aqueous solutions (B).24–27 | ||
The question arises though why for ISA-D7 and its homologues at the different interfaces the unusual pentameric cycles are more favorable than the anticipated hexameric cycles. We suggest that in the hexameric cycles, the void space in the middle would be too large to host one ISA residue, while the central void in the pentameric cycles fits ideally to the size of the ISA residue (Fig. 3). In fact, in the case of ISA-D10, hexameric structures are also observed, but they are strongly distorted by the two guest ISA residues (Fig. 2 and 3). We expect that the longer alkyl chains of ISA-D10 make the 2D assembly process less dynamic due to the stronger molecule–substrate interaction, and thus the most stable ordering may not be reached within the timescale of the experiment.
Finally, the cyclic structures tend to form a hexagonal lattice, which can be connected both with the hexagonal symmetry of the underlying Au(111) and HOPG surfaces and/or because these lattices allow the tightest packing of these structures. Thus, the cyclic assemblies observed for ISA-D15, ISA-D10 and ISA-D7 at different interfaces appear as 2D analogues of the inverted hexagonal phase of lipids.24–27 In both cases, the cyclic structures are organized in a hexagonal lattice, where the core of the cycles is formed by polar headgroups, while the periphery is occupied by the apolar alkyl chains. Moreover, both our 2D assemblies (Fig. 7A) and the inverted hexagonal phase (Fig. 7B) are formed by molecules with a triangular (or conical in 3D) shape having a small polar head group and a large hydrophobic part.24,25
It is also clear that, in the case of the Au(111)–water interface at a substrate potential close to the PZC, as well as for the Au(111)–tetradecane interface, ISA-D7 molecules form much more uniform and ordered patterns than ISA-D10 and ISA-D15, while the most disordered organization is observed for ISA-D15. Thus, the shorter chains of ISA-D7 favor the formation of more ordered structures. This chain length dependent phenomenon may have two causes: (1) faster dynamics of ISA-D7 molecules at the interface facilitates the prompt formation of the thermodynamically most stable structure, featuring the highest symmetry and (2) shorter chains probably allow ISA residues to organize into cyclic pentameric structures, while the longer alkyl chains force the molecules to form lamellae-like structures.
To further explore the assembly of the ISA derivatives at the Au(111)–water interface, we have studied the effect of substrate potential. In the case of ISA-D15 and ISA-D10, a change in the potential does not affect the molecular packing or simply destroys it. Indeed, both molecules reveal an unchanged assembly in the range of potentials 200–500 mV (vs. SCE). Above this potential we observed disordered aggregates (data not shown). Importantly, the potential-driven assembly–disassembly process is fully reversible. ISA-D7 exhibits remarkable and also fully reversible changes in 2D assembly as a function of substrate potential (Fig. 8). At potentials more negative than the potential of zero charge, where the gold reconstruction is expected, we observed the formation of lamellar structures (Fig. 8A). The distance between the lamellae corresponds to the “open” orientation of the alkyl chains. Indeed, the distance between the lamellae is 1.8–2.0 nm, which corresponds well with the estimated distance between the two ends of the alkyl chains in the “open” form of ISA-D7 (2.2 nm) (see also model in Fig. 9A). In this case the ISA residue probably “stands up” from the surface, hence its carboxylic acid moieties point away from the surface. Changes in the orientation of aromatic groups at electrified surfaces at certain substrate potentials have already been reported for other molecules.13,28 In our case, the negative substrate potential could stabilize this ISA orientation, where the positive pole of the ISA dipole is localized at the oxygens of the 4- and 6-alkoxy groups, while the negative pole is at the carboxylic groups. Moreover, the formation of the lamellar assembly at negative potentials could be connected with the gold reconstruction, which is known to favor the formation of the lamellae, due to higher commensurability of the reconstructed gold substrate with the alkyl chains.18,29,30 However, because we did not evaluate the effect of an organic adlayer on the onset potential of the Au(111) reconstruction, it is hard to estimate whether this reconstruction was completed under our conditions. In contrast, at more positive potentials with respect to the PZC, we observed a zig-zag organization of bright spots (Fig. 8C). The obtained pattern is very similar to that observed for ISA-D7 at the Au(111)–tetradecane interface (see ESI Fig. S1†). However in the latter case, this assembly was less frequently observed compared to the pentameric one. The nearest neighbor distance between the bright spots is 1.00 ± 0.05 nm, while the next nearest neighbor distance measures 1.50 ± 0.05 nm. The angle formed by the bright spots within the zig-zag is about 95 ± 8°. The nearest neighbor distance corresponds well to the distance between two H-bonded ISA residues. In contrast, the ISA residues located at 1.5 nm distance are not H-bonded. Moreover, the distance between the zig-zags d2 = 1.7 ± 0.1 nm, while the width of the zig-zag d3 = 0.70 ± 0.05 nm (Fig. 8C). It is impossible to resolve alkyl chains in this assembly, indicating that they are probably not well packed and not well immobilized on the surface. According to our model (Fig. 9C), the zig-zag pattern of bright spots is formed by ISA residues connected by double H-bonds. However, if we assume that the center of the bright spots corresponds to the center of the aromatic ring of the ISA residue then the expected angle formed by the bright spots within a zig-zag should be 120°. Since we observe a somewhat smaller angle (95 ± 8°), we conclude that the center of the bright spots in the STM images is probably shifted to the 5-carbon atom of the ISA residue. This shift is possibly connected with the larger electronic density that one expects at the 5-position compared to the 2-position of 4,6-dialkoxy-substituted ISA. The neighboring zig-zag rows pack close to each other, probably because both alkyl chains in an ISA-D7 molecule are not parallel. In this case, the alkyl chains do not show any particular direction on the surface, but interact by hydrophobic and van der Waals interactions. This packing is less dense than the pentamer-based packing. According to our calculations, the area per molecule in the zig-zag arrangement is 1.30 ± 0.05 nm2, while for the pentamer-based assembly it is 1.10 ± 0.05 nm2. The less dense packing at more positive potentials could be induced by the adsorption of anions filling the non-occupied area of the substrate. It has already been established that at substrate potentials above and below the PZC, the interactions of ions and dipoles with the surface dominate and thus remove hydrophobic residues from the surface.31–33 In the present case, more positive potentials slightly decrease the packing density of ISA-D7 molecules, thus favoring a zig-zag arrangement. It should be noted, that at even more positive (> 550 mV) or negative (< 200 mV) substrate potentials the ordered organization of ISA-D7 molecules on the surface is lost, probably because the interactions of ions and dipoles of the electrolyte with the surface are too strong. Thus, the assembly of ISA-D7 molecules at the Au(111)–water interface can be switched from pentameric to lamellar and zig-zag type by changing the substrate potential from the PZC towards negative and positive potentials, respectively (Fig. 8 and 9). It should be noted that the ISA derivatives are not charged, so their interaction with the substrate should not depend strongly on its potential. Moreover, the ordered structures are observed on Au(111) at potentials close to the PZC as well as on non-electrified interfaces. Therefore, the observed effects of substrate potential on the 2D assembly of the alkylated ISA derivatives at the Au(111) surface are probably connected with the influence of this potential on the interaction of perchlorate ions and water dipoles with this surface. This competition mechanism is different from that reported for chloride34 and iodide ions,10 which adsorb strongly on metal surfaces and serve as a 2D template for the subsequent assembly of organic molecules.
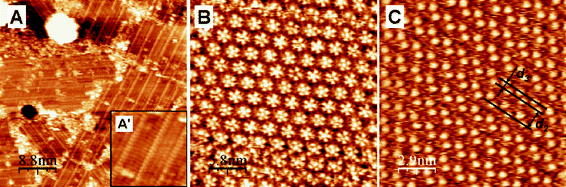 | ||
| Fig. 8 STM images of the 2D lattice of ISA-D7 at the Au(111)–water (0.1 M HClO4) interface at different substrate potentials (Ew): 200 mV (A), 350 mV (B) and 550 mV (C) vs. SCE. It = 1 nA; Ubias was −150 mV (A), −300 mV (B) and −500 mV (C). Image A′ is a magnified part of image A. | ||
 | ||
| Fig. 9 Proposed models for the assembly of ISA-D7 at the Au(111)–water interface at negative (A), around zero (B) and positive (C) potentials, with respect to the PZC. | ||
Conclusions
In conclusion, the self-assembly of three dialkoxylated ISA derivatives has been studied at the Au(111)–water interface using electrochemical STM as well as at Au(111)–tetradecane and HOPG–tetradecane interfaces using conventional STM at a liquid–solid interface. The formation of 2D structures analogous to the inverted hexagonal phase of lipids has been found at different interfaces. Indeed, the molecules form (pentameric and hexameric) cyclic structures where the core of the cycle is formed from the polar ISA residues, while the periphery is occupied by the hydrophobic alkyl chains. The best order at Au(111)–liquid interfaces has been found for the derivative with the shorter (heptyl) alkyl chains, presenting predominantly pentameric cyclic structures. Remarkably, the latter derivative shows at the Au(111)–water interface a strong and reversible variation of its packing as a function of substrate potential. In contrast to the pentamer-based packing at the PZC, at potentials negative to the PZC assembly a lamellar pattern is formed, while at more positive potentials a less dense zig-zag packing dominates. Thus, in contrast to mono 5-alkoxy-ISA derivatives exhibiting exclusively a lamellar assembly, dialkoxylated-ISA derivatives, due to their triangular shape, preferentially form cyclic assemblies at different interfaces, resembling the inverted hexagonal phase formed by detergents and lipids with small polar head groups and large hydrophobic chains.Acknowledgements
This work has been funded by the Fund of Scientific Research – Flanders (FWO), K.U. Leuven (GOA) and the Belgian Federal Science Policy Office through IAP-6/27.References
- D. Philp and J. F. Stoddart, Angew. Chem., Int. Ed. Engl., 1996, 35, 1154–1196 CrossRef.
- F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491–1546 CrossRef CAS.
- R. Madueno, M. T. Räisänen, C. Silien and M. Buck, Nature, 2008, 454, 618–621 CrossRef CAS.
- G. Binnig, H. Rohrer, Ch. Gerber and E. Weibel, Phys. Rev. Lett., 1982, 49, 57–61 CrossRef CAS.
- S. De Feyter and F. C. De Schryver, Chem. Soc. Rev., 2003, 32, 139–150 RSC.
- S. De Feyter and F. C. De Schryver, J. Phys. Chem. B, 2005, 109, 4290–4302 CrossRef.
- L.-J. Wan, Acc. Chem. Res., 2006, 39, 334–342 CrossRef CAS.
- P. Wu, Q. D. Zeng, S. D. Xu, C. Wang, S. X. Yin and C.-L. Bai, ChemPhysChem, 2001, 2, 750–754 CrossRef CAS.
- S. De Feyter, A. Gesquière, M. Klapper, K. Müllen and F. C. De Schryver, Nano Lett., 2003, 3, 1485–1488 CrossRef CAS.
- K. Itaya, Prog. Surf. Sci., 1998, 58, 121–247 CrossRef CAS.
- D. M. Kolb, Surf. Sci., 2002, 500, 722–740 CrossRef CAS.
- C. Safarowsky, L. Merz, A. Rang, P. Broekmann, B. A. Hermann and C. A. Schalley, Angew. Chem., Int. Ed., 2004, 43, 1291–1294 CrossRef CAS.
- T. Dretschkow, D. Lampner and T. Wandlowski, J. Electroanal. Chem., 1998, 458, 121–138 CrossRef CAS.
- Y. He, T. Ye and E. Borguet, J. Am. Chem. Soc., 2002, 124, 11964–11970 CrossRef CAS.
- G. Pan, L. Wan, Q. Zheng and C.-L. Bai, Chem. Phys. Lett., 2003, 367, 711–716 CrossRef CAS.
- N. Tao, C. Li and H. He, J. Electroanal. Chem., 2000, 492, 81–93 CrossRef CAS.
- S. Yoshimoto, Bull. Chem. Soc. Jpn., 2006, 79, 1167–1190 CrossRef CAS.
- A. S. Klymchenko, S. Furukawa, K. Müllen, M. Van der Auweraer and S. De Feyter, Nano Lett., 2007, 7, 791–795 CrossRef CAS.
- A. S. Klymchenko, S. Furukawa, M. Van der Auweraer, K. Müllen and S. De Feyter, Nano Lett., 2008, 8, 1163–1168 CrossRef CAS.
- N. T. M. Hai, M. Van der Auweraer, K. Müllen and S. De Feyter, J. Phys. Chem. C, 2009, 113, 11567–11574 CrossRef CAS.
- M. Wilms, M. Kruft, G. Bermes and K. Wandelt, Rev. Sci. Instrum., 1999, 70, 3641–3650 CrossRef CAS.
- A. F. Silva and A. Martins, in Interfacial Electrochemistry, Theory, Experiment and Applications, ed. A. Wieckowski, Marcel Dekker, New York, 1999, pp. 449–462 Search PubMed.
- B. Han, Z. Li, S. Pronkin and T. Wandlowski, Can. J. Chem., 2004, 82, 1481–1494 CrossRef CAS.
- J. N. Israelachvili, Intermolecular and Surface Forces, Academic Press, New York, 1985 Search PubMed.
- J. N. Israelachvili, D. J. Mitchell and B. W. Ninham, J. Chem. Soc., Faraday Trans. 2, 1976, 72, 1525–1568 RSC.
- J. M. Seddon, Biochim. Biophys. Acta, 1990, 1031, 1–69 CAS.
- I. Koltover, T. Salditt, J. O. Radler and C. R. Safinya, Science, 1998, 281, 78–81 CrossRef CAS.
- S. K. Shaw, A. Lagutchev, D. D. Dlott and A. A. Gewirth, J. Phys. Chem. C, 2009, 113, 2417–2424 CrossRef CAS.
- H.-M. Zhang, Z.-X. Xie, B.-W. Mao and X. Xu, Chem.–Eur. J., 2004, 10, 1415–1422 CrossRef CAS.
- Y. F. He, T. Ye and E. Borguet, J. Phys. Chem. B, 2002, 106, 11264–11271 CrossRef CAS.
- N. Ivosevic, V. Zutic and J. Tomaic, Langmuir, 1999, 15, 7063–7068 CrossRef CAS.
- J. Lipkowski and L. Stolberg, in Adsorption of Molecules at Metal Electrodes, ed. J. Lipkowski and P. N. Ross, VCH, New York, 1992, p. 171 Search PubMed.
- D. Bizzotto and J. Lipkowski, Prog. Surf. Sci., 1995, 50, 237–246 CrossRef CAS.
- D. T. Pham, K. Wandelt and P. Broekmann, ChemPhysChem, 2007, 8, 2318–2320 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Video S1: Ordering of ISA-D10 at the Au(111)–water (0.1 M HClO4) interface as a function of time. Video S2: Ordering of ISA-D7 at the Au(111)–water (0.1 M HClO4) interface as a function of time. STM images of the self-assembly at the Au(111)–tetradecane interface. STM images of the self-assembly at the graphite–tetradecane interface. Cyclic voltammetry on the Au(111) substrate in HClO4. See DOI: 10.1039/c0nr00176g |
| ‡ Present address: ERATO Kitagawa Integrated Pores Project, Japan Science and Technology Agency (JST), Kyoto Research Park Bldg#3, Shimogyo-ku, Kyoto 600-8815, Japan. |
| This journal is © The Royal Society of Chemistry 2010 |
