Silica-based nanoparticles for photodynamic therapy applications
Pierre
Couleaud
b,
Vincent
Morosini
c,
Céline
Frochot
bd,
Sébastien
Richeter
a,
Laurence
Raehm
a and
Jean-Olivier
Durand
*a
aInstitut Charles Gerhardt Montpellier, UMR 5253 CNRS-UM2-ENSCM-UM1, Equipe Chimie Moléculaire et Organisation du Solide, CC1701 Place Eugène Bataillon, 34095, Montpellier Cedex 05, France. E-mail: durand@univ-montp2.fr
bLaboratoire Réactions et génie des Procédés, UPR 3349, Nancy-Université, 1, rue Grandville, 54000, Nancy, France
cCentre de Recherche en Automatique de Nancy (CRAN), UMR 7039, Nancy-Université, CNRS, Centre Alexis Vautrin, Vandœuvre-lès-Nancy, France
dGDR 3049 “Médicaments Photoactivables – Photochimiothérapie (PHOTOMED)”
First published on 25th May 2010
Abstract
Silica-based nanoparticles for applications in photodynamic therapy (PDT) have emerged as a promising field for the treatment of cancer. In this review, based on the pathway the photosensitizer is entrapped inside the silica matrix, the different methods for the synthesis of silica-based nanoparticles are described from the pioneering works to the latest achievements which concern multifunctional nanoplatforms, up-converting nanoparticles, two-photon PDT, vectorization and in vivo applications.
 Pierre Couleaud | P. Couleaud received a Master’s Research degree in chemistry specializing in biomolecules, organic synthesis, medical and environment applications in 2007 from the University of Limoges. He is currently pursuing his PhD in the Laboratory of Reactions and Process Engineering at the University of Nancy under the supervision of Dr Céline Frochot and Dr Muriel Barberi-Heyob. His research is focused on the study and the development of targeted silica multifunctional nanoparticles and their applications in cancer treatment by vascular targeted photodynamic therapy. |
 Vincent Morosini | Vincent Morosini received his Bachelor of Sciences (2004) in organic chemistry and master degree (2006) in macromolecular, biomolecular and molecular chemistry and physic chemistry from the Henri Poincaré University in Nancy, France. He is currently pursuing his PhD at the Research Center for Automatic Control, Nancy University. His research interest is focused on the synthesis of biomolecule-conjugated quantum dots designed for anticancer photodynamic therapy. |
 Céline Frochot | C. Frochot graduated from the Ecole Nationale Supérieure des Industries Chimiques, Nancy, and obtained her PhD in macromolecular chemistry and physical chemistry in 1997. In 2000, she became a CNRS researcher and her interest is to develop novel photo-activable compounds for nanomedicine and photodynamic therapy. Particularly, the main field of her research at the Laboratory of Reactions and Process Engineering concerns the synthesis and photophysical properties of targeted photosensitizers designed for anti-vascular photodynamic therapy applications. Another part of her research includes the use of fluorescence probes to analyze the behaviour of macromolecules in complex media. |
 Sébastien Richeter | Sébastien Richeter received his PhD degree from Strasbourg University (France) under the guidance of Dr Henry Callot in 2003. During his PhD, he studied the functionalization of metalloporphyrins and the synthesis and properties of multiporphyrinic systems. He joined the Scripps Research Institute (La Jolla, California, USA) and studied the chemistry of cavitands in the research group of Pr. Julius Rebek Jr. Since September 2004, he has been Maître de Conférences at the University of Montpellier 2 (France). His current research interests are the synthesis of new porphyrinoids and the use of porphyrin derivatives in materials science. |
 Laurence Raehm | Laurence Raehm got her PhD in Strasbourg (France) in 1999 under the supervision of Jean-Pierre Sauvage and Jean-Marc Kern for studies on molecular machines. She spent two more years handling catenanes as a postdoctoral researcher in the group of J. K. M. Sanders in Cambridge, UK. Her career as Maître de Conférences (assistant professor) began in Paris in 2001, and was still dedicated to supramolecular chemistry. She is currently working in Montpellier (Université Montpellier 2), France. Her research interests now involve the use of mesoporous silica nanoparticles for applications in imaging and therapy. |
 Jean-Olivier Durand | Jean-Olivier Durand graduated from the Ecole Nationale Supérieure de Chimie de Paris in 1990 and obtained his PhD in organic chemistry with Prof. J. P. Genêt in 1993. He spent a 20 month postdoctoral position with Prof. W. Oppolzer at the University of Geneva (Switzerland). After two other postdoctoral studies in Rennes (Prof. Le Corre) and Paris (Dr M. Larchevêque), he was appointed a CNRS researcher in 1996 at the Laboratoire de Chimie Moléculaire et Organisation du Solide, Institut Charles Gerhardt Montpellier. His research interests consist of developing mesoporous silica nanoparticles for photodynamic therapy and drug delivery. |
Over the last few years, photodynamic therapy (PDT) has emerged as an alternative to chemo and radiotherapy for the treatment of various diseases including cancer.1,2 It involves the use of light and photosensitizers (PS) that accumulate in the tumor tissue. Photodynamic sensitizers are drugs that can transfer their energy from their triplet excited state to neighboring oxygen molecules3 when activated by light of a specific wavelength. Singlet oxygen (1O2) and other cytotoxic Reactive Oxygen Species (ROS) are formed and lead to the destruction of cancer cells by both apoptosis and necrosis. The efficiency of PDT can be related to the formation of ROS and it is commonly accepted that 1O2 is the main cytotoxic species that destroys tumour cells. PS described so far in the literature present several disadvantages. Mainly, they are hydrophobic or have a limited solubility in water and therefore aggregate in aqueous media such as blood which leads to the modification of their photophysical properties and particularly to the decrease of 1O2 quantum yield. Moreover, in order to avoid destruction of healthy cells, PS are required to accumulate selectively in tumor cells. Even if many efforts are focused on developing third generation PS with a covalently linked vector to target receptors over-expressed in cancer cells, very few have been evaluated for clinical applications mainly because their in vivo selectivity was not high enough.4 In order to address these issues, PS have been encapsulated in nanoparticles (NPs). Indeed, encapsulation should lead to the administration of the PS in monomeric form and without loss of activity. More importantly, nanoparticles are a means to allow selective accumulation of the PS in cancer cells due to the enhanced permeability and retention (EPR) effect of tumor tissues.5 Therefore, nanoparticles have been prepared to improve the efficiency of PDT. Polymeric, metallic, semi-conductor-based or silica-based nanoparticles have been described and have been reviewed recently by some of us and others.6–8 Among the variety of nanoparticles, silica-based nanomaterials have very recently emerged as promising vectors for PDT applications. Indeed silica nanoparticles are chemically inert and the silica matrix porosity is not susceptible to swell or change with a varying pH. Furthermore, a variety of precursors and methods are available for their syntheses allowing flexibility and thus numerous PDT drugs to be encapsulated. Moreover, particles size, shape, porosity and mono-dispersibility can be easily controlled during their preparation.9 The surface modification with specific biomolecules for tumor-cell targeting is another possibility offered by silica nanoparticles and this research area has been explored recently. In this review we describe the two synthetic methods used for the immobilization of the PS inside silica-based nanoparticles. The most frequently described method concerns immobilization through non-covalent interactions. The other method leads to a covalent linkage of the PS and appears to be more efficient, as no release of the PS from the nanoparticles occurs. However, this method requires more chemistry to functionalize the PS with an anchoring function.
Many methods to prepare silica-based nanoparticles have been applied to design nano-objects for PDT applications. The Stöber procedure10 leads to nanoparticles of about 100 nm diameter whereas the reverse microemulsion (water in oil) method, which uses surfactants, gives smaller nano-objects.11 Alternatively direct microemulsion (oil in water) procedure leads to mesoporous silica nanoparticles which have just emerged as promising vectors for PDT.12–14
The very recent developments of silica nanoparticles concern up-conversion or two-photon PDT which opens new promising ways to treat deeper tissue regions.15,16 Most of the studies with silica nanoparticles have been carried out in vitro, but in vivo PDT is currently being investigated.17 Another field which grows rapidly with silica-based nanoparticles consists of combining several applications within one nano-object, such as MRI, fluorescence imaging and PDT.18–20 Such multifunctional nanoparticles emerge as important theranostic nano-objects. We thus describe the exploitation of silica-based nanoparticles in research from the beginning of the century to the recent advances, based on the way the PS is being encapsulated in the particle (Table 1).
I. Non covalent encapsulation of PS in silica nanoparticles
a) Silica nanoparticles for PDT
Following the pioneering work of Prasad and Kopelman groups,21,22 several researchers have tried to encapsulate PS inside silica nanoparticles in order to vectorize them in cancer cells. In 2003, the precursor work of Kopelman lead to the encapsulation of mesometa-tetra(hydroxyphenyl)chlorin (m-THPC, Fig. 1), or Foscan®, a medicinal product used clinically, into silica nanoparticles.22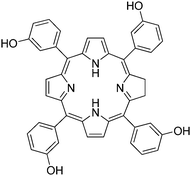 | ||
| Fig. 1 m-THPC. | ||
| Synthesis | Size | PS | C/E | λ absmax | Φ f | Φ Δ | 1O2 detection | Phototoxicity | Reference |
|---|---|---|---|---|---|---|---|---|---|
| a Size: diameter in nm; PS: photosensitizer; C/E: C covalently coupled, E encapsulated; λabsmax: maximum absorption wavelength; Φf: fluorescence quantum yield; ΦΔ: singlet oxygen formation quantum yield for the NP; ΦΔPS: singlet oxygen formation quantum yield for the free PS; 1O2: detection of 1O2 with a chemical probe; hν: 1O2 detection by direct luminescence at 1270 nm. MB: methylene blue; HPPH: 2-devinyl-2-(1-hexyloxyethyl)pyropheophorbide; PHPP: 2,7,12,18-tetramethyl-3,8-di(1-propoxyethyl)-13,17-bis-(3-hydroxypropyl)porphyrin; PPIX: protoporphyrin IX; PdTPP: palladium tetraphenyl porphyrin; HP: hematoporphyrin; ADBA: 9,10-anthracenedipropionic acid; DPBF: 1,3-diphenylisobenzofuran; RNO: N,N-dimethyl-4-nitrosoaniline with imidazole as a chemical trap; ABDM: 9,10-anthracenecliybis(methylene)dimalonic acid; ESR spin trapping technique with TEMP (2,2,6,6-tetraethyl-4-piperridone) as a spin-trapping agent. | |||||||||
| ORMOSIL | 180 | m-THPC | E | 410 | — | >ΦΔPS | ADPA | — | 22 |
| ORMOSIL or Stöber | 190/160 | MB | E | 650 | — | — | ADPA | C6 (rat glioma)—positive PDT results (confocal microscopy) | 23 |
| Hollow ORMOSIL | 130 | Hypocrellin A | E | — | >ΦfPS | >ΦΔPS | EPR with TEMP | HeLa—phototoxicity | 24 |
| Hollow ORMOSIL-coated HA-NP | 110 | Hypocrellin A (HA) | E | 490 | >ΦfPS | >ΦΔPS | ADPA | HeLa—phototoxicity | 25 |
| AOT/1-butanol/H2O ORMOSIL | 30 | HPPH | E | 420 | — | — | ADPA, hν | Growth inhibition 90% (UCl-107), 100% (HeLa) | 21 |
| AOT/1-butanol/H2O ORMOSIL | 25 | PPIX | E | 405 | — | — | DPBF | Destruction of HeLa cells structures | 26 |
| AOT/1-butanol/H2O ORMOSIL | 30 | m-THPC | E | 420 | — | — | — | 100% loss of KYSE 510 cells viability | 27 |
| Tween 80/1-butanol/H2O ORMOSIL | 25–30 | Pc4 | E | 674 | — | — | ABMD ESR withTEMP | Phototoxicity on A375 and B16F10 cells | 28 |
| Tween 80/1-butanol/H2O ORMOSIL | 10;25;60 | PPIX | E | — | — | — | APF ROS detection kit | HT-29, HCT 116, A431, LLBC37, MDA-MB-231, MCF7—phototoxicity | 29 |
| AOT/1-butanol/H2O ORMOSIL | 25.7 INP, 24.5 VNP | HPPH | E | 420 | Φ fINP < ΦfVNP | Φ ΔINP = 1.7 × ΦΔVNP | hν | Phototoxicity of INP > VNP (RIF-1 tumor) | 30 |
| W/O ORMOSIL | 105 | MB | E | — | 6 × ΦfPS | 0.049 | DPBF | HeLa cells—growth inhibition 90% in vivo (balb/c nude mice) | 17 |
| Stöber coated Fe3O4 | 30 | MB | E | 590 | — | 0.03 < ΦΔPS = 0.5 | DPBF, hν | — | 18 |
| W/cottonseed O ORMOSIL coated Fe3O4 | 20–30 | Purpurin-18 | E | 668 | — | <ΦΔPS | RNO | — | 20 |
| AOT/1-butanol/H2O ORMOSIL coated Fe3O4 | 20–30 | PHPP | E | 409 | — | — | RNO | 40% SW480 cell viability lost | 32 |
| Fe3O4 -loaded MSN | 60–120 | ZnPc | E | 670 | — | — | — | — | 33 |
| Silica-coated Fe3O4 | 30–50 | HPPH | E | 420 | — | — | RNO | — | 34 |
| Mesoporous silica coated NaYF4 | 35 × 60 | ZnPc | E | — | — | ABDA | — | 35 | |
| Stöber-coated PUNP | 60 to 120 | Merocyanin | E | 420 | — | — | ADPA | Phototoxicity on MCF7/AZ cells | 36 |
| W/O SiO2 | 60 | Two-photon sensitizer | E | 370 | 0.51 | hν | Two-photon toxicity on macrophage | 16 | |
| AOT/1-butanol/H2O ORMOSIL | 30 | HPPH–BDSA | E | 420–400 | 0.17 | — | — | Two-photon toxicity on HeLa Cells | 15 |
| Grafting on Silica | 10 + C60 | C60 | C | — | — | — | — | — | 37 |
| Stöber | 77 ± 12 | PPIX | C | 400 | 0.9 | DPBF, hν | — | 38 | |
| Tween 80/1-butanol/H2OORMOSIL | 20 | Iodobenzylpyropheophorbide | C | 410 | — | — | ADPA, hν | Colon-26—phototoxicity (confocal microscopy) | 39 |
| O/W MSN | 110 | PPIX | C | 408 | — | =ΦΔPS | DPBF, hν | Growth inhibition 100% (HeLa cells) | 14 |
| O/W MSN | — | PdTPP | C | 400 | — | — | DPBF, hν | Changes in MDA-MB-231 cell morphology (TEM)—phototoxicity | 13 |
| O/W MSN | 160 | Anionic porphyrin | C | 418 | 0.57 | hν | Phototoxicity on MDA-MB-231 cells | 12 | |
| O/W MSN coated W/O ORMOSIL | 57 core–shell, 37 silica | HP | C | 400 | — | >ΦΔPS | ABDM | HO-8910PM (only imaging) | 40 |
| W/O ORMOSIL-coated Fe3O4 | 55 | Ir(III) complex | C | 350 | 0.62 | — | — | Phototoxicity on Hela cells | 19 |
To avoid the leaching of the PS from the silica matrix, the synthesis was performed by first using aminopropyltriethoxysilane as the reagent, then by initiating hydrolysis polycondensation of tetramethylorthosilicate. An increased amount of hydrogen bonds between PS and amino groups were probably involved to explain the higher efficiency of the encapsulation process than the Stöber procedure. An average nanoparticle diameter of 180 nm was observed by SEM. A good spectral (UV-vis) correspondence between free and embedded m-THPC was observed. To characterize 1O2 formation, anthracene-9,10-dipropionic acid (ADPA) was used as it is easily converted to an endoperoxide and the fluorescence of ADPA was followed vs. the time of irradiation. The rate constant of the reaction of 1O2 with ADPA was determined for PS in solution and for PS embedded inside the nanoparticles. This rate was higher for the nanoparticles, suggesting that 1O2 production from nanoparticles exceeded that of free PS.
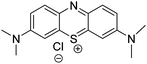
The same group published the encapsulation of methylene blue (Fig. 2), a promising drug for PDT applications.23 Intravenous administration of methylene blue has been approved by the Food and Drug Administration for methemoglobinemia. However, clinical use of methylene blue is limited partly because of the poor penetration of this drug in the cellular compartment of the tumor. Furthermore, methylene blue is usually inactivated via the reduction of the cation to the neutral leukomethylene blue which has negligible photodynamic activity. Encapsulation of methylene blue inside silica nanoparticles is a way to vectorize it and protect it from degradation.
 | ||
| Fig. 2 Methylene blue. | ||
Two methods were performed for its encapsulation. The Stöber procedure was successful for the immobilization of the PS inside pure silica giving nanoparticles with a diameter of 190 nm. Alternatively organically modified silica (ORMOSIL) nanoparticles were prepared by combining methyltrimethoxysilane and phenyltrimethoxysilane as precursors. This procedure resulted in nanoparticles with a diameter of 160 nm revealing a hydrophobic outer shell and a hydrophilic inner core. The loading of PS was higher with the Stöber procedure than with ORMOSIL nanoparticles. However, reaction of 1O2 with ADPA after irradiation of the nanoparticles at 650 nm showed a higher kinetic rate with ORMOSIL than with Stöber nanoparticles, although the production of 1O2 with the Stöber nanoparticles was higher. The microenvironment in the double shell matrix of ORMOSIL could explain this result. Those nanoparticles were however not tested in vitro.
Other types of silica particles have been used for the encapsulation of PS for PDT. Wei and collaborators24 have elaborated porous hollow silica nanospheres of 130 nm diameter. The nanoparticles were prepared by careful hydrolysis of N-(β-aminoethyl)-α-aminopropyl triethoxysilane catalyzed by ammonia. The embedding rather than surface adsorption of hypocrellin A (Fig. 3) was demonstrated by fluorescence quenching experiments.
 | ||
| Fig. 3 Hypocrellin A. | ||
The particles were found to have good light and thermal stabilities. Such silica nanoparticles were efficiently taken up by HeLa tumor cells and presented more PDT efficiency than hypocrellin A. Higher 1O2 generation quantum yield was found for the encapsulated PS than for free hypocrellin A.
The same collaborators later reported25 the preparation of hypocrellin A nanoparticles by reprecipitation followed by encapsulation of these particles using N-(β-aminoethyl)-α-aminopropyl triethoxysilane to form 110 nm silica nanovehicles. The resulting embedded hypocrellin A also showed superior light-stability and singlet oxygen generation ability than free hypocrellin A. Fluorescence images of Hela cells demonstrated the active uptake of the drug-doped nanovehicles into the tumor cells The mitochondrial localization of the particles was evidenced using Rhodamine-123. In vitro experiments also showed that the particles destroyed the mitochondria membrane. The photoxicity of the nanovehicles was studied and suggested that they could be an effective drug delivery system for killing tumor cells in vitro by PDT.
In 2003, the Prasad group also prepared ORMOSIL nanoparticles for the entrapment of 2-devinyl-2-(1-hexyloxyethyl)pyropheophorbide (HPPH, Fig. 4), a photosensitizer in phase I/II clinical trials for eosophageal cancer.21
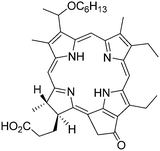 | ||
| Fig. 4 2-Devinyl-2-(1-hexyloxyethyl)pyropheophorbide (HPPH). | ||
The microemulsion technique was performed with AOT and butanol as surfactants and vinyltriethoxysilane and aminopropyltriethoxysilane as the reagents for the gelation procedure. The nanoparticles exhibited a diameter of 30 nm which is smaller than in previously described procedures. 1O2 production measured by its emission at 1270 nm and by reaction with ADPA was very efficient. The nanoparticles were taken up by HeLa and UCI-107 cancer cells as confirmed by fluorescence of HPPH at 665 nm. The nanoparticles were very efficient in killing cancer cells after irradiation at 650 nm as analyzed by MTT assay.
Qian et al. encapsulated Protoporphyrin IX (PpIX, Fig. 5) in ormosil nanoparticles.26 In PDT, an alternative to the administration of exogenous photosensitizers is to stimulate the cellular synthesis of endogenous ones. 5-Aminolaevulinic acid (ALA) is a metabolic precursor in the biosynthesis of haem and PpIX is the immediate precursor to haem, and thus PpIX can be considered as a natural photosensitizer.
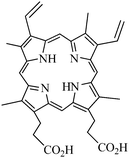 | ||
| Fig. 5 Protoporphyrin IX (PpIX). | ||
PpIX encapsulated into ormosil nanoparticles were synthesized following Prasad's method. Monodispersed and spherical nanoparticles with a 25 nm diameter were obtained. Extinction and fluorescence spectra of free PpIX in DMSO and encapsulated PpIX were similar, even if PpIX encapsulating nanoparticles had a non negligible scattering efficiency (due to the 25 nm diameter) and the fluorescence was 2 nm red-shifted due to the different surroundings. 1O2 was detected indirectly with 1,3-diphenylisobenzofuran (DPBF). In vitro PDT was performed on HeLa cells by a 532 nm light source irradiation (2 mW cm−2, 2 min). After 8 min of irradiation, changes could be observed in the HeLa cells and the cell structures were destroyed.
The cellular uptake of m-THPC (Fig. 1) entrapped in organically modified silica nanoparticles was shown to be mediated by serum proteins. The team of Reddi investigated the encapsulation of m-THPC into organic-modified silica nanoparticles of 33 ± 9 nm diameter following Prasad's method.27 They showed that m-THPC was entrapped in a monomeric form and produced 1O2 with a high efficiency. In aqueous media with high salt concentrations, the nanoparticles underwent aggregation and precipitation but their stability could be preserved in the presence of foetal bovine serum (3%). The in vitro studies were realized on human oesophageal cancer cells KYSE 510 and the entrapped m-THPC was compared to m-THPC diluted into the standard solvent ethanol/poly(ethyleneglycol) 400/water (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, by vol). In the absence of light, the two formulations did not affect the viability of the cells up to an m-THPC concentration of 1.75 mM. Phototoxicity was determined after 24 h dark incubation and irradiation with a light (600–700 nm, PTL Penta quartz halogen lamp, 2 mW cm−2) of 0.12 J cm−2. All cells were killed after incubation with 1.25 mM m-THPC. The dose–response curves obtained by incubating the cells with increasing concentrations of m-THPC delivered by the two formulations were identical but the nanoparticles formulation reduced the cellular uptake of m-THPC by about 50% in comparison to standard solvent. With an elegant Fluorescence Resonance Energy Transfer (FRET) approach, using a cyanine as an acceptor covalently linked to the nanoparticles, the authors demonstrated that m-THPC is transferred from the nanoparticles to serum proteins of the medium. To prevent this transfer to proteins it was necessary to coat the nanoparticles surface with poly(ethylene glycol).
50, by vol). In the absence of light, the two formulations did not affect the viability of the cells up to an m-THPC concentration of 1.75 mM. Phototoxicity was determined after 24 h dark incubation and irradiation with a light (600–700 nm, PTL Penta quartz halogen lamp, 2 mW cm−2) of 0.12 J cm−2. All cells were killed after incubation with 1.25 mM m-THPC. The dose–response curves obtained by incubating the cells with increasing concentrations of m-THPC delivered by the two formulations were identical but the nanoparticles formulation reduced the cellular uptake of m-THPC by about 50% in comparison to standard solvent. With an elegant Fluorescence Resonance Energy Transfer (FRET) approach, using a cyanine as an acceptor covalently linked to the nanoparticles, the authors demonstrated that m-THPC is transferred from the nanoparticles to serum proteins of the medium. To prevent this transfer to proteins it was necessary to coat the nanoparticles surface with poly(ethylene glycol).
Another type of PS based on hydrophobic silicon phthalocyanine Pc4 (Fig. 6) was encapsulated in ORMOSIL by the method originally developed by Prasad's group with some modifications (replacement of AOT by Tween 80 as surfactant) which led to nanoparticles with a 25–30 nm diameter.28 Compared to the free drug, encapsulation not only improved the aqueous solubility, stability and delivery of the drug but the PDT efficiency as well. Indeed cell viability measurements on A-375 and B16–F10 melanoma cells demonstrated that the nanoparticles were more phototoxic than the free drug. Apoptosis was the major pathway of cell death. Evaluation of the level of intracellular protein-derived peroxides in A-375 melanoma cells after internalization of the nanoparticles and PDT suggested a type II mechanism (phototoxicity through formation of 1O2). Tracking with specific markers showed that more nanoparticles were localized in mitochondria and lysosomes than free PS.
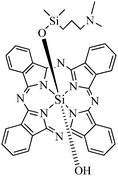 | ||
| Fig. 6 Encapsulated Pc4 photosensitizer. | ||
Simon et al. recently published29 the synthesis and properties of PpIX, (Fig. 5) ORMOSIL nanoparticles prepared by Prasad's method, and performed in vitro and in vivo studies. In vitro experiments showed that for the tested NPs (10 nm, 25 nm and 60 nm), no significant difference of internalization kinetic and total amount of NPs uptake (around 200 fmol per 1000 cells after 1 h of incubation) could be observed. The exact mechanism of internalization remains unknown, but both active and a high proportion of passive process are involved, and it is cell-dependant. PpIX silica NPs effects on cell survival were tested on the HCT 116 colon cancer cell line. The results obtained showed that a 3 h incubation with PpIX silica NPs and 20 min of illumination (50 μW cm−2) gave the best results for cancer cells destructions, i.e. EC50 at 0.44 μM for all sizes of NPs. Moreover, in all tumor types, PpIX silica NPs were more efficient (more than one log of difference) than free PpIX. For both systems, a loss of efficiency was found 5 h after incubation (no residual activity after 24 h). The cell clearance of PpIX was proved to be responsible for the loss of efficiency and the clearance process seems to begin early, before two hours.
There is a strong relationship between the sites of subcellular localization of the PpIX and photodamage to nearby organelles involved in cell death. The intracellular accumulation of PpIX silica NPs takes place in the cytoplasm of cells. Quantification of cell internalization could be realized by measuring the ROS generation by fluorescence spectroscopy, since the ROS generation is correlated to the presence of PpIX silica NPs in both HCT 116 and HT-29 colon cancer cells. Higher ROS amounts cause larger cell damages leading to cell destruction.
In vivo studies on nude tumor-bearing mice were performed to observe the biodistribution of PpIX silica NPs loaded with DID (dioctadecyl tetramethyl indodicarbocyanine chloro benzene) tracer through whole body optical imaging. Images of the biodistribution were recorded after NPs tail vein injection (from 0 to 24 h). For the three cancer models studied (HCT 116, A 549 and glioblastoma), a high tumor upkate of PpIX silica NPs was observed, but the maximal accumulations were reached at different times: 2 h for glioblastoma, 16 h for A549 and 20 h for HCT 116 models. A549 and HCT 116 tumor models behaved similarly, i.e. a slow accumulation to reach a maximum without image changes until 24 h. For glioblastoma, the NPs uptake took place quickly after the injection to reach the maximum after 2 h, while a marked decrease was observed immediately later. NPs also accumulate in healthy tissues such as liver, for which a very high accumulation was observed (higher than that within the tumor mass), but also in spleen, lymph nodes, ovaries, lungs, and adrenal glands. The fluorescence intensities decreased over time in these organs, except for the liver. No urine elimination was observed throughout 24 h.
Recently, the Prasad group used ORMOSIL nanoparticles, embedded with HPPH (Fig. 4) but covalently iodine-concentrated.30 These particles were prepared using (3-iodopropyl)trimethoxysilane and vinyltriethoxysilane. The properties of these nanoparticles were compared with noniodinated nanoparticles (prepared from vinyltriethoxysilane) and it was shown that the intraparticle external heavy-atoms significantly enhanced the efficiency of 1O2 generation and the in vitro PDT efficiency. Indeed, the quantity of 1O2 generated by the iodinated nanoparticles was found to be increased by roughly 1.7 times over the non iodinated nanoparticles. Cell viability assay was carried out for RIF-1 tumor cells and nanoparticles with iodine were the most efficient for PDT applications.
Wang and collaborators entrapped methylene blue (Fig. 2) in a phosphonate-terminated silica matrix by controlled synchronous hydrolysis of tetraethoxysilane and trihydroxyl silyl propyl methyl phosphonate in a water-in-oil microemulsion.17 Spherical nanoparticles were obtained with a mean diameter of 105 nm. 1O2 generation was evaluated using DPBF and estimated to be 0.049. In vitro PDT studies were investigated on HeLa cells. Cytotoxicity was first evaluated. The dead cell percentage of HeLa cells was higher than 80% for concentrations superior to 0.7 mg mL−1. Cell phototoxicity was performed and 90% of the cells were killed with 1 mg mL−1 methylene blue doped nanoparticles upon 30 min light exposure (635 nm laser, 27.5 mW cm−2). Real-time in vivo imaging experiments on BALB/c nude mice bearing MB-encapsulating nanoparticles were performed. The methylene blue doped nanoparticles were injected intravenously into the tail for near-infrared in vivo imaging. Moreover, 12 h post injection, PDT experiments (635 nm laser, 500 mW cm−2) have been realized with success: the tumors got gradually necrosed.
b) Multifunctional silica nanoparticles for PDT and diagnosis
A marked advantage of using nanoparticles is that they can be used as multi-tasks platforms. Indeed, their chemical composition can be modulated to: (i) couple or encapsulate photoactivable units for PDT treatment; (ii) target the nanoparticles to tumour cells or neovascularization by coupling vector units; as well as (iii) use them as a diagnostic agent by incorporating a contrast enhancer; (iv) use them for other therapies such as hyperthermia. For biomedical applications, multifunctional nano-objects combine two or more functions, such as fluorescent markers or photothermal therapy agents with MRI contrast agents or hyperthermia therapy agents.An example of a single particle platform that combines two functions has been described by Rossi and collaborators18 in 2007. Silica-coated magnetic nanoparticles containing methylene blue (Fig. 2) as a PS have been prepared and therefore combine therapy (PDT) and diagnostic (MRI contrast agent) possibilities. The particles were composed of silica spheres of about 30 nm diameter containing 11 nm-diameter magnetic particles. The PS, methylene blue, was added to the silica precursor tetraorthosilicate during the growth of the silica layer and was therefore entrapped in the silica matrix. The magnetic cores were prepared by coprecipitation of Fe2+/Fe3+ ions under alkaline conditions followed by stabilization with tetraethylammonium oxide. The immobilized drug could generate 1O2, detected by its characteristic phosphorescence decay curve in the near infra-red and by a chemical method using DPBF to trap 1O2. The encapsulation of the PS in the silica led to a 1O2 quantum yield of 3 ± 2%, while the quantum yield of methylene blue free in acetonitrile was 50%. This difference was also reported by Tang et al.31 This can be due to scattering of nanoparticles, local sequestration of 1O2 and/or intrinsic lower encapsulated methylene blue 1O2 quantum yield. The magnetization curve confirmed the superparamagnetic behavior of the particle. This type of platform has yet to be tested for in vitro experiments of uptake in cells and cell viability.
Another example of potentially interesting magnetic nanocarriers for PDT was reported by Zhou and collaborators.20 Fe3O4 nanoparticles were coated with a silica layer using tetraethoxysilane in a cottonseed oil using reverse microemulsion method in presence of purpurin-18 (Fig. 7) as a PS.
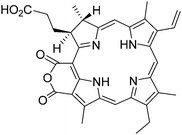 | ||
| Fig. 7 Purpurin-18. | ||
Nanoparticles with a 20–30 nm diameter were obtained. The characterization of the PS in particle was achieved by UV-visible spectroscopy. The generation of 1O2 was followed by N,N-dimethyl-4-nitrosoaniline (RNO) bleaching assay, i.e. the measure of the decrease of absorbance at 440 nm of RNO, a selective scavenger which was oxidized into RNO2 in the presence of an imidazole unit as a chemical trap, was found to be less effective for the encapsulated purpurin than for the free PS.
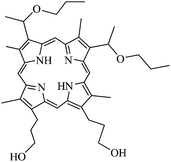
Further studies were conducted by Chen, Zhou and co-workers.32 Silica covered Fe3O4 magnetic nanoparticles including 2,7,12,18-tetramethyl-3,8-di(1-propoxyethyl)-13,17-bis-(3-hydroxypropyl)porphyrin (PHPP, Fig. 8) as a PS were prepared. Fe3O4 nanoparticles were prepared by co-precipitation and were about 10 ± 2 nm in diameter. AOT/1-butanol/water micelles method was combined with sol–gel method in presence of PHPP to coat the magnetic particles with silica. The resulting particles were about 20–30 nm in diameter but some agglomeration could be observed, as shown by TEM images. The encapsulation of the PS was checked by FT-IR measurements and photoluminescence of PHPP. The efficiency of PHPP encapsulation was estimated by UV-visible spectroscopy after destruction of Fe3O4 cores of the nanoparticles and was found to be around 21%. The RNO-bleaching method allowed a significant 1O2 generation. In vivo photodynamic efficacy was evaluated on SW480 colon carcinoma cells. No significant dark toxicity was detected even for a concentration as high as 80 μmol L−1. PDT experiments were performed on the same cell lines and significant PDT efficacy (40% cell viability lost) was found (24 h incubation time, 10 min 488 nm laser irradiation, 4.35 J cm−2). The potential of the magnetic core for imaging and therapy is yet to be tested.
Zinc(II) phthalocyanines (ZnPc, Fig. 9) are second generation photosensitizers useful for PDT applications on HeLa cancer cells. However, the low solubility of these compounds result in the formation of aggregates in aqueous media, and thus in a loss of efficiency. H.-J. Kim et al.33 included ZnPc into Fe3O4-loaded mesoporous silica nanoparticles (MSN). MSN have been used for various biomedical applications such as cell markers or drug and gene delivery platform. MSN possess advantages such as large surface area and pore volume, as well as uniform pore size. They are both a suitable carrier for hydrophobic molecules and a way to protect the PS from degradation. ZnPc- and Fe3O4-loaded MSN (ZnPc/Fe3O4/MSN) were prepared through a one-pot procedure under basic conditions in the presence of cetyltrimethylammonium bromide CTAB as structuring agent and Fe3O4 nanoparticles. Near spherical or rounded cubic nanoparticles were obtained with a size in the range of 60–120 nm. According to narrow-angle X-ray diffraction pattern of MSN and ZnPc/Fe3O4/MSN, a slightly disordered mesoporous structure was observed. A widening of the pore size from 3.6 nm to ∼4 nm was also calculated. Wide-angle X-ray diffraction pattern of ZnPc/Fe3O4/MSN displayed the characteristic peaks of the amorphous silica matrix and the cubic inverse spinel Fe3O4. Magnetic measurements showed a superparamagnetic behavior with high saturation magnetization of 1.8 emu g−1 at 1.0 T, which is enough for MRI and magnetic hyperthermia applications. UV-visible spectroscopy showed the presence of non-aggregated ZnPc molecules entrapped in the silica matrix. However, experiments concerning singlet oxygen generation or PDT still have to be carried out. Drug delivery experiments were performed with ibuprofen as a guest molecule. The loading of ibuprofen was realized by dispersing and stirring MSN or ZnPc/Fe3O4/MSN in a solution of ibuprofen in hexane and the release of ibuprofen in distilled water could be monitored by UV-visible spectroscopy. It appeared that ZnPc/Fe3O4/MSN showed a more delayed and steady release of ibuprofen compared to MSN. This effect could be attributed to the interaction between ibuprofen and the ZnPc entrapped in the silica matrix.
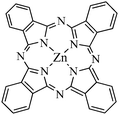 | ||
| Fig. 9 Zinc(II) phthalocyanines (ZnPc). | ||
Liu et al.34 described the preparation of photodynamic drug nanocarriers consisting of magnetite and photosensitizer (PHPP, Fig. 8), surrounded by a silica shell through a sol–gel process in a micellar media. TEM analyses showed nanocarriers with a nearly spherical shape in the range of 30–50 nm which tend to aggregate. XRD analyses showed the typical patterns of magnetite in addition to the broad diffraction band at ca. 2θ = 20° corresponding to the amorphous silica matrix. The photosensitizer entrapped in the silica matrix was detected by FT-IR and UV-visible spectroscopy. Upon irradiation using 637 nm laser, 1O2 was produced and released from the nanocarriers. The RNO-bleaching method, allowed the determination the 1O2 generating efficiency. Compared to the free PHPP, the results obtained showed that: (1) the photosensitivity of the nanocarriers was less but still effective for potential PDT applications; and (2) the presence of Fe3O4 and silica shell had no critical effect on the production and release of 1O2.
NIR-to-visible up-conversion nanomaterials emit higher energy photons after absorbing lower energy photons. Qian et al.35 described the synthesis of mesoporous-silica-coated NaYF4@silica nanoparticles with a core–shell structure containing ZnPc (Fig. 9) molecules as photosensitizer in the mesoporous silica matrix. The absorption peak of ZnPc located at 670 nm overlaps with the red emission peak of the up-conversion of the NaYF4 nanocrystals, what is a suitable situation for the activation of ZnPc and the further production of 1O2. NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb/Er nanocrystals (35 nm width × 60 nm length) were coated by an amorphous silica shell (10 nm ± 1.5 nm) via a microemulsion method, and then by a mesoporous silica layer (11 nm ± 1.5 nm) onto the NaYF4
Yb/Er nanocrystals (35 nm width × 60 nm length) were coated by an amorphous silica shell (10 nm ± 1.5 nm) via a microemulsion method, and then by a mesoporous silica layer (11 nm ± 1.5 nm) onto the NaYF4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Yb/Er@silica nanoparticles. N2-Absorption/desorption isotherms confirmed the mesoporous nature of those outer silica shell with a BET surface area of 770 m2 g−1. Pore size distribution has a mean size of 2 nm. A good biocompatibility was observed for these nanoparticles. After the incubation of the nanoparticles with MB49 bladder cancer cells for 24 h and washing, the subsequent intracellular localization of the nanoparticles could be realized using a confocal microscope equipped with a 980 nm NIR laser. Fluorescence of the nanoparticles was mainly observed in the cytoplasm. The introduction of a PS such as ZnPc into the mesoporous silica shell was realized by soaking the nanoparticles in a concentrated solution of ZnPc in pyridine. Then, the release of ZnPc in a different solvent was studied. It appeared that ZnPc was released within one hour in ethanol, but no release was observed in aqueous media, such as DI water, PBS or cell culture media. The production of 1O2 for further PDT applications was shown by exposing the NaYF4/ZnPC nanoparticles to NIR laser irradiation in the presence of 9,10-anthracenediylbis(methylene)dimalonic acid (ABDA) which can react with 1O2 to form the endoperoxide. The decreased amount of ABDA, and thus the efficiency in 1O2 formation by the NaYF4/ZnPC nanoparticles, could be estimated by measuring fluorescence decay at 431 nm when excited at 380 nm. The data obtained show an increased destruction of ABDA with the exposure time. In contrast, the fluorescence of ABDA remained unchanged when the same experiments were carried out with the NaYF4 nanoparticles without ZnPc. In vitro experiments showed that the viability of cells incubated with the NaYF4/ZnPc nanoparticles and exposed to NIR irradiation for 5 min was significantly lower than those incubated with ZnPc-free NaYF4 nanoparticles.
Yb/Er@silica nanoparticles. N2-Absorption/desorption isotherms confirmed the mesoporous nature of those outer silica shell with a BET surface area of 770 m2 g−1. Pore size distribution has a mean size of 2 nm. A good biocompatibility was observed for these nanoparticles. After the incubation of the nanoparticles with MB49 bladder cancer cells for 24 h and washing, the subsequent intracellular localization of the nanoparticles could be realized using a confocal microscope equipped with a 980 nm NIR laser. Fluorescence of the nanoparticles was mainly observed in the cytoplasm. The introduction of a PS such as ZnPc into the mesoporous silica shell was realized by soaking the nanoparticles in a concentrated solution of ZnPc in pyridine. Then, the release of ZnPc in a different solvent was studied. It appeared that ZnPc was released within one hour in ethanol, but no release was observed in aqueous media, such as DI water, PBS or cell culture media. The production of 1O2 for further PDT applications was shown by exposing the NaYF4/ZnPC nanoparticles to NIR laser irradiation in the presence of 9,10-anthracenediylbis(methylene)dimalonic acid (ABDA) which can react with 1O2 to form the endoperoxide. The decreased amount of ABDA, and thus the efficiency in 1O2 formation by the NaYF4/ZnPC nanoparticles, could be estimated by measuring fluorescence decay at 431 nm when excited at 380 nm. The data obtained show an increased destruction of ABDA with the exposure time. In contrast, the fluorescence of ABDA remained unchanged when the same experiments were carried out with the NaYF4 nanoparticles without ZnPc. In vitro experiments showed that the viability of cells incubated with the NaYF4/ZnPc nanoparticles and exposed to NIR irradiation for 5 min was significantly lower than those incubated with ZnPc-free NaYF4 nanoparticles.
Another approach has been published by Zhang et al. describing a new concept based on photon up-converting nanoparticles (PUNPs).36 Up-conversion, in which excitation light at a longer wavelength produces emission at a shorter wavelength is very promising since with PUNPs, it becomes possible to excite the photosensitizers in the near IR. NaYF4/Yb3+, Er3+nanoparticles have been used as PUNP and coated with a PS (Merocyanine 540, Fig. 10)-encapsulating porous thin layer of silica. A mouse monoclonal antibody, anti-MUC1/episialin which is highly specific towards MCF-7/AZ cancer cells was covalently attached to the silica surface. The generation of 1O2 was detected chemically using ADPA. In vitro PDT tests were performed to confirm the PDT cytotoxicity of the particles towards MCF-7/AZ breast cancer cells, thus demonstrating the potential of these IR-region excitable nanoparticles.
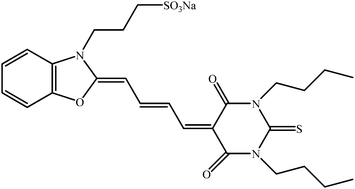 | ||
| Fig. 10 Merocyanine 540. | ||
Two-photon absorption (TPA)-induced excitation of photosensitizers is another promising approach to increase light penetration. Indeed, the photosensitizers can absorb simultaneously two less-energetic photons, the excitation in the near infra-red (IR) region avoids tissue absorbing or scattering and induces a deeper light penetration in the tissue.
Velusamy et al.16 synthesized new quadrupolar chromophores for two-photon absorption (Fig. 11) with a very high two-photon absorption cross-section of 7080 GM at 800 nm in toluene and a fluorescence quantum yield of 0.25. The compound was insoluble in water and was thus encapsulated in SiO2 nanoparticles following the reverse microemulsion method. The nanoparticles were of a 60 nm diameter. The fluorescence quantum yield dropped to 0.08 with a red shift of λem to 539 nm. The quantum yield of 1O2 generation of the nanoparticles was determined to be 0.51 in D2O. Those nanoparticles were incubated with macrophages and shown to be tightly bound to the membrane with no toxicity as determined by MTT assay. Macrophage cells were then irradiated with a two-photon laser at 2.6 W cm−2 (800 nm) for 3 min. The results indicated a significant and NP-dose responsive cell death, thus demonstrating the suitability of the nanoparticles for two-photon PDT.
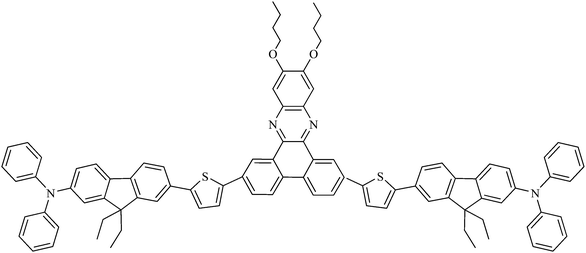 | ||
| Fig. 11 2-Photon PS. | ||
The Prasad group described the synthesis15 of organically modified silica nanoparticles co-encapsulating HPPH (Fig. 4) and an excess amount of BDSA (9,10-bis(4′-(4′′-aminostyryl)styryl)anthracene, Fig. 12), a highly two-photon active molecule (TPA cross section in the aggregated state at 750 nm = 217 GM) as a donor. The size of nanoparticles entrapping 1.1 wt % HPPH and 1.1 wt % HPPH/20 wt % BDSA was 25 ± 7 nm and 30 ± 6 nm, respectively. HPPH absorption in nanoparticles had a significant overlap with the fluorescence of BDSA aggregates which enabled an efficient energy transfer through FRET (Förster Resonance Energy Transfer) mechanisms. After indirect two-photon excitation (850 nm) the authors proved that the energy of the near-IR light is efficiently up-converted by BDSA aggregates to excite HPPH. After excitation at 532 nm in D2O, the characteristic 1O2 emission at 1270 nm was observed under photoexcitation of the dispersion of nanoparticles co-encapsulating HPPH and BDSA. For the two-photon sensitization of 1O2 in water, ADPA was used and the authors could also observe the formation of 1O2.
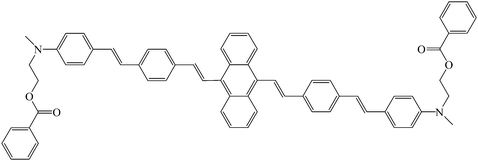 | ||
| Fig. 12 9,10-Bis(4′-(4′′-aminostyryl)styryl)anthracene (BDSA). | ||
Hela cells were treated with the nanoparticles and two-photon laser scanning microscopic images revealed an intense fluorescence signal from the cytoplasm. The intracellular FRET efficiency was estimated up to 36%. Drastic changes of the morphology of the cells were observed after the cells were treated overnight and irradiated in the same conditions. This is in good agreement with the formation of 1O2 after two-photon excitation. As a conclusion, this new concept of two-photon induced intra-particle FRET offers a simple and proper methodology for developing formulations with better photophysical properties.
II. Covalent encapsulation of PS in silica nanoparticles
When physically entrapped inside the silica network, the PS can be prematurely released from the carrier which can lead to a reduced efficiency of treatment and to side-effects. Covalent coupling of the PS inside the nanoparticles is expected to overcome these drawbacks.Davydenko et al.37 studied the sensibilization of C60 fullerene anchored through covalently bonded spacers to hydrophilic non-porous pyrogenic silica nanoparticles of 10 nm diameter modified by γ-aminopropyl groups. The ability of fullerene to generate active forms of oxygen was demonstrated using photosenstitive organic molecules. No in vitro or in vivo studies have been done, but these first results suggested that these systems might be useful as fluorescent probes and active species for PDT.
Rossi and collaborators have covalently encapsulated PpIX by coupling the acid groups with aminopropyltriethoxysilane (Fig. 13).38 The Stöber procedure was used and led to particles of 77 ± 12 nm. 1O2 generation was characterized by its phosphorescence spectra at 1270 nm and by reaction with DPBF. The efficiency of 1O2 generation was determined to be 0.9 ± 0.06 for the nanoparticles. This efficiency was higher than the free porphyrin in CCl4 solution (1O2 quantum yield of 0.77). These nanoparticles were not tested in vitro.
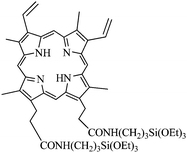 | ||
| Fig. 13 Dimethyl-8,13-divinyl-3,7,12,17-tetramethyl-21H,23H-porphyrine-2,18-dipropylamidepropyltriethoxysilane (silyl-PpIX). | ||
Prasad and collaborators first reported the covalent incorporation of PS molecules into ORMOSIL nanoparticles.39 Iodobenzylpyrosilane (IPS, Fig. 14), a precursor for ORMOSIL was synthesized with the linked PS iodobenzylpyropheophorbide (IP).
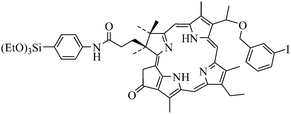 | ||
| Fig. 14 Iodobenzylpyrosilane (IPS). | ||
The coprecipitation of IPS with the commonly used ORMOSIL precursor vinyltriethoxysilane in the nonpolar core of Tween-80/water microemulsion led to the formation of a monodispersed aqueous dispersion of ORMOSIL with covalently linked IP. The particles were of about 20 nm diameter. Since the covalently incorporated PS molecules were found to retain their spectroscopic and functional properties such as absorption or fluorescence independently of the IPS/VTES ratio used, the authors concluded that no aggregation occurred and that it could be possible to increase the relative content of IPS within the nanoparticles to obtain more 1O2. To be sure that the generated 1O2 was not deactivated within the nanoparticles, they used the 1O2 mediated bleaching of ADPA. They proved that 1O2 was mainly deactivated outside the nanoparticles. Upon photoirradiation, cytotoxic 1O2 molecules were generated. Therefore, the nanoparticles were tested for cellular uptake in vitro, on Colon-26 cells and proved to exhibit a manifest phototoxic effect on the cultured cells proportional to the cellular uptake. In addition, the highest photodynamic effect was associated to the highest content of the PS within the nanoparticles, thus demonstrating the potential of the nanoparticles for PDT.
Mou and collaborators have reported conjugation of PpIX (Fig. 5) with MSN through covalent bonding to yield PpIX-modified MSN (Fig. 15) for PDT studies.14
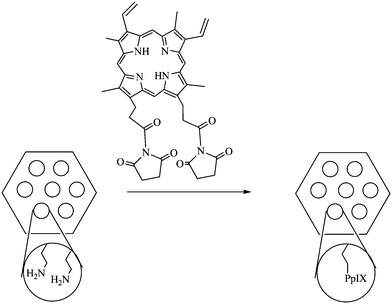 | ||
| Fig. 15 PpIX-modified pores of MSN. | ||
These particles were obtained by using classic MCM-41 type direct microemulsion synthesis, leading to 110 nm diameter objects. The MSN were then functionalized with 3-aminopropyltrimethoxysilane, allowing the grafting of activated PpIX (Fig. 11). The porous nature of the particles lead to site-isolation of the PS which avoided self-quenching of aggregated PpIX. The absorption spectrum of the PS-grafted nanoparticles was similar to that of the free PpIX, showing no change of the chromophore upon entrapment in the MSN. Moreover, samples of PpIX grafted to MSN were shown to have a better integrity under laser irradiation compared to free PpIX. 1O2 generation was checked both by monitoring the 1O2 luminescence and by using DPBF as a detector. Dark cytotoxicity was estimated for HeLa cells and the cell viability was not affected at dosage up to 80 μg mL−1. Uptake of the PpIX-modified particles by HeLa cells was quite efficient (higher than 70% for 20–80 μg mL−1 concentrations after a total of 6 h incubation). An almost linear relationship between cellular uptake and dosage was obtained. Phototoxicity was studied and all the cells were killed (6 min tungsten lamp irradiation, 115 mW cm−2, 80 μg mL−1). Both dosage- and irradiation time-dependent cell viability were observed. Depending on the concentration, both necrosis and apoptosis could be observed.
The same group prepared MSN then grafted a phosphorescent Pd-meso-tetra(4-carboxyphenyl) porphyrin (PdTPP, Fig. 16) as the PS.13 This metalloporphyrin is frequently used to measure oxygen distribution in tissues via oxygen-dependent quenching of phosphorescence.
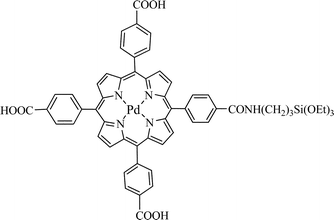 | ||
| Fig. 16 Pd-meso-tetra(4-carboxyphenyl) porphyrin (PdTPP). | ||
1O2 generation formed by photoirradiation of the nanoparticles was measured both by direct luminescence at 1270 nm in D2O and EtOH and by its reaction with DPBF. A significant photo-induced cytotoxicity at 532 nm was observed for MDA-MB-231 breast cancer cells. However, the reason for this photo-induced toxicity was not very clear but could be explained by the high concentration of PdTPP in the MSN as well as the fact that MSN facilitated the endocytotic cell uptake that dramatically increased the intracellular density of 1O2 upon photoirradiation.
Further progress in the field of silica nanoparticles for PDT was achieved when multifunctional particles were functionalized by a biomolecule able to specifically target cancer cells. Active targeting represents an obvious improvement for PDT. Until now, most of the efforts in the development of tumor targeting-photosensitizers have focused on the targeting of markers over-expressed by tumor cells themselves, even if anti-vascular strategies are being more-and-more important.4,7
In order to specifically target cancer cells, MSN were functionalized by a biomolecule able to target cancer cells. As specific bioreceptors are overexpressed at the surface of cancer cells in many tumors, functionalizing the particle in order to target these receptors would enhance the uptake of the nanoparticles by these cells. Novel MSN12 were synthesized combining covalent anchoring of the water soluble photosensitizer (Fig. 17) to the mesoporous silica matrix and targeting of cancer cells with mannose attached on the surface of MSN. Targeting breast cancer cells (MDA-MB-231) with mannose was essential to get a high PDT efficiency. Indeed the involvement of mannose receptors in the active endocytosis of mannose-functionalized MSN was demonstrated.
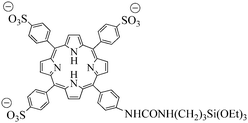 | ||
| Fig. 17 Water-soluble porphyrin. | ||
In the promising field of using silica nanoparticles as multifunctional platform for imaging and therapy, R. Zhang et al.40 described the synthesis of a highly efficient multifunctional nanocomposite material that contains both a nonporous FITC-doped silica core for fluorescence imaging and a mesoporous silica shell containing hematoporphyrin (HP) as a photosensitizer. Non porous FITC@SiO2 core were synthesized via a water-in-oil reverse microemulsion. The hematoporphyrin conjugated mesoporous silica nanoparticles were synthesized with TEOS and APTS-HP (Fig. 18) via a sol–gel O/W co-condensation reaction. The non porous silica core isolated the fluorescent dye from the external environment to avoid photobleaching. On the contrary, the mesoporous silica shell allowed the diffusion of 1O2 outside of the nanoparticle. TEM images revealed a monomodal particle size distribution around 57 nm for mesoporous core–shell nanoparticles and 37 nm for silica cores. Disodium 9,10-anthracenediyl-bis-(methylene)dimalonic acid (ABMD) was used to chemically check the capacity of such material to produce 1O2. A significant decrease of ABMD absorption for SiO2@HP and HP alone was observed, but for the same amount of HP, the decrease was more important for SiO2@HP or FITC@SiO2@HP than for HP alone, due to a higher stability of PS covalently linked in a silica shell. Moreover, the FITC inside the non-porous silica shell was well protected from the damage by 1O2 (no photobleaching). Finally, the imaging capability was demonstrated by cell imaging on HO-8910 PM cells. Confocal microscopy on such cells showed that most of the nanoparticles were localized in the membrane of the cell and a few inside the cell.
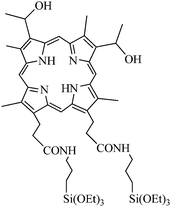 | ||
| Fig. 18 APTS-HP. | ||
Hsiao and collaborators reported the synthesis of a multifunctional nanomaterial conjugating a functionalized iridium complex with a Fe3O4/SiO2 core/shell nanocomposite.19 The magnetic core provides capability for MRI. The silica shell allows the embedment of the PS, with sufficient space for oxygen diffusion. A third row transition metal (Ir(III)) complex was chosen as the PS to serve both as a photosensitizer and as a luminescent moiety. An hydrophilic iridium(III) complex (Fig. 19) was developed to generate a good affinity with the silica shell.
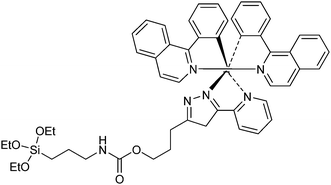 | ||
| Fig. 19 Ir(III) complex. | ||
The size of the nanoparticles was determined by TEM, 55 ± 5 nm for Fe3O4/SiO2(Ir) nanoparticles compared to 12 ± 1 nm for Fe3O4. Upon aeration, both emission intensity and observed lifetime decreased drastically, suggesting an O2 quenching process presumably resulting in 1O2 production. PDT experiments were performed on HeLa cells. The cellular uptake and imaging ability were confirmed by MRI revealing that the cellular-uptake was dose-responsive and that the uptake could be imaged by MRI at low concentration (6.25 μg mL−1). The cytotoxicity of the particles towards HeLa cells was demonstrated upon irradiation. Evidence of 1O2-induced apoptosis was found. This is the first example of a multifunctional particle, combining MRI, phosphorescence and PDT properties. The main drawback of this approach is the 366 nm irradiation, which is not really compatible with a good penetration of light in the tissue.
Conclusion
Simple and proper methodologies to develop formulations of silica nanoparticles applicable for PDT applications are currently being developed. Depending on the synthetic method, nanoparticles of different controlled size and porosity can be elaborated. Moreover, thanks to the easy surface modification of silica particles, addition to cancer biomarkers can guide the particle to specifically targeted cancer cells resulting in less damage of healthy cells. Recent developments concern the use of silica nanoparticles that can be excited by IR or two-photon in order to treat tissues more deeply. New researches are also focused on multifunctional nanoparticles that could serve both as diagnosis tools and therapeutic agents. Thus, we believe that nanoparticles gathering: (i) organized porosity; (ii) covalent coupling of the photosensitizer; (iii) specific core that allow for example MRI for diagnosis; and (iv) a vector may be an important advancement for the future of PDT.Abbreviations
| ABDA | 9,10-Anthracenediyl-bis(methylene)dimalonic acid |
| ABMD | Disodium 9,10-anthracenediyl bis(methylene)dimalonic acid |
| ADPA | Anthracene-9,10-dipropionic acid |
| APTS | 3-(Aminopropyl)triethoxysilane |
| ALA | 5-Aminolaevulinic acid |
| AOT | Sodium bis(2-ethylhexyl) sulfosuccinate |
| BDSA | 9,10-bis (4′-(4′′-Aminostyryl)styryl)anthracene |
| DID | Dioctadecyl tetramethyl indodicarbocyanine chloro benzene |
| DMSO | Dimethylsulfoxide |
| DPBF | 1,3-Diphenylisobenzofuran |
| EPR | Enhanced permeability and retention |
| FITC | Fluorescein isothiocyanate |
| FRET | Förster resonance energy transfer |
| HP | Hematoporphyrin |
| HPPH | 2-Devinyl-2-(1 hexyloxyethyl)pyropheophorbide |
| IP | Iodobenzylpyropheophorbide |
| IPS | Iodobenzylpyrosilane |
| MSN | Mesoporous silica nanoparticles |
| MRI | Magnetic resonance imaging |
| m-THPC | meso meta-Tetra(hydroxyphenyl)chlorin |
| NPs | Nanoparticles |
| ORMOSIL | Organically modified silica |
| PDT | Photodynamic therapy |
| PpIX | Protoporphyrin IX |
| PS | Photosensitizers |
| ROS | Reactive oxygen species |
| NP | Nanoparticle |
| PdTPP | Pd-meso-tetra(4-carboxyphenyl) porphyrin |
| PHPP | 2,7,12,18-Tetramethyl-3,8-di(1-propoxyethyl)-13,17-bis-(3-hydroxypropyl)porphyrin |
| PUNP | Photon up-converting nanoparticle |
| RNO | N,N-Dimethyl-4-nitrosoaniline |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| TPA | Two-photon absorption |
| TEOS | Tetraethoxysilane |
| XRD | X-Ray diffraction |
| ZnPc | Zinc(II) phthalocyanine |
References
- T. Maisch, Mini-Rev. Med. Chem., 2009, 9, 974–983 CAS.
- C. A. Robertson, D. H. Evans and H. Abrahamse, J. Photochem. Photobiol., B, 2009, 96, 1–8 CrossRef CAS.
- B. Ortel, C. R. Shea and P. Calzavara-Pinton, Front. Biosci., 2009, 4157–4172 CrossRef CAS.
- J. P. Taquet, C. Frochot, V. Manneville and M. Barberi-Heyob, Curr. Med. Chem., 2007, 14, 1673–1687 CrossRef CAS.
- A. S. Hoffman, J. Controlled Release, 2008, 132, 153–163 CrossRef CAS.
- R. R. Allison, H. C. Mota, V. S. Bagnato and C. H. Sibata, Photodiagn. Photodyn. Ther., 2008, 5, 19–28 CrossRef CAS.
- D. Bechet, P. Couleaud, C. Frochot, M.-L. Viriot, F. Guillemin and M. Barberi-Heyob, Trends Biotechnol., 2008, 26, 612–621 CrossRef CAS.
- D. K. Chatterjee, L. S. Fong and Y. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1627–1637 CrossRef CAS.
- S. Wang, R. Gao, F. Zhou and M. Selke, J. Mater. Chem., 2004, 14, 487–493 RSC.
- W. Stober, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef.
- Y. Piao, A. Burns, J. Kim, U. Wiesner and T. Hyeon, Adv. Funct. Mater., 2008, 18, 3745–3758 CrossRef CAS.
- D. Brevet, M. Gary-Bobo, L. Raehm, S. Richeter, O. Hocine, K. Amro, B. Loock, P. Couleaud, C. Frochot, A. Morere, P. Maillard, M. Garcia and J. O. Durand, Chem. Commun., 2009, 1475–1477 RSC.
- S.-H. Cheng, C.-H. Lee, C.-S. Yang, F.-G. Tseng, C.-Y. Mou and L.-W. Lo, J. Mater. Chem., 2009, 19, 1252–1257 RSC.
- B. H.-L. Tu, Y.-S. Lin, Y. Hung, L.-W. Lo, Y.-F. Chen and C.-Y. Mou, Adv. Mater., 2009, 21, 172–174 CrossRef.
- S. Kim, T. Y. Ohulchanskyy, H. E. Pudavar, R. K. Pandey and P. N. Prasad, J. Am. Chem. Soc., 2007, 129, 2669–2675 CrossRef CAS.
- M. Velusamy, J. Y. Shen, J. T. Lin, Y. C. Lin, C. C. Hsieh, C. H. Lai, C. W. Lai, M. L. Ho, Y. C. Chen, P. T. Chou and J. K. Hsiao, Adv. Funct. Mater., 2009, 19, 2388–2397 CrossRef CAS.
- X. He, X. Wu, K. Wang, B. Shi and L. Hai, Biomaterials, 2009, 30, 5601–5609 CrossRef CAS.
- D. B. Tada, L. L. R. Vono, E. L. Duarte, R. Itri, P. K. Kiyohara, M. S. Baptista and L. M. Rossi, Langmuir, 2007, 23, 8194–8199 CrossRef CAS.
- C.-W. Lai, Y.-H. Wang, C.-H. Lai, M.-J. Yang, C.-Y. Chen, P.-T. Chou, C.-S. Chan, Y. Chi, Y.-C. Chen and J.-K. Hsiao, Small, 2008, 4, 218–224 CrossRef CAS.
- F. Liu, X. Zhou, Z. Chen, P. Huang, X. Wang and Y. Zhou, Mater. Lett., 2008, 62, 2844–2847 CrossRef CAS.
- I. Roy, T. Y. Ohulchanskyy, H. E. Pudavar, E. J. Bergey, A. R. Oseroff, J. Morgan, T. J. Dougherty and P. N. Prasad, J. Am. Chem. Soc., 2003, 125, 7860–7865 CrossRef CAS.
- F. Yan and R. Kopelman, Photochem. Photobiol., 2003, 78, 587–591 CrossRef CAS.
- W. Tang, H. Xu, R. Kopelman and M. A. Philbert, Photochem. Photobiol., 2005, 81, 242–249 CrossRef CAS.
- J. Zhou, L. Zhou, C. Dong, Y. Feng, S. Wei, J. Shen and X. Wang, Mater. Lett., 2008, 62, 2910–2913 CrossRef CAS.
- L. Zhou, J.-H. Liu, J. Zhang, S.-H. Wei, Y.-Y. Feng, J.-H. Zhou, B.-Y. Yu and J. Shen, Int. J. Pharm., 2010, 386, 131–137 CrossRef CAS.
- J. Qian, A. Gharibi and S. He, J. Biomed. Opt., 2009, 14, 014012 CrossRef.
- C. Compagnin, L. Bau, M. Mognato, L. Celotti, G. Miotto, M. Arduini, F. Moret, C. Fede, F. Selvestrel, I. M. R. Echevarria, F. Mancin and E. Reddi, Nanotechnology, 2009, 20, 345101 CrossRef.
- B. Z. Zhao, J. J. Yin, P. J. Bilski, C. F. Chignell, J. E. Roberts and Y. Y. He, Toxicol. Appl. Pharmacol., 2009, 241, 163–172 CrossRef CAS.
- V. Simon, C. Devaux, A. Darmon, T. Donnet, E. Thiénot, M. Germain, J. Honnorat, A. Duval, A. Pottier, E. Borghi, L. Levy and J. Marill, Photochem. Photobiol., 2010, 86, 213–222 CrossRef CAS.
- S. Kim, T. Y. Ohulchanskyy, D. Bharali, Y. H. Chen, R. K. Pandey and P. N. Prasad, J. Phys. Chem. C, 2009, 113, 12641–12644 CrossRef CAS.
- W. Tang, H. Xu, R. Kopelman and M. A. Philbert, Photochem. Photobiol., 2005, 81, 242–249 CrossRef CAS.
- Z. L. Chen, Y. Sun, P. Huang, X. X. Yang and X. P. Zhou, Nanoscale Res. Lett., 2009, 4, 400–408 Search PubMed.
- H. J. Kim, K. J. Shin, M. K. Han, K. An, J. K. Lee, I. Honma and H. Kim, Scr. Mater., 2009, 61, 1137–1140 CrossRef CAS.
- F. Liu, X. Zhou, S. Ni, X. Wang, Z.Y. and Z. Chen, Curr. Nanosci., 2009, 5, 293–296 Search PubMed.
- H. S. Qian, H. C. Guo, P. C. L. Ho, R. Mahendran and Y. Zhang, Small, 2009, 5, 2285–2290 CrossRef CAS.
- P. Zhang, W. Steelant, M. Kumar and M. Scholfield, J. Am. Chem. Soc., 2007, 129, 4526–4527 CrossRef CAS.
- M. O. Davydenko, E. O. Radchenko, V. M. Yashchuk, I. M. Dmitruk, Y. I. Prylutskyy, O. P. Matishevska and A. A. Golub, J. Mol. Liq., 2006, 127, 145–147 CrossRef CAS.
- L. M. Rossi, P. R. Silva, L. L. R. Vono, A. U. Fernandes, D. B. Tada and M. S. Baptista, Langmuir, 2008, 24, 12534–12538 CrossRef CAS.
- T. Y. Ohulchanskyy, I. Roy, L. N. Goswami, Y. Chen, E. J. Bergey, R. K. Pandey, A. R. Oseroff and P. N. Prasad, Nano Lett., 2007, 7, 2835–2842 CrossRef CAS.
- R. R. Zhang, C. L. Wu, L. L. Tong, B. Tang and Q. H. Xu, Langmuir, 2009, 25, 10153–10158 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |