Controlling the volumetric parameters of nitrogen-doped carbon nanotube cups†
Brett L.
Allen
ab,
Matthew B.
Keddie
a and
Alexander
Star
*ab
aDepartment of Chemistry, University of Pittsburgh, Pittsburgh, PA 15260, USA. E-mail: astar@pitt.edu; Tel: +412-624-6493
bNational Energy Technology Laboratory, Pittsburgh, PA 15236, USA
First published on 17th March 2010
Abstract
Analogous to multiwalled carbon nanotubes, nitrogen-doped carbon nanotube cups (NCNCs) have been synthesized with defined volumetric parameters (diameter and segment lengths) by controlling the catalyst particle size and the concentration of nitrogen precursor utilized in the chemical vapor deposition (CVD) reaction, allowing for tailored interior cavity space of cross-linked NCNCs, i.e. nanocapsules.
Chemical doping of carbon nanomaterials, especially carbon nanotubes (CNTs), has attracted much interest because of the ability to modify their electronic properties.1 For instance, such modification has been performed by B and N doping.2 It has been theoretically modeled and experimentally confirmed that N-doped single-walled carbon nanotubes exhibit metallic behavior.3 Furthermore, the incorporation of nitrogen atoms induces structural defects resulting in novel bamboo-like fibers.4 Essentially, induction of nitrogen during the synthesis of graphitic CNTs results in the formation of pentagons (as opposed to hexagons) increasing the curvature of the lattice,5 observed as defects and bamboo-like morphology. If this concentration is further increased, it is possible to synthesize stacked cup-like fibers comprised of individual conical segments.6
Previously, we have demonstrated the synthesis of multiwalled, stacked cup-like carbon nanotubes, known as nitrogen-doped carbon nanotube cups (NCNCs), through the incorporation of a nitrogen precursor during the CVD reaction.7 These fibers, presumably because of the presence of nitrogen functionalities, exhibited electrocatalytic activity towards oxygen reduction.8 Furthermore, treatment through mechanical grinding allowed for the separation of individual segments or “cups” from the fiber. Studies by atomic force microscopy (AFM) revealed the presence of a large concentration of nitrogen functionalities decorating the open basal plane of individual cups, allowing for the possibility of bioconjugation.7 By the addition of glutaraldehyde, a suspension of hollow, separated cups can be cross-linked to form nanocapsules capable of encapsulating a variety of cargo such as gold nanoparticles (GNPs) or quantum dots.9 Such studies demonstrate the versatility of synthesized nanostructures in terms of both morphology and electronic properties; however, the ability to control the volumetric parameters of synthesized NCNCs and, in turn, the interior cavity space of nanocapsules has yet to be demonstrated. In this paper, the volumetric parameters (diameter and segment length) of NCNCs were controlled by the use of monodispersed iron nanoparticles and varying the nitrogen precursor (NH3) concentration. We demonstrate that the diameter of individual cups can be tuned by the use of defined diameter catalyst particles. Moreover, segment length is demonstrated to be inversely related to nitrogen precursor concentration.
Iron nanoparticles (FeNPs) were synthesized by the thermal decomposition of iron pentacarbonyl (Fe(CO)5) as reported previously.10 Briefly, approximately 2 mmol of Fe(CO)5 was mixed with 5 mmol of a fatty acid consisting of either oleic, lauric, or octanoic acid in 10 mL of dioctyl ether. The solution was then refluxed at 286 °C under an N2 atmosphere for 1 h (Fig. 1). Particles were precipitated by the addition of 20 mL of isopropyl alcohol (IPA) and centrifuged at 3400 rpm. This was followed by several washings with IPA and subsequent resuspension in hexane for further use in CVD reactions.
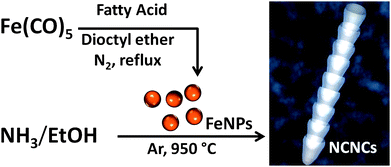 | ||
| Fig. 1 Synthesis of nitrogen-doped carbon nanotube cups (NCNCs) by chemical vapor deposition (CVD) reaction from NH3 and EtOH using monodispersed Fe nanoparticles (FeNPs) as a catalyst. | ||
Synthesized particles were further spin-coated at 1400 rpm on to a bare quartz plate (2.5 cm × 2.5 cm) and placed in a sealed quartz tube for CVD reaction. As illustrated in Fig. 1, NCNCs were synthesized from a liquid precursor of 5% NH3 (wt/wt) in EtOH. Specifically, a three-foot long, sealed quartz tube (2.5 cm i.d.) was placed in a Lindberg/Blue tube furnace and heated to 950 °C. Carrier gases of Ar and H2 with flow rates of 422 sccm and 125 sccm, respectively, were used to remove O2 from the system. The liquid precursor of 5% NH3 was injected at 5 mL h−1 for 1 h, followed by termination of the reaction by stopping the precursor. H2 was then shut off and the system was allowed to cool in an Ar atmosphere for 1 h prior to collection of the synthesized product by sonicating the quartz plate in 15 mL of N,N-dimethylformamide (DMF).
Transmission electron microscopy (TEM) was used to characterize as-synthesized FeNPs and the resultant NCNCs. As shown in Fig. 2, the NCNCs' diameter distributions appear to be directly proportional to FeNP diameter. Using octanoic acid in the thermal decomposition of Fe(CO)5, FeNPs of 16 ± 3 nm are produced yielding NCNCs of 33 ± 5 nm in diameter. Likewise, FeNPs synthesized with lauric acid result in an average diameter of 8 ± 2 nm, with an average NCNC diameter of 26 ± 5 nm. Synthesized FeNPs from oleic acid have an average diameter of 5 ± 2 nm with NCNCs' average diameter of 16 ± 4 nm; however, it is important to note the shape of NCNCs utilizing oleic acid-synthesized FeNPs. As shown in the top inset of Fig. 2c, NCNCs deviate from their typical conical morphology in exchange for a “tear-drop” shape. Presumably, because of the high radius of curvature from oleic acid-synthesized catalyst particles, the resulting tear-drop morphology is observed. Such divergence from a conical segment structure alludes to a size threshold for radius of curvature in FeNPs in NCNC synthesis.
 | ||
| Fig. 2 TEM micrographs of NCNCs synthesized from (a) octanoic acid-, (b) lauric acid-, and (c) oleic acid-capped FeNPs. Diameter distributions of NCNCs are observed to depend on catalyst particle size (shown lower left insets, scale bar 50 nm). Inset in (c) illustrates shape deformation from a conical “cup-like” structure (scale bar = 20 nm). | ||
Table 1 provides the resulting statistical measurements for synthesized FeNPs and the corresponding NCNC diameter distributions. Moreover, dynamic light scattering (DLS) was performed to assess size discriminations in FeNP diameter distributions inherently witnessed by TEM (see the ESI†). As listed, slight variance between TEM and DLS measurements of FeNPs is observed. Because NCNCs are fibrous 1D nanostructures, DLS measurements were not performed, as spherical estimations would prove disingenuous. As shown in the ESI,† AFM was also used to assess the size of synthesized FeNPs of varying diameter. Briefly, particles were spin-coated from hexane on a bare piece of mica. Tapping-mode imaging was then implemented to visualize particles, followed by cross-sectional height analysis to measure 20.8 nm, 12.4 nm, and 2.7 nm for FeNPs synthesized using octanoic, lauric, and oleic acids, respectively.
| Fatty acid | Diameter of FeNPs | Diameter of NCNCs |
|---|---|---|
| a TEM data. b DLS data. | ||
| Octanoic acid | 16 ± 3 nma (12.5 ± 1.8 nm)b | 33 ± 5 nm |
| Lauric acid | 8 ± 2 nma (7.1 ± 0.8 nm)b | 26 ± 5 nm |
| Oleic acid | 5 ± 2 nma (3.1 ± 0.6 nm)b | 16 ± 4 nm |
We further controlled the volumetric parameters of NCNCs and experimentally elucidated the role of N-doping on structural morphology by altering the nitrogen concentration in the liquid precursor at a “fixed” monodispersed nanoparticle distribution. For our studies, octanoic acid-synthesized particles were used for CVD reaction possessing the diameter distribution listed above (16 ± 3 nm). Additionally, stock solutions of 1%, 5%, and 9% NH3 in EtOH were prepared as liquid precursors for NCNC growth.
As described previously, CVD was performed using octanoic acid-synthesized FeNPs spin-coated from hexane on a bare quartz plate. The NH3-containing precursor was injected at a rate of 5 ml h−1 for 1 h under an inert atmosphere at 950 °C, followed by termination of the reaction and sonication of the product into DMF. TEM analysis was then used to examine the structural morphology due to the variation of NH3 concentrations in the feedstock. Fig. 3 illustrates the corresponding low- and high-resolution TEM micrographs of NCNCs synthesized with 1%, 5%, and 9% NH3, respectively. Additionally, electron energy loss spectroscopy (EELS) was performed and confirmed a range of 2–7 atomic % N in each sample (see the ESI†). It is also important to note that since the same FeNPs were used for each variance in N concentration, all three runs resulted in NCNCs with similar diameters (38 ± 9 nm) but different segment lengths. As can be observed from the micrograph indicating 1% NH3, an individual segment length is approximately 750 nm. Of interest to note is that some structures exhibited no segments at all, but were represented by the morphology of a multiwalled carbon nanotube. As the concentration of NH3 in the feedstock increased to 5%, however, conical segments of the type illustrated previously were observed. Using 9% NH3 as the liquid precursor, smaller segment lengths were observed in the range of 31 ± 8 nm, as indicated in the corresponding table.
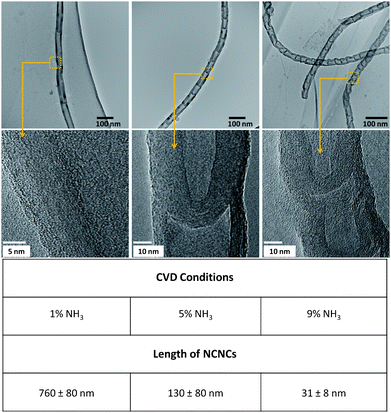 | ||
| Fig. 3 Varying the NH3 concentration during the CVD reaction (uniform particle size, 16 ± 3 nm) resulted in alteration of the segment lengths of NCNCs. Nominal length values and ranges are listed under each concentration 1%, 5%, and 9% respectively. | ||
Such control of segment length has been theoretically predicted.11,12 Two models were studied in which (1) carbon atoms in the wall of the NCNCs were substituted or (2) nitrogen atoms were adsorbed at the open edge of the NCNCs. Results from this study conclude that dopant nitrogen atoms exhibit a preference for edge adsorption, as opposed to side-wall incorporation. Thus, once enough N atoms are present on the open edge of the NCNC, carbon atoms will not attach to this site, effectively acting as a “stopper” for further growth of longer nanotubes. Our experimental results support this hypothesis. As the NH3 concentration in the liquid precursor is increased, available N atoms are also increased resulting in faster saturation of open edge planes and shorter segment lengths. The converse holds true as well, as indicated by longer segment lengths using 1% NH3.
Defining these parameters (segment length and diameter) enables us to tailor the volumetric capacity of individual NCNCs. As we have previously demonstrated,9 separated NCNCs can be cross-linked by the addition of glutaraldehyde. Moreover, cargo such as gold nanoparticles (GNPs) and quantum dots can be encapsulated by their addition prior to cross-linkage, presumably through a capillary action effect. However, in facilitating this work, polydispersed structures were often found, each with its own intrinsic loading capacity. Utilizing NCNCs of controlled diameter and segment length should provide uniform and tailored interior volume for encapsulation of a variety of cargo. Indeed, it was observed that variation in these parameters effectively increased or decreased the loading capacity. Briefly, 0.1 mg of NCNCs doped by 1%, 5%, or 9% NH3 were mechanically separated through grinding with a mortar and pestle and resuspended through sonication in EtOH for 5 min (Fig. 4). Approximately 2 mL of the separated NCNC suspension was mixed with 500 μL of commercially available GNPs (Sigma Aldrich, 5 nm nominal diameter, diluted 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with EtOH) and further sonicated for an additional 5 min. Subsequently, 500 μL of 4% glutaraldehyde (GA) was added, and the suspension was incubated for 24 h. Fig. 4 shows the TEM micrographs from encapsulated GNPs. Using TEM, the morphology of the resultant nanocapsules, as well as the number of GNPs encapsulated within their interior cavity was examined. For 5% NH3-synthesized nanocapsules, 9 ± 4 GNPs were found within the interior of 25 measured nanostructures. In contrast, 9% NH3-synthesized nanocapsules revealed the encapsulation of 3 ± 1 particles within the interior cavity. Moreover, an increased distribution of particles was found for the former. This suggests that while 5% NH3-synthesized nanocapsules can posses tens of particles, a critical value of ∼4 GNPs can be encapsulated within the small interior cavity of 9% NH3-synthesized nanocapsules, confirming a direct correlation between segment length and interior loading capacity. Results from 1% NH3-synthesized nanocapsules deviated from this trend, however. We expected that increased segment length would result in an increased number of particles encapsulated. On average, no GNPs were found within the interior of formed nanocapsules. Additionally, capsule formation was not observed in many cases, presumably the result of non-covalent π–π interactions dominating over covalent bond formation. Therefore, with the exception of NCNCs made using 1% NH3 precursor, interior cavity space can be estimated based upon the number of encapsulated GNPs. Each GNP of 5 nm diameter occupies a volume of 65 nm3. Thus, for 5% NH3-synthesized nanocapsules, the average interior volume is approximately 600 nm3, while that of 9% NH3 is 200 nm3.
1 with EtOH) and further sonicated for an additional 5 min. Subsequently, 500 μL of 4% glutaraldehyde (GA) was added, and the suspension was incubated for 24 h. Fig. 4 shows the TEM micrographs from encapsulated GNPs. Using TEM, the morphology of the resultant nanocapsules, as well as the number of GNPs encapsulated within their interior cavity was examined. For 5% NH3-synthesized nanocapsules, 9 ± 4 GNPs were found within the interior of 25 measured nanostructures. In contrast, 9% NH3-synthesized nanocapsules revealed the encapsulation of 3 ± 1 particles within the interior cavity. Moreover, an increased distribution of particles was found for the former. This suggests that while 5% NH3-synthesized nanocapsules can posses tens of particles, a critical value of ∼4 GNPs can be encapsulated within the small interior cavity of 9% NH3-synthesized nanocapsules, confirming a direct correlation between segment length and interior loading capacity. Results from 1% NH3-synthesized nanocapsules deviated from this trend, however. We expected that increased segment length would result in an increased number of particles encapsulated. On average, no GNPs were found within the interior of formed nanocapsules. Additionally, capsule formation was not observed in many cases, presumably the result of non-covalent π–π interactions dominating over covalent bond formation. Therefore, with the exception of NCNCs made using 1% NH3 precursor, interior cavity space can be estimated based upon the number of encapsulated GNPs. Each GNP of 5 nm diameter occupies a volume of 65 nm3. Thus, for 5% NH3-synthesized nanocapsules, the average interior volume is approximately 600 nm3, while that of 9% NH3 is 200 nm3.
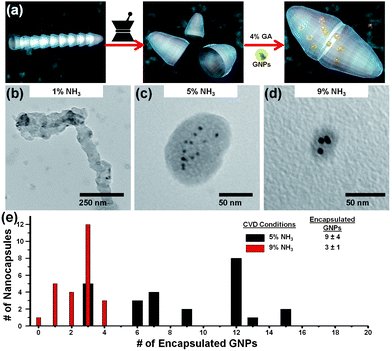 | ||
| Fig. 4 (a) Schematic illustration of stacked NCNC segment separation by mechanical grinding, followed by cross-linkage with 4% glutaraldehyde (GA) in the presence of gold nanoparticles (GNPs), resulting in encapsulated GNPs. TEM micrographs demonstrate the encapsulation of GNPs with cross-linked NCNCs for (b) 1%, (c) 5%, and (d) 9% NH3-synthesized NCNCs. (e) The corresponding bar graph shows the statistical distribution of encapsulated GNPs. 1% NH3-synthesized nanocapsules did not contain a significant number of encapsulated GNPs. | ||
While we demonstrate the successful control of NCNCs by CVD using monodispersed FeNPs, similar types of nanostructures can be synthesized by other methods. For instance, Chun et al. utilized an anodized aluminium oxide (AAO) template and performed CVD reactions using acetylene to deposit graphitic carbon within the template wells.13 The resultant product was a film of carbon nanocups that could be separated through the use of Ar milling. With this methodology, a higher degree of control of physical dimensions can be attained; however, there are no intrinsic N-functionalities with which to perform subsequent reactions. Our protocol, in lieu of an exact degree of dimensional tailoring, incorporates a variety of N-functionalities for chemical reactions. As a result, we have demonstrated the successful cross-linkage of NCNCs to form nanocapsules, a task not easily accomplished without the inherent presence of N-functionalities.
The ease of encapsulating cargo, such as GNPs, in graphitic nanocapsules offers significant advantages over current nano-encapsulation methods. Typically, alternative systems utilize layer-by-layer adsorption of oppositely charged macromolecules to a colloidal template.14 Polymer nanocapsules have been able to encapsulate species such as quantum dots and magnetic nanoparticles; however, these systems suffer from complexity of compositional processes or limited stability in external chemical environments, as varying pH may make such capsules more or less permeable.14 In contrast, NCNC-formed nanocapsules encapsulate cargo presumably through capillary action, followed by a chemical crosslinking reaction. Once encapsulated, the mulitwalled graphitic shell provides an inert barrier between external environments and interior cargo, offering high stability and versatility for a variety of applications.
Conclusions
Finite control over structural and morphological properties of nanomaterials through chemical doping is essential for their engineered properties. We have demonstrated the novel utility of nitrogen doping to achieve the desired volumetric parameters of a hybrid carbon nanomaterial. Furthermore, imparted functionality is observed in the fabrication of nanocapsules of defined interior capacity for the encapsulation of cargo. Such immediate applications of this type of material may include their use in drug delivery where the inherent nitrogen functionalities permit biocompatibility, and tailored cavity space may be utilized for medicinal cargo.Acknowledgements
The authors would like to thank the Nanoscale Fabrication and Characterization Facility of the Petersen Institute of Nanoscience and Engineering for access to characterization instrumentation. This work was supported by an NSF CAREER Award No. 0954345.References
- R. Saito, M. Fujita, G. Dresselhaus and M. S. Dresselhaus, Appl. Phys. Lett., 1992, 60, 2204 CrossRef CAS.
- O. Stephan, P. M. Ajayan, C. Colliex, Ph. Redlich, J. M. Lambert, P. Bernier and P. Lefin, Science, 1994, 266, 1683 CAS.
- M. Terrones, P. M. Ajayan, F. Banhart, X. Blase, D. L. Carroll, J. C. Charlier, R. Czerw, B. Foley, N. Grobert, R. Kamalakaran, P. Kohler-Redlich, M. Rühle, T. Seeger and H. Terrones, Appl. Phys. A: Mater. Sci. Process., 2002, 74, 355 CAS.
- X. Ma and E. G. Wang, Appl. Phys. Lett., 2001, 78, 978 CrossRef CAS.
- R. Kurt and A. Karimi, ChemPhysChem, 2001, 2, 388 CrossRef CAS.
- X. Ma, E. G. Wang, R. D. Tilley, D. A. Jefferson and W. Zhou, Appl. Phys. Lett., 2000, 77, 4136 CrossRef CAS.
- B. L. Allen, P. D. Kichambare and A. Star, ACS Nano, 2008, 2, 1914 CrossRef CAS.
- Y. Tang, B. L. Allen, D. R. Kauffman and A. Star, J. Am. Chem. Soc., 2009, 131, 13200 CrossRef CAS.
- B. L. Allen, C. M. Shade, A. M. Yingling, S. Petoud and A. Star, Adv. Mater., 2009, 21, 4692 CAS.
- C. L. Cheung, A. Kurtz, H. Park and C. M. Lieber, J. Phys. Chem. B, 2002, 106, 2429 Search PubMed.
- E. G. Wang, J. Mater. Res., 2006, 21, 2767 CrossRef CAS.
- G. L. Zhao, D. Bagayoko and E. G. Wang, Mod. Phys. Lett. B, 2003, 17, 375 CrossRef CAS.
- H. Chun, M. G. Hahm, Y. Homma, R. Meritz, K. Kuramochi, L. Menon, L. Ci, P. M. Ajayan and Y. J. Jung, ACS Nano, 2009, 3, 1274 CrossRef CAS.
- Y. Ma, W.-F. Dong, M. A. Hempenius, H. Möhwald and G. J. Vancso, Nat. Mater., 2006, 5, 724 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: AFM and DLS of FeNPs, high-resolution TEM and EELS analysis, and TEM of statistical distributions. See DOI: 10.1039/c0nr00043d |
| This journal is © The Royal Society of Chemistry 2010 |
