Effect of doping on the morphology and multiferroic properties of BiFeO3 nanorods
Dimple P.
Dutta
*a,
O. D.
Jayakumar
a,
A. K.
Tyagi
*a,
K. G.
Girija
a,
C. G. S.
Pillai
a and
G.
Sharma
b
aChemistry Division, Bhabha Atomic Research Centre, Mumbai, 400085, India. E-mail: dimpled@barc.gov.in; aktyagi@barc.gov.in
bMechanical Metallurgy Section, Bhabha Atomic Research Centre, Mumbai, 400085, India
First published on 25th May 2010
Abstract
In this study we report the synthesis of BiFeO3 nanorods using a sonochemical technique. The nanorods had a diameter of 20–50 nm, a length of 100–500 nm and exhibit aspect ratios in the range of 5–10. However, after doping, the TEM images of Bi0.9Ba0.1Fe0.9Mn0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 samples show that the aspect ratios of both the double doped samples have reduced considerably, while retaining the crystallinity of the particles. BiFeO3 nanorods show a weak ferromagnetic order at room temperature, which is quite different from the linear M–H relationship reported for bulk BiFeO3. The saturation magnetization of these BiFeO3 nanostructures has been found to increase on doping with various metal ions (Ba2+, Ca2+, Mn2+, Cr3+), reaching a maximum value of 1.35 emu g−1 for the Bi0.9Ba0.1Fe0.9Mn0.1O3 nanostructures. However, saturation of electric polarization was observed only in case of the Bi0.9Ca0.1Fe0.9Cr0.1O3 nanostructures.
Introduction
Multiferroic materials yield simultaneous effects of ferroelectricity, ferromagnetism or ferroelasticity in the same material due to their unique and strong coupling of electric, magnetic, and structural order parameters.1,2 Hence, they offer a wide opportunity for potential applications in information storage, magnetic recording media, spintronic devices and sensors.3–5 Among all the multiferroic materials studied so far, BiFeO3 (BFO) which exhibits the coexistence of ferroelectric and antiferromagnetic (AFM) orders has received great attention as it has the potential to be one of the prime candidates for room-temperature magneto electric applications due to its high ferroelectric Curie point (TC ∼ 1103 K) and the antiferromagnetic (AFM) Néel point (TN ∼ 647 K). However, both its spontaneous polarization and saturation magnetization are disappointingly low when compared to many standard ferroelectrics and ferromagnets. This is due to the superimposition of a spiral spin structure on BFO's antiferromagnetic order. In this spiral spin structure, the antiferromagnetic axis rotates through the crystal with an incommensurate long-wavelength period of 62 nm, which cancels the macroscopic magnetization and also inhibits the observation of the linear magnetoelectric effect in bulk BFO.6,7 Hence, for novel electronics applications of BFO, its magnetic and electric properties must be enhanced.Several studies aimed at upgrading the magnetic and ferroelectric properties of BiFeO3 have ensued in the last five years. Improvement in magnetic properties at room temperature has been observed in single crystalline bismuth ferrite nanoparticles which show strong size-dependent magnetic properties.8–10 Also the ferroelectric saturation polarization Ps and remnant polarization Pr of BiFeO3 nanoparticles have been reported to be higher than that of the bulk material.11 Introducing suitable dopant ions in BiFeO3 films has also been reported to be a potential method for enhancing its magnetic, electric and magnetoelectric properties.5,12–17 Hence it was of interest to synthesize doped BiFeO3 nanoparticles and study the effect of the dopant ions on its magnetic and electric properties at room temperature.
It has always been a challenge to synthesize pure BiFeO3 as the product is mostly contaminated with secondary phases such as Bi2O3 and Bi2Fe4O9.18 In the present work, we have synthesized phase pure undoped BiFeO3 and co-doped Bi0.9Ba0.1Fe0.9Mn0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 nanostructures through a sonochemical route. Sonochemical synthesis is based on acoustic cavitation resulting from the continuous formation, growth and implosive collapse of the bubbles in a liquid.19,20 Undoped BiFeO3 nanoparticles synthesized using the sonochemical technique has been reported earlier but the products showed the presence of some unidentified peaks in the powder X-ray diffraction pattern.21 The choice of the dopant ions was based on the fact that replacing Fe3+ ions in BiFeO3 thin films with other transition metal ions such as Cr3+ and Mn3+ that have better electronic stability is expected to increase the resistance by reducing valence fluctuations in Fe3+.22–25 Also, weak ferromagnetism has been observed at room temperature for single doped BiFeO3 nanoparticles where divalent cations, viz. Ba2+ and Ca2+, substitute trivalent cations of Bi3+.26 We have also evaluated the magnetic and ferroelectric properties of the sonochemically synthesized Bi0.9Ba0.1Fe0.9Mn0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 nanoparticles and the details of this work are discussed herein.
Results and discussion
Undoped BiFeO3, single doped BiFeO3 (Bi0.9Ba0.1FeO3, BiFe0.9Mn0.1O3, Bi0.9Ca0.1FeO3, BiFe0.9Cr0.1O3) and double doped BiFeO3 (Bi0.9Ba0.1Fe0.9Mn0.1O3, Bi0.9Ca0.1Fe0.9Cr0.1O3) particles were synthesized using the sonochemical technique. The XRD patterns of the undoped BiFeO3 as well as that of Bi0.9Ba0.1FeO3, BiFe0.9Mn0.1O3 and Bi0.9Ba0.1Fe0.9Mn0.1O3 are shown in Fig. 1, while those of Bi0.9Ca0.1FeO3, BiFe0.9Cr0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 are shown in Fig. 2, respectively. The peaks in all the XRD pattern corresponded to the rhombohedral structure of BiFeO3 with R3c space group (JCPDS no: 71-2494). The observed broadening of the peaks in the XRD patterns of all the above samples, compared to that normally seen for bulk BiFeO3 obtained via solid state synthesis is typical for nanoparticles. No additional peaks were observed in any of the XRD patterns, confirming the formation of phase pure BiFeO3. Earlier reports on alkaline earth metal doped BiFeO3 nanoparticles synthesized using a sol–gel technique showed the presence of some unidentified impurity phases.26 The lattice parameters of all the samples are given in Table 1. The lattice parameters of undoped BiFeO3 is relatively close to the reported literature values (a = 5.587 Å, c = 13.860 Å). However, the lattice parameter values change on doping BiFeO3. The results suggest that the rhombohedral BiFeO3 structure undergoes distortion on doping, which involves Fe or Bi displacements relative to the cubic perovskite parent structure. The (104) and (110) peaks are overlapping in case of BiFe0.9Mn0.1O3 and Bi0.9Ba0.1Fe0.9Mn0.1O3, but can be distinguished in pure BiFeO3 and for all the other doped samples.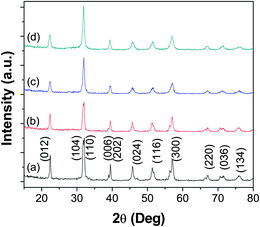 | ||
| Fig. 1 XRD patterns of (a) BiFeO3, (b) Bi0.9Ba0.1FeO3, (c) BiFe0.9Mn0.1O3 and (d) Bi0.9Ba0.1Fe0.9Mn0.1O3. | ||
 | ||
| Fig. 2 XRD patterns of (a) Bi0.9Ca0.1FeO3, (b) BiFe0.9Cr0.1O3, and (c) Bi0.9Ca0.1Fe0.9Cr0.1O3. | ||
| Sample | a/Å | c/Å |
|---|---|---|
| BiFeO3 | 5.5811(1) | 13.8589(2) |
| Bi0.9Ba0.1FeO3 | 5.5799(2) | 13.8575(1) |
| BiFe0.9Mn0.1O3 | 5.5817(4) | 13.8598(1) |
| Bi0.9Ba0.1Fe0.9Mn0.1O3 | 5.5778(5) | 13.8264(2) |
| Bi0.9Ca0.1FeO3 | 5.5673(5) | 13.7712(3) |
| BiFe0.9Cr0.1O3 | 5.5713(3) | 13.7932(5) |
| Bi0.9Ca0.1Fe0.9Cr0.1O3 | 5.5768(5) | 13.8231(4) |
To further investigate the microstructure and topography, we used transmission electron microscope to image the undoped and doped BiFeO3 samples on carbon coated copper TEM grids. Fig. 3A shows the TEM image of undoped BiFeO3 sample obtained via sonochemical synthesis followed by heat treatment at 400 °C for 1 h. It can be seen that the obtained nanocrystalline BiFeO3 are rod-like with a diameter of 20–50 nm and a length of 100–500 nm. These pure BiFeO3 nanorods exhibit aspect ratio in the range of 5–10. There are reports on faceted BiFeO3 nanoparticles synthesized using a sol–gel technique, nanospindles synthesized using a hydrothermal route and also nanowires and nanotubes synthesized using template assisted synthesis.8,27–29 However, to the best of our knowledge, this is the first report on the synthesis of BiFeO3 nanorods. The energy dispersive spectrum of the BiFeO3 nanorods shown in Fig. 4 confirms the presence of Bi, Fe and O in our prepared sample. The atomic ratio of Bi to Fe is approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Spectra taken at a number of selected positions of the sample show the presence of the same constituents. The Cu and C signals arise from the TEM grid. Fig. 3B shows the selected area electron diffraction (SAED) pattern taken from the BiFeO3 nanorod, which exhibits its highly crystalline structure. The indexing of the concentric rings corresponds to the rhombohedral BiFeO3 structure. The TEM images of Bi0.9Ba0.1Fe0.9Mn0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 samples are shown in Fig. 3C and 3D, respectively. It is clear from the images that the aspect ratios of both the double doped samples have reduced considerably, while retaining the crystallinity of the particles. In case of Bi0.9Ba0.1Fe0.9Mn0.1O3, very few particles with a nanorod shape are seen, as a majority of them have a faceted morphology (Fig. 3C). Compared to Bi0.9Ba0.1Fe0.9Mn0.1O3, the Bi0.9Ca0.1Fe0.9Cr0.1O3 samples show the presence of more nanorods (Fig. 3D). This clearly indicates that the nanorod shape of the BiFeO3 particles is destabilized under the influence of various dopant ions. Such an effect has also been observed in cobalt doped ZnO nanostructures.30
1. Spectra taken at a number of selected positions of the sample show the presence of the same constituents. The Cu and C signals arise from the TEM grid. Fig. 3B shows the selected area electron diffraction (SAED) pattern taken from the BiFeO3 nanorod, which exhibits its highly crystalline structure. The indexing of the concentric rings corresponds to the rhombohedral BiFeO3 structure. The TEM images of Bi0.9Ba0.1Fe0.9Mn0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 samples are shown in Fig. 3C and 3D, respectively. It is clear from the images that the aspect ratios of both the double doped samples have reduced considerably, while retaining the crystallinity of the particles. In case of Bi0.9Ba0.1Fe0.9Mn0.1O3, very few particles with a nanorod shape are seen, as a majority of them have a faceted morphology (Fig. 3C). Compared to Bi0.9Ba0.1Fe0.9Mn0.1O3, the Bi0.9Ca0.1Fe0.9Cr0.1O3 samples show the presence of more nanorods (Fig. 3D). This clearly indicates that the nanorod shape of the BiFeO3 particles is destabilized under the influence of various dopant ions. Such an effect has also been observed in cobalt doped ZnO nanostructures.30
 | ||
| Fig. 3 (A) TEM image of BiFeO3, (B) SAED pattern of BiFeO3, (C) TEM image of Bi0.9Ba0.1Fe0.9Mn0.1O3 and (D) TEM image of Bi0.9Ca0.1Fe0.9Cr0.1O3. | ||
 | ||
| Fig. 4 EDX spectrum of BiFeO3 nanorods showing Bi, Fe and O peaks. Note: The Cu and C peaks are due to the carbon coated copper grids used for dispersing the sample. | ||
To investigate the magnetic order at room temperature of our undoped bismuth ferrite nanorods, magnetic measurements were done using vibrating sample magnetometer (VSM). For all the samples we can observe sizable hysteresis with a finite value of the coercive field, remanent magnetization and saturation magnetization that are tabulated in Table 2. A DC magnetization loop of the BiFeO3 nanorods, recorded at 300 K, is shown in Fig. 5. The data represent the average of all random orientations of the BiFeO3 nanorods used for the measurements. BiFeO3 nanorods show a weak ferromagnetic order at room temperature, which is quite different from the linear M–H relationship reported for bulk BiFeO3.31 The weak ferromagnetic order was also observed in BiFeO3 films, nanoparticles, nanowires and nanotubes.26,28,29,32–34 The weak ferromagnetic order generally observed in BFO films and in nanoparticles has been attributed to the size effect. It is well known that the incommensurate spiral spin structure of bulk BFO, with a period of 62 nm, cancels the macroscopic magnetization. The BFO nanostructures with typical dimensions below 62 nm can possess favorable magnetic properties due to their grain size confinement, an effect that has been found to partially destroy the long-range spiral spin structure of bulk BFO. The diameters of our BiFeO3 nanorods are in the range of 20–50 nm, which is less than the wavelength of the incommensurate spiral spin structure of the bulk material. This would lead to partial destruction of the spiral spin structure in the BiFeO3 nanorods and hence the incomplete spin compensation becomes measurable, resulting in weak FM behaviors. The maximum magnetization, MS, measured at the maximum applied field of Happl = 8 kOe corresponds to MS ∼ 0.277 emu g−1 (0.016 μB/Fe) for the BiFeO3 nanorods. This is less than that observed in case of BiFeO3 nanowires (MS ∼ 0.534 emu g−1) but more than that reported for BiFeO3 nanotubes (MS ∼ 0.125 emu g−1).28,29 This may be attributed to the different shape anisotropy, magnetocrystalline anisotropy and different extents of defects present in the various nanoforms. The coercive field of the nanorods is quite small (Hc ∼ 382 Oe). A shift in the hysteresis loops is also observed in the M–H curves of BiFeO3 nanorods (shown as inset in Fig. 4). This can be ascribed to the presence of exchange coupling between the ferromagnetic surfaces and the antiferromagnetic cores. The hysteresis loops of undoped BiFeO3 nanorods exhibit very small remnant magnetization and a lack of proper saturation. This can be attributed to the presence of exchange and dipolar interparticle interactions in our system.
| Sample | Coercive field/Oe | Remanant magnetization/emu g−1 | Maximum magnetization (at 8 kOe)/emu g−1 |
|---|---|---|---|
| BiFeO3 | 382 | 0.01 | 0.28 |
| Bi0.9Ba0.1FeO3 | 1918 | 0.04 | 0.31 |
| BiFe0.9Mn0.1O3 | 574 | 0.02 | 0.32 |
| Bi0.9Ba0.1Fe0.9Mn0.1O3 | 1942 | 0.25 | 1.35 |
| Bi0.9Ca0.1FeO3 | 350 | 0.11 | 0.68 |
| BiFe0.9Cr0.1O3 | 350 | 0.05 | 0.47 |
| Bi0.9Ca0.1Fe0.9Cr0.1O3 | 1142 | 0.13 | 1.06 |
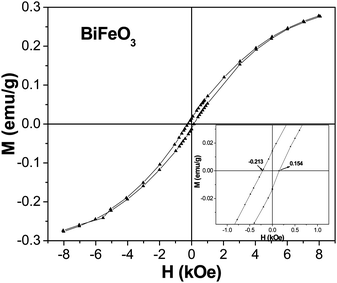 | ||
| Fig. 5 Field variation of magnetization over ±8 kOe at room temperature for the undoped BiFeO3 nanorods. Inset (right) enlarges M(H) curve showing the presence of hysteresis. | ||
Fig. 6 shows the room temperature magnetic hysteresis curves for the single doped Bi0.9Ba0.1FeO3, BiFe0.9Mn0.1O3 and double doped Bi0.9Ba0.1Fe0.9Mn0.1O3 samples. There is a small increase in the maximum magnetization (MS) of single doped BiFeO3 nanostructures with Mn and Ba doping (MS ∼ 0.31 emu g−1) compared to that observed in BiFeO3 nanorods. These effects may originate from the variable valence and dissimilar transition metal ions. The highest value of MS (1.35 emu g−1), MR (0.25 emu g−1) and coercive field Hc (1.94 kOe) occurs in the double doped Bi0.9Ba0.1Fe0.9Mn0.1O3 nanostructures. The high coercive field value obtained in case of single doped Bi0.9Ba0.1FeO3 nanostructures was comparable to that reported for Bi0.95Ba0.05FeO3 nanoparticles.26 However, Bi0.9Ba0.1FeO3 and Bi0.9Ba0.1Fe0.8Mn0.2O3 samples synthesized via pyrolysis of xerogel precursors exhibited higher MS and coercive field values compared to our nanostructures. This may be due to the presence of P4mm phase in their samples, which is absent in our case, since in systems like BiFeO3, different synthesis methods often lead to different competing structures. The M–H loops for Bi0.9Ca0.1FeO3, BiFe0.9Cr0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 are shown in Fig. 7. In this case also, though the highest value of MS (1.06 emu g−1), MR (0.13 emu g−1) and coercive field Hc (1.14 kOe) occurs in the double doped Bi0.9Ba0.1Fe0.9Mn0.1O3, it is less than that observed for Bi0.9Ba0.1Fe0.9Mn0.1O3 nanostructures. Bi0.9Ca0.1FeO3 show the highest MS (0.68 emu g−1) value among all the single doped samples though it is less than that reported for Bi0.9Ca0.1FeO3 nanoparticles synthesized using a sol–gel route.26
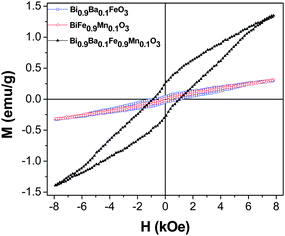 | ||
| Fig. 6 Field variation of magnetization over ±8 kOe at room temperature for the Bi0.9Ba0.1FeO3, BiFe0.9Mn0.1O3 and Bi0.9Ba0.1Fe0.9Mn0.1O3 samples. | ||
 | ||
| Fig. 7 Field variation of magnetization over ±8 kOe at room temperature for the Bi0.9Ca0.1FeO3, BiFe0.9Cr0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 samples. | ||
Fig. 8 shows the ferroelectric properties of the undoped BiFeO3 nanorods and double doped Bi0.9Ba0.1Fe0.9Mn0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 nanostructures, investigated by the P–E loop measurements. At a maximum applied electric field of ±500 V cm−1, the remanent polarization (Pr) is 0.21 μC cm−2 and the coercive field (Ec) is 155 V cm−1 for the BiFeO3 nanorods. The Pr value is much less than that reported in case of BiFeO3 nanoparticles and thin films, but higher than that observed in case of bulk BiFeO3.11,35,36 Saturation of polarization is not observed in case of the BiFeO3 nanorods as well as Bi0.9Ba0.1Fe0.9Mn0.1O3 nanostructures at a maximum applied electric field of ±500 V cm−1 and ±2000 V cm−1, respectively. However, a high saturation polarization (Ps) of 10.5 μC cm−2, Pr of 7 μC cm−2 and Ec of 1957 V cm−1 is observed in case of the Bi0.9Ca0.1Fe0.9Cr0.1O3 nanostructures. The P–E hysteresis loops of all samples indicate their ferroelectric nature but with lossy features. This leaky feature is the least in case of Bi0.9Ca0.1Fe0.9Cr0.1O3.
 | ||
| Fig. 8 FE hysteresis loop for BiFeO3 nanorods, Bi0.9Ba0.1Fe0.9Mn0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 nanostructures. | ||
To understand this behavior, we have to first note that in BiFeO3, small amounts of Fe2+ ions and oxygen vacancies exist.37 Incidentally, BiFeO3 shows p-type conductivity,38 which can be understood by considering the substitution of a small amount Fe2+ ions in Fe3+ positions (acceptor doping of Fe3+ by Fe2+). When Ca2+/Ba2+ and Cr3+/Mn2+ is added to BiFeO3, Ca2+/Ba2+ is supposed to substitute Bi3+ because of the close ionic radii of Ca2+/Ba2+ and Bi3+. Such acceptor doping of Bi3+ by Ca2+/Ba2+ is expected to generate oxygen vacancies without the liberation of electrons.39 Normally, the oxygen partial pressure in the ambience is sufficient to incorporate oxygen into the structure to nullify the oxygen vacancies and show p-type conductivity. The hole generated can be consumed by Fe2+ in Fe3+ position resulting in lower acceptor doping of Fe3+ by Fe2+ in BiFeO3 with consequent decrease in conductivity. When Cr3+ substitutes Fe3+, the acceptor doping of Fe3+ by Fe2+ is further reduced since Cr3+ is very stable electronically and this further causes a decrease in the conductivity. Hence, Ca2+ and Cr3+ co-doped BiFeO3 show comparatively non-lossy ferroelectric hysteresis loops compared to undoped BiFeO3 and Bi0.9Ba0.1Fe0.9Mn0.1O3 nanostructures.
Experimental
All the reactions were carried out at room temperature under ambient conditions. High purity bismuth nitrate [Bi(NO3)3·5H2O], ferric nitrate [Fe(NO3)3·9H2O], manganese acetate [Mn(OOCCH3)2·4H2O], chromium nitrate [Cr(NO3)3·9H2O], barium nitrate [Ba(NO3)2] and calcium chloride [CaCl2] were obtained from commercial sources (Aldrich). In a typical procedure, an aqueous solution of Bi(NO3)3·6H2O and Fe(NO3)3·9H2O was sonicated for 20 min. To the mixture, 5 ml of tetraethylene glycol (TEG) was added and sonicated for further 10 min. The pH of the solution was then adjusted to ∼8 by adding ammonia solution and the resultant mixture was irradiated with a high intensity (100 W cm−2) ultrasonic radiation operating at 20 kHz, under air at room temperature for 100 min. This was done by the direct immersion of a titanium horn (13 mm diameter) to a depth of 6 cm in the solution. The subsequently formed white precipitate was centrifuged, washed with distilled water and finally with acetone. The product was heated under air in furnace at 400 °C for 1 h.For the doped BiFeO3 nanostructures, the method is similar to that reported for undoped BiFeO3 but here a stoichiometric amount of barium nitrate/manganese acetate or calcium chloride/chromium nitrate was also added to the reaction mixture. We have prepared Bi0.9Ba0.1FeO3, BiFe0.9Mn0.1O3, Bi0.9Ba0.1Fe0.9Mn0.1O3, Bi0.9Ca0.1FeO3, BiFe0.9Cr0.1O3 and Bi0.9Ca0.1Fe0.9Cr0.1O3 nanostructures using the sonochemical technique. The products obtained were subjected to thermal treatment, and resultant residues were characterized by XRD.
Characterization
X-Ray diffraction (XRD) measurements were carried out on a Philips Instrument, operating with Cu-Kα radiation (λ = 1.5406 Å) and employing a scan rate of 0.02° s−1 in the scattering angular range (2θ) of 15° to 80°. Silicon was used as an external standard for correction due to instrumental broadening. The average crystallite size was calculated from the diffraction line width based on Scherrer's relation: d = 0.9λ/Bcosθ, where λ denotes the wavelength of X-rays and B is the corrected full width at half maxima (FWHM). EDS analyses was were carried out using an Inca Energy 250 instrument coupled to Vega MV2300t/40 scanning electron microscope. Conventional TEM micrographs were recorded on JEOL 2000FX. The particulates obtained, were dispersed in methanol solution and then deposited on the carbon coated copper grids for TEM/SAED studies. Magnetization of powder samples was measured using an EG&G P.A.R. vibrating sample magnetometer (model 4500). The magnetic hysteresis loops (Mvs.H) was measured at 300 K with H = ±8 kOe. Electric field (P–E) hysteresis loops of the samples (5 mm diameter pellet with sputtered gold as top electrode and silver paste as bottom electrode) were measured by the modified Sawyer–Tower circuit at a 1 kHz ac frequency.Conclusion
Phase pure BiFeO3 nanorods have been successfully synthesized using a facile sonochemical technique. These nanorods show a weak ferromagnetic order at room temperature, which is quite different from the linear M–H relationship reported for bulk BiFeO3. On doping, the nanorod shape of the BiFeO3 particles is destabilized and we get nanostructures with a reduced aspect ratio. Addition of various dopants in BiFeO3 nanorods, alters their magnetic as well as ferroelectric properties to different extents. The highest value of MS (1.35 emu g−1), MR (0.25 emu g−1) and coercive field Hc (1.94 kOe) occurs in the double doped Bi0.9Ba0.1Fe0.9Mn0.1O3 nanostructures. However, saturation of electric polarization was observed only in case of Bi0.9Ca0.1Fe0.9Cr0.1O3 which exhibited a Ps of 10.5 μC cm−2, Pr of 7 μC cm−2 and Ec of 1957 V cm−1. This material also showed enhanced ferromagnetic properties compared to the bulk BiFeO3. Thus, fine tuning of the concentration of these dopants in BiFeO3 nanostructures can lead to better materials for different potential applications.References
- H. Schmid, Ferroelectrics, 1994, 162, 19–25 CrossRef.
- W. Eerenstein, N. D. Mathur and J. F. Scott, Nature, 2006, 442, 759–765 CrossRef CAS.
- M. Fiebig, T. Lottermoser, D. Fröhlich, A. V. Goltsev and R. V. Pisarev, Nature, 2002, 419, 818–820 CrossRef CAS.
- N. Hur, S. Park, P. A. Sharma, J. S. Ahn, S. Guha and S.-W. Cheong, Nature, 2004, 429, 392–395 CrossRef CAS.
- J. Wang, J. B. Neaton, H. Zheng, V. Nagarajan, S. B. Ogale, B. Liu, D. Viehland, V. Vaithyanathan, D. G. Schlom, U. V. Waghmare, N. A. Spaldin, K. M. Rabe, M. Wuttig and R. Ramesh, Science, 2003, 299, 1719–1722 CrossRef CAS.
- I. Sosnowska, T. Peterlin-Neumaier and E. Streichele, J. Phys. C: Solid State Phys., 1982, 15, 4835 CrossRef CAS.
- Y. F. Popov, A. K. Zvezdin, G. P. Vorob'ev, A. M. Kadomtseva, V. A. Murashev and D. N. Rakov, JETP Lett., 1993, 57, 69.
- Tae-J. Park, G. C. Papaefthymiou, A. J. Viescas, A. R. Moodenbaugh and S. S. Wong, Nano Lett., 2007, 7, 766–772 CrossRef CAS.
- R. Mazumder, S. Ghosh, P. Mondal, D. Bhattacharya, S. Dasgupta, N. Das, A. Sen, A. K. Tyagi, M. Sivakumar, T. Takami and H. Ikuta, J. Appl. Phys., 2006, 100, 033908 CrossRef.
- R. Mazumder, P. S. Devi, D. Bhattacharya, P. Choudhury, A. Sen and M. Raja, Appl. Phys. Lett., 2007, 91, 062510 CrossRef.
- Y.-Q. Kang, M.-S. Cao, J. Yuan and X.-L. Shi, Mater. Lett., 2009, 63, 1344–1346 CrossRef CAS.
- M. Azuma, K. Tanaka, T. Saito, S. Ishitwata, Y. Shimakawa and M. Takano, J. Am. Chem. Soc., 2005, 127, 8889 CrossRef CAS.
- J. B. Li, G. H. Ra, J. K. Liang, J. Luo and J. Chen, Appl. Phys. Lett., 2007, 90, 162513 CrossRef.
- B. Yu, M. Li, J. Liu, D. Guo, L. Pei and X. Zhao, J. Phys. D: Appl. Phys., 2008, 41, 065003 CrossRef.
- G. l. Yuan, S. O. Wing and H. L. W. Chan, J. Appl. Phys., 2007, 101, 064101 CrossRef.
- D. H. Wang, W. C. Goh, M. Ning and C. K. Ong, Appl. Phys. Lett., 2006, 88, 212907 CrossRef.
- V. A. Khomchenko, D. A. Kiselev, J. M. Vieira, A. L. Kholkin, M. A. Sá and Y. G. Pogorelov, Appl. Phys. Lett., 2007, 90, 242901 CrossRef.
- J.-C. Chen and J.-M. Wu, Appl. Phys. Lett., 2007, 91, 182903 CrossRef.
- S. Sundar Manoharan and M. Rao, Encyclopedia of Nanoscience and Nanotechnology, ed. H. S. Nalwa, American Scientific Publishers, USA, p. 67 Search PubMed.
- K. S. Suslick, Science, 1990, 247, 1439 CrossRef CAS.
- N. Das, R. Majumdar, A. Sen and H. S. Maiti, Mater. Lett., 2007, 61, 2100–2104 CrossRef CAS.
- J. K. Kim, S. S. Kim, W.-J. Kim, A. S. Balla and R. Guo, Appl. Phys. Lett., 2006, 88, 132901 CrossRef.
- S. U. Lee, S. S. Kim, H. K. Jo, M. H. Park, J. W. Kim and A. S. Bhalla, J. Appl. Phys., 2007, 102, 044107 CrossRef.
- D. H. Kim, H. N. Lee, M. D. Biegalski and H. M. Christen, Appl. Phys. Lett., 2007, 91, 042906 CrossRef.
- M. Azuma, H. Kanda, A. A. Belik, Y. Shimakawa and M. Takano, J. Magn. Magn. Mater., 2007, 310, 1177 CrossRef CAS.
- B. Bhushan, A. Basumallick, S. K. Bandopadhyay, N. Y. Vasanthacharya and D. Das, J. Phys. D: Appl. Phys., 2009, 42, 065004 CrossRef.
- J.-T. Han, Y.-H. Huang, X.-J. Wu, C.-L. Wu, W. Wei, B. Peng, W. Huang and J. B. Goodenough, Adv. Mater., 2006, 18, 2145–2148 CrossRef CAS.
- J. Wei, D. Xue and Y. Xu, Scr. Mater., 2008, 58, 45–48 CrossRef CAS.
- F. Gao, Y. Yuan, K. F. Wang, X. Y. Chen, F. Chen and J. M. Liu, Appl. Phys. Lett., 2006, 89, 102506 CrossRef.
- O. D. Jayakumar, C. Sudakar, C. Persson, V. Sudarsan, T. Sakuntala, R. Naik and A. K. Tyagi, Cryst. Growth Des., 2009, 9, 4450–4455 CrossRef CAS.
- S. T. Zhang, M. H. Lu, D. Wu, Y. F. Chen and N. B. Ming, Appl. Phys. Lett., 2005, 87, 262907 CrossRef.
- F. Gao, X. Chen, K. Yin, S. Dong, Z. Ren, F. Yuan, T. Yu, Z. Zou and J.-M. Liu, Adv. Mater., 2007, 19, 2889–2892 CrossRef CAS.
- P. Kharel, S. Talebi, B. Ramachandran, A. Dixit, V. M. Naik, M. B. Sahana, C. Sudakar, R. Naik, M. S. R. Rao and G. Lawes, J. Phys.: Condens. Matter, 2009, 21, 036001 CrossRef.
- O. D. Jayakumar, S. N. Achary, K. G. Girija, A. K. Tyagi, C. Sudakar, G. Lawes, R. Naik, J. Nisar, X. Peng and R. Ahuja, Appl. Phys. Lett., 2010, 96, 032903 CrossRef.
- V. R. Palkar, J. John and R. Pinto, Appl. Phys. Lett., 2002, 80, 1628–1630 CrossRef CAS.
- M. M. Kumar, V. R. Palkar, K. Srinivas and S. V. Suryanarayana, Appl. Phys. Lett., 2000, 76, 2764–2766 CrossRef CAS.
- V. R. Palkar and R. Pinto, Pramana, 2002, 58, 1003 CrossRef CAS.
- A. S. Poghossian, H. V. Abovian, P. B. Avakian, S. H. Mkrtchian and V. M. Haroutunian, Sens. Actuators, B, 1991, 4, 545 CrossRef.
- A. J. Moulson and J. M. Herbert, Electroceramics: Materials, Properties & Application, Chapman & Hall, London, 1997 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
