Preparation, stability and cytocompatibility of magnetic/PLA-PEG hybrids†
Aristides
Bakandritsos
a,
George
Mattheolabakis
b,
Radek
Zboril
c,
Nikolaos
Bouropoulos
ad,
Jiri
Tucek
c,
Dimitrios G.
Fatouros
e and
Konstantinos
Avgoustakis
*b
aMaterials Science Department, School of Natural Sciences, University of Patras, Rio 26504, Patras, Greece
bLaboratory of Pharmaceutical Technology, Department of Pharmacy, University of Patras, Rio 26500, Patras, Greece. E-mail: avgoust@upatras.gr
cDepartments of Physical Chemistry and Experimental Physics and Nanomaterial Research Centre, Palacky University, Svobody 26, 77146, Olomouc, Czech Republic
dInstitute of Chemical Engineering and High Temperature Chemical Processes (ICEHT), Foundation for Research and Technology-Hellas (FORTH), 26504, Patras, Greece
eSchool of Pharmacy and Biomedical Sciences, University of Portsmouth, Portsmouth, UK P01 2DT
First published on 25th January 2010
Abstract
Hybrid nanocolloids based on biodegradable polymers of poly(lactide) (PLA) or poly(lactide)-block-poly(ethylene glycol) (PLA-PEG) and hydrophobic iron oxide magnetic nanoparticles (MNPs) of ca. 5 nm are prepared via a self-assembly route. The magnetic nanoparticles are organized in superclusters inside the hydrophobic core of PLA-PEG micelles or cholate-stabilized PLA nanospheres. The hydrodynamic diameter of MNPs-loaded PLA nanospheres is ∼250 nm, whereas that of MNPs-loaded PLA-PEG micelles is much lower (∼100 nm) and thus compatible with applications where prolonged blood circulation is required. The PLA-PEG micelles exhibit high encapsulation efficiency for the MNPs, imparting a saturation magnetization value to the hybrid of 8.4 emu g−1. Both hybrid colloids display magnetic properties of a non-interacting assembly of superparamagnetic particles and a low blocking temperature, both of which are key attributes for colloidally stable ferrofluids. Furthermore, the PLA-PEG magnetic hybrids display remarkable colloidal stability at high ionic strength, temperature and in human blood plasma, while the estimated critical micelle concentration of ca. 2 × 10−5 mM (0.3 mg L−1) indicates the low probability of the colloids dissociation in the blood compartment. They were also found to be non-toxic to human cells in vitro. The results underline the potential of the magnetic/PLA-PEG hybrids and encourage further research for their in vivo biomedical applications.
Introduction
Amphiphilic block-copolymers are a subject of interest in materials science for their ability to self-assemble into supramolecular architectures, either in solid or liquid state, giving rise to various phases and morphologies, depending on the medium's affinity for the individual blocks, concentration and temperature.1 These properties, and particularly their ability to form stable micelles at low concentrations, paved the way for their utilization in biomedical nanotechnologies.2–5 Therefore, amphiphilic polymers such as poly(ethylene oxide)-block-poly(propylene oxide) type polymers, with the commercial name pluronics and poloxamers, have been studied for their potential as nanocarriers for hydrophobic drugs with promising results.6–8 Nevertheless, the synthesis of biocompatible and biodegradable analogues, such as poly(lactide)-block-poly(ethylene glycol) (PLA-PEG) based polymers, represented a step forward with regard to the clinical application of such systems.9–11 Other biodegradable block co-polymers are of similar importance.12–14One of the major challenges in the contemporary drug administration technologies is the targeted delivery of the drug molecules, in order to suppress their systemic distribution and, in turn, the ensuing undesirable side-effects. Furthermore, selective drug delivery minimizes the doses required for disease treatment, reducing the pharmacotherapy costs. An effective strategy towards a more site-specific drug delivery could be the entrapment of drug in magnetic carriers.15,16 Thus, incorporation of magnetic nanoparticles (MNPs) in the core of drug-loaded polymeric micelles enables targeting to the desirable area by application of external magnetic fields. Apart from drug targeting, MNPs are investigated in other biomedical applications, such as magnetic resonance imaging (MRI) for diagnostic purposes15,16 and cancer treatment with hyperthermia, where the magnetic component is not only a means for targeting but a therapeutic agent as well.17 Magnetically triggered drug release applications have also been considered.18
The use of PLA-PEG block-copolymers as carrier vehicles for MNPs to the bloodstream and to the biological tissues offers several advantages in comparison to other approaches, such as the use of conventional polymeric carriers.19 The PEG corona is covalently attached to the hydrophobic core, sterically stabilizing the particles very effectively. Nanocarriers based on PLA-PEG copolymers exhibit prolonged blood circulation following intravenous administration and passive targeting (i.e. enhanced permeability and retention due to leaky vasculature) to sites of tumour or inflammation.20,21 For these reasons, the direct surface functionalization of MNPs with PEG moieties has been successfully performed with promising results.22 However, the encapsulation of MNPs into PLA-PEG further improves the functionality of the resulting hybrid carriers, offering the potential of a multiple payload into the PLA core. Furthermore, the biodegradability and amorphous nature23 of the PLA core guarantees the release of the drug and provides control over the release kinetics.
Important attributes of MNPs-loaded PLA-PEG nanocarriers are their size and content in magnetic material. The size determines, among others, the biodistribution properties of intravenously administered nanocarriers.24 The amount of MNPs loaded into the PLA-PEG nanocarriers controls the efficacy of their magnetic manipulation in targeting-related applications and determines the contrast enhancement in magnetic resonance imaging techniques. In addition, other important properties of hybrid PLA-PEG carriers such as their colloidal stability in media with a relatively high ionic strength and temperature (blood resembling media) have to be addressed.
There are a few reports in the literature that have emphasized the importance of MNPs encapsulation in PLA-PEG based particulate carriers. Ren et al. (2005) encapsulated Fe3O4 in PLA-PEG micelles using a “phase separation” process, but due to the hydrophilic nature of the utilized Fe3O4 MNPs, low loading was obtained (1–1.5% w/w). The size of the micelles was found to range between 100–200 nm depending on the molecular weight of PEG and the PLA/PEG ratio.25 Nasongkla et al. (2006), in a significant contribution in the field, used hydrophobic iron oxide nanoparticles (8 nm in diameter) instead of hydrophilic iron oxide and achieved higher loading (6.7% w/w) of MNPs into doxorubicin-loaded and “actively”-targeted PLA-PEG micelles. Increased uptake by tumour SLK cells and increased cytotoxicity were observed with the targeted micelles compared to their non-targeted counterparts.23 Liu et al. (2007) developed targetable magnetic nanospheres based on mixtures of poly(lactide-co-glycolide) (PLGA) and PLA-PEG-maleimide. High magnetite loading (40–60% w/w) into the nanospheres was achieved using superparamagnetic and hydrophobic oleic acid-coated magnetite gel. Although the targeting ability of the nanospheres was not reported, their size (360–370 nm) appeared to be prohibitively large to display prolonged blood residence and efficient targeting.26
In this work organophilic iron oxide nanoparticles were entrapped with high efficiency (15.3% w/w iron oxide loading for a 17.2% theoretical (feed) loading) in PLA-PEG micelles. The hydrodynamic diameter of the micelles was tuned at ca. 100 nm by appropriately selecting the polymer composition, bearing in mind that carrier sizes in this range are considered suitable for applications where prolonged blood circulation is required.20,21 The colloidal stability, magnetic properties and cytocompatibility of the MNPs-loaded PLA-PEG micelles were investigated and compared to those of non-pegylated counterparts (MNPs-loaded cholate stabilized PLA nanospheres). The objective of this work is to establish the synthetic path which leads to well-defined hybrid assemblies of this type and studying some of the key properties with which magnetic carriers, suitable for biomedical applications such as magnetic targeted drug delivery and MRI, should be endowed with.
Experimental
Chemicals
The following chemicals were used for the preparation of the products: FeCl3 (Sigma–Aldrich), oleic acid 90% tech. grade (Alfa Aesar), oleylamine 70% tech. grade (Fluka), diphenyl ether for synthesis (Merck), NaOH in pellets (Merck), NaCl (Merck), absolute ethanol for analysis (Merck), THF for liquid chromatography (Merck) and acetone (POCK SA). DL-Lactide (LE) was purchased from Boehringer Ingelheim (Germany). It was recrystallized twice from ethyl acetate and dried under high vacuum at room temperature before use. Monomethoxy-poly(ethyleneglycol) (mPEG, molecular weight: 5 000) was obtained from Sigma (St. Louis, MO) and dried under high vacuum at room temperature before use.Human umbilical vein endothelial cells (HUVEC) were kindly provided by the Laboratory of Molecular Pharmacology, Department of Pharmacy, University of Patras (Dr E. Papadimitriou). Human blood plasma was a kind gift from the University Hospital of Patras.
Synthesis of magnetic nanoparticles (MNPs)
Organophilic magnetic iron oxide nanoparticles were synthesized following previously reported thermolytic processes.27,28 Oleic acid (10 mmol in 15 cm3 EtOH) and then NaOH (40 mmol in 10 cm3 H2O) were added in an ethanolic solution of FeCl3 (6 mmol, 10 cm3) under vigorous magnetic stirring. The red precipitate formed (ferric oleate) was washed and centrifuged four times with (i) H2O, (ii) H2O–EtOH = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (iii) H2O–EtOH = 50
50 (iii) H2O–EtOH = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and (iv) acetone. The dried oleate salt was transferred to a spherical flask at ∼120 °C, containing diphenyl ether (30 cm3), oleylamine (1.9 mmol) and oleic acid (3.5 mmol). The temperature was increased to the boiling point of diphenyl ether (250 °C) within 1 h, where it was maintained for another 1 h. The mixture was then left to equilibrate at room temperature and precipitated with a mixture of acetone/ethanol. The product, abbreviated as MagC18, was isolated from the soluble unreacted reagents by centrifugation. The purification procedure was repeated four times. The product afforded fine colloidal dispersions in a variety of organic solvents such as hexane, heptane, toluol, chloroform and tetrahydrofuran (THF).
50 and (iv) acetone. The dried oleate salt was transferred to a spherical flask at ∼120 °C, containing diphenyl ether (30 cm3), oleylamine (1.9 mmol) and oleic acid (3.5 mmol). The temperature was increased to the boiling point of diphenyl ether (250 °C) within 1 h, where it was maintained for another 1 h. The mixture was then left to equilibrate at room temperature and precipitated with a mixture of acetone/ethanol. The product, abbreviated as MagC18, was isolated from the soluble unreacted reagents by centrifugation. The purification procedure was repeated four times. The product afforded fine colloidal dispersions in a variety of organic solvents such as hexane, heptane, toluol, chloroform and tetrahydrofuran (THF).
Synthesis of polymers
PLA (Mw = 103 kDa) and PLA(10)PEG(5) (the number in parentheses represents the molecular weight of the respective block in kDa, as determined by gel-permeation chromatography) were synthesized by ring opening polymerization of DL-lactide.29 Dry toluene (50 cm3) was transferred to a three-necked polymerization flask equipped with condenser and stirrer and connected to a nitrogen source. Lactide (10 g), or lactide and PEG in the case of the copolymer, were added in the flask and stirring begun. The temperature was increased to 120 °C and the catalyst (0.1% w/v stannous octoate) was added. The reaction discontinued after 4 h and the synthesized polymer was purified by dissolving it in dichloromethane and precipitating in diethylether. The purified polymers were stored under vacuum at room temperature.Preparation of MNPs-loaded polymeric hybrids
Magnetically modified PLA nanospheres (MagC18/PLA) were prepared by the emulsion-solvent evaporation technique.30 PLA (50 mg) and MagC18 (5 mg) (i.e. 7% w/w in Fe2O3) were dispersed in dichloromethane (2 cm3) and the organic phase was emulsified in 6 cm3 of an aqueous sodium cholate solution (12 mM) using probe sonication (Bioblock Model 75038) at 15 watt for 2 min. The organic solvent was removed by evaporation under mild vacuum in a rotary evaporator (Buchi R114). The magnetically modified PLA(10)-PEG(5) micelles (MagC18/PLA-PEG) were prepared with the solvent-diffusion/precipitation/solvent-evaporation technique (nanoprecipitation).31 In a typical preparation, PLA(10)-PEG(5) (36 mg) and MagC18 (2–10 mg) (i.e. 4–17.2% w/w in Fe2O3) were dissolved in THF (0.5 cm3) and added slowly into water (5 cm3) under sonication (Branson 2510 100W, 42kHz). The aqueous phase immediately turned brown, however, remained optically clear. THF was selected because it is an effective solvent for both the polymer and the magnetic nanoparticles and it is miscible with water. The mixture was left at 40 °C until the THF evaporation was completed and the total volume was readjusted if needed at 5 cm3 with water. In both preparations, non-encapsulated MagC18 and aggregates possibly formed were removed by mild centrifugation.Characterization
Powder X-ray diffraction analysis was performed on a Siemens D-5000 diffractometer with Cu Kα radiation (1.54 Å). Infrared spectra were recorded with a Digilab Excallibur series FTS 3000 spectrometer in a KBr pellet form. The spectra were measured with a resolution of 2 cm−1 and were the sum of 64 scans. Electrophoretic measurements for the determination of zeta-potential values of the suspensions were performed with a Malvern Instrument Nano ZetaSizer equipped with a variable temperature measurement compartment and with a 4 mW He–Ne laser, operating at a wavelength of 633 nm and having an avalanche photodiode as a detector. Data were acquired with laser Dopler velocimetry and with the phase analysis light scattering mode (PALS), after equilibration at 25 °C. Dynamic light scattering (DLS) was performed using the same instrument, where scattered light was collected at a fixed angle of 173°. The size-distribution diagrams are the intensity-weighted results, and the reported mean hydrodynamic diameters (Dh) are the z-average calculated values. The reported polydispersity index (PdI) values, ranging between 0 for an ideally monodispersed sample and 1 for a system with very broad size distribution, have been derived from the formula PdI = σ2/Dh2, where σ is the standard deviation of the distribution (nm). Standard deviations (s.d.) denote the width where the majority of the experimental points lie, while error bars in certain figures were obtained from the measurements of three different batches. The DLS and zeta-potential data were obtained at dilutions of 0.05 w/w with respect to the hybrid content.Transmission electron microscopy (TEM) measurements were carried out on a JEM 2010 microscope operating at 200 kV and scanning electron microscopy (SEM) measurements on a Zeiss SUPRA 35VP-FEG (accelerating voltage 5–20 kV). A superconducting quantum interference device (SQUID, MPMS XL-7, Quantum Design) was used for the magnetic measurements. The hysteresis loops were collected at temperatures of 2 and 300 K in external magnetic fields of up to 7 T. The zero-field-cooled (ZFC) and field-cooled (FC) magnetization curves were recorded on heating in the temperature range from 5 to 300 K and in an external magnetic field of 1000 Oe after cooling in a zero magnetic field and in a field of 1000 Oe, respectively. The colloidal stability of the nanospheres was evaluated from their resistance to aggregation induced by increasing concentrations of NaCl. Samples from the nanospheres (50 mm3) were mixed with aqueous NaCl solutions (950 mm3) of different concentration (range 0–3 M) and the mixtures were transferred to a mildly shaking waterbath (25 °C). After 6 h, the size characteristics of the nanospheres were determined by DLS. The effect of temperature on the colloidal stability of the nanospheres was also evaluated at constant NaCl concentrations (0.14 M and 2 M). The pH in all measurements was adjusted at 7.4. The iron oxide content was determined thermogravimetrically in air using the TG/DTA analyzer (STA 449C Jupiter- Netzsch) and/or spectrophotometrically (see ESI).† The critical micelle concentration (c.m.c.) of PLA-PEG in water was determined by fluorescence spectroscopy. The fluorescence emission spectra of pyrene (concentration of 6 × 10−7 M) in the presence of various concentrations of PLA-PEG at 25 °C were recorded at 90° and with an excitation wavelength of 338 nm on a Hitachi F-2500 fluorescence spectrophotometer. The c.m.c. determination was based on the fact that at c.m.c. the ratio of the areas of the bands at 373 nm (I) and 384 nm (I′) undergoes a substantial decrease.32 The I/I′ ratio at each PLA-PEG concentration was estimated after deconvolution of the pertinent spectra. The micelle formation for the c.m.c. determination was conducted with the nanoprecipitation technique, similarly with the MagC18/PLA-PEG preparation.
Stability in the presence of human blood plasma and cytocompatibility of MagC18/PLA and MagC18/PLA-PEG hybrids
Magnetic colloids (0.2 cm3) were added in human blood plasma (0.8 cm3) in microcentrifuge vials and left for 24 h at 37 °C under mild agitation. The vials were centrifuged at 10000 rpm (RCF= ∼6500 g, Kendro Laboratory Products, Heraeus, Biofuge pico) for 30 min. The pellets were resuspended in H2O, vortexed for 5 min and re-centrifuged. This washing step was repeated a second time to remove any loosely bound plasma components on the surface of the colloids. The DLS signal was recorded before and after incubation with plasma.The cytocompatibility of the hybrids was evaluated from the viability of human umbilical vein endothelial cells (HUVEC) in the presence of different concentrations of hybrids using the MTT test as previously described.33 The cells were seeded in 24-well plates at a density of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in 500 mm3 RPMI-1640 supplemented with 10% v/v fetal bovine serum. Twenty four hours after plating, different amounts of hybrids were added in the wells. After 24 h of incubation at 37 °C, 50 mm3 of MTT solution (5 mg cm−3 in PBS pH 7.4) were added into each well and plates were incubated at 37 °C for 2 h. The medium was withdrawn and 200 mm3 acidified isopropanol (0.33 cm3 HCl in 100 cm3 isopropanol) was added in each well and agitated thoroughly to dissolve the formazan crystals. The solution was transferred to 96-well plates and immediately read on a microplate reader (Biorad, Hercules, CA, USA), at a wavelength of 490 nm. The experiments were performed in triplicate and repeated three times. Cytocompatibility was deduced by %cell viability, which was calculated from the ratio of the absorbance from cells treated with the hybrids and that from non-treated cells (control).
000 cells per well in 500 mm3 RPMI-1640 supplemented with 10% v/v fetal bovine serum. Twenty four hours after plating, different amounts of hybrids were added in the wells. After 24 h of incubation at 37 °C, 50 mm3 of MTT solution (5 mg cm−3 in PBS pH 7.4) were added into each well and plates were incubated at 37 °C for 2 h. The medium was withdrawn and 200 mm3 acidified isopropanol (0.33 cm3 HCl in 100 cm3 isopropanol) was added in each well and agitated thoroughly to dissolve the formazan crystals. The solution was transferred to 96-well plates and immediately read on a microplate reader (Biorad, Hercules, CA, USA), at a wavelength of 490 nm. The experiments were performed in triplicate and repeated three times. Cytocompatibility was deduced by %cell viability, which was calculated from the ratio of the absorbance from cells treated with the hybrids and that from non-treated cells (control).
Results and discussion
Magnetic nanoparticles
The organophilic oleate-functionalized nanoparticles (MagC18), readily responding to a Neodymium permanent hand magnet, correspond to the inverse spinel structure of maghemite (or magnetite) according to their XRD pattern which is shown in Fig. 1a. Mössbauer measurements performed in similarly prepared MNPs revealed the formation of maghemite (γ-Fe2O3).27 From TEM microscopy (see Fig. 2b and 3b), a mean crystallite size of 4.5 nm was deduced, in good agreement with the value of 5 nm obtained from the Scherrer equation.34 The state of the nanocrystallites in their THF dispersion was evaluated with DLS (Fig. 1b), from which a Dh = ∼12 nm was recorded, suggesting that the particles exist as single entities rather than aggregates, corroborating their fine surface functionalization.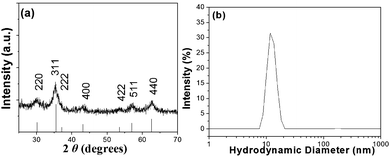 | ||
| Fig. 1 (a) X-Ray diffraction pattern from the oleate-coated magnetic nanocrystallites (bottom lines represent the diffraction pattern from the iron oxide spinel structure, JCPDS no. 19-0629) and (b) the corresponding Dh distribution after their dispersion in THF. | ||
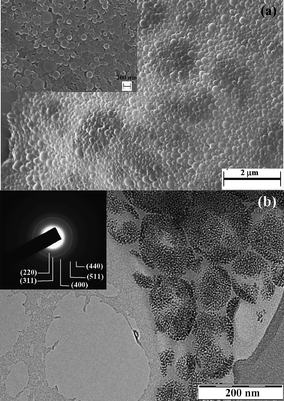 | ||
| Fig. 2 (a) SEM and (b) TEM micrographs from MagC18/PLA nanospheres. The inset in (a) corresponds to the same sample at higher magnification. The inset in (b) represents the corresponding selected area electron diffraction pattern and the numbers in parentheses are the Miller indices corresponding to the lattice planes of the spinel iron oxide structure. | ||
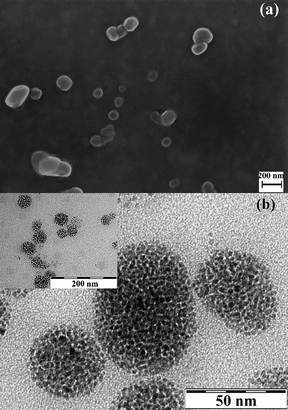 | ||
| Fig. 3 (a) SEM and (b) TEM micrographs from MagC18/PLA-PEG micelles. The inset corresponds to the same sample at lower magnification. | ||
The oleate sheath imparts to the inorganic particles high solubility in various organic solvents up to concentrations of 5% w/v, confirming the successful grafting of the organic molecules on the surface atoms of the nanoparticles, which is further substantiated from FT-IR measurements (provided in the ESI).† From the spectrophotometric analysis measurements, the inorganic content of MagC18 was estimated at 75% w/w, that is a 25% w/w is attributed to the oleate capping molecules.
Physicochemical properties of the MagC18/PLA and MagC18/PLA-PEG hybrids
| Sample | Fe2O3 in feed (% w/w) | Fe2O3 in hybrids (% w/w) |
![[D with combining macron]](https://www.rsc.org/images/entities/i_char_0044_0304.gif) SEM/nm ± s.d.
SEM/nm ± s.d. |
![[D with combining macron]](https://www.rsc.org/images/entities/i_char_0044_0304.gif) h/nm ± s.d
h/nm ± s.d |
PdI |
|---|---|---|---|---|---|
| a The higher final loading in Fe2O3 than the maximum possible loading based on the initial feed should be attributed to the removal of some polymer aggregates during the last centrifugation step of the preparation. b A mean value of 40 ± 9 nm was estimated from TEM. | |||||
| PLA | — | — | 100 ± 30 | 150 ± 46 | 0.1 |
| MagC18/PLA | 7 | 7.5a | 140 ± 58 | 255 ± 103 | 0.19 |
| PLA-PEG | — | — | 30 ± 11 | multimodal | 0.3 |
| MagC18/PLA-PEG | 4 | 4.2a | — | 102 ± 45 | 0.2 |
| MagC18/PLA-PEG | 9.1 | 8.5 | — | 102 ± 39 | 0.15 |
| MagC18/PLA-PEG | 17.2 | 15.3 | 70 ± 21b | 102 ± 32 | 0.1 |
General aspects-size. The successful transfer and the fine dispersion of the highly organophilic MagC18 nanocrystallites in water with the aid of the polymers was macroscopically manifested by the immediate brown coloration of the aqueous phase. It is noted that MagC18/PLA-PEG hybrids could spontaneously assemble without the sonication step, and could also be prepared with the emulsion-solvent evaporation method, but the size obtained was slightly higher. The colloidal stability the MagC18/PLA-PEG system was high, i.e. no precipitation or Dh increase for more than 8 months since the first batch was prepared. Average hydrodynamic diameters of 255 ± 10 nm and 102 ± 3 nm, for MagC18/PLA and MagC18/PLA-PEG, respectively, were recorded with DLS (Table 1). Their size distribution diagrams are depicted in Fig. 4, along with those of the nanospheres produced without incorporation of MNPs. The Dh of the MagC18/PLA-PEG colloid is considerably smaller than that of MagC18/PLA, providing a significant advantage for the former particles in the straggle of evading the rapid clearance from the systemic blood circulation following intravenous administration.20,21 The higher size values estimated from DLS compared to those obtained from SEM are expectable since the hydrated and expanded PEG corona contributes to the Dh recorded values, unlike in microscopy techniques. The difference also observed between the mean diameters from SEM (70 nm) and TEM (40 nm) for MagC18/PLA-PEG can probably be attributed to the fact that in TEM the PEG sheath and the PLA outer shell of the cores are not visible, and therefore a size of 40 nm corresponds solely to the size of the MNP superclusters (Table 1).
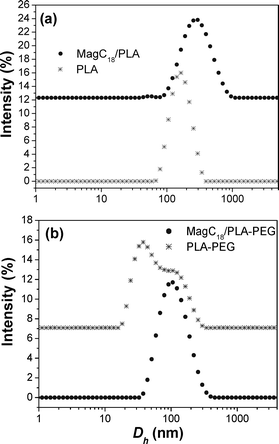 | ||
| Fig. 4 D h distribution diagrams from (a) the plain PLA nanospheres and their magnetic analogues and (b) the plain PLA-PEG micelles and their magnetic analogues. | ||
Both plain PLA-PEG and MagC18/PLA-PEG colloids were practically optically clear, corroborating the small size of the hybrid nano-entities produced, while the cholate-stabilized PLA system appears as a milky emulsion. The PLA-PEG micelles display high polydispersity, where the hydrodynamic diameter of the majority of the micelles lies in the region of 30 nm and another part in the region of ∼100 nm (Fig. 4b). The z-average Dh of the distribution was estimated at 55 nm, as it has been previously reported.30 First, it should be noted that all colloids were prepared at PLA-PEG concentrations far above their critical micelle concentration of 0.3 mg L−1 (pertinent data and other information about the c.m.c. are available in the ESI).† The low Dh component certainly does not originate from the presence of unimers, since unimers' blob size is much smaller than the ∼30 nm entities observed here (their presence though is indisputable since such micellar systems are always in a dynamic equilibrium with unimers). The bimodal distribution can neither be explained on the basis of different polymer chain lengths (i.e. high polydispersity of the parent polymer), since there is no reason for a higher Mw macromolecule to preferentially aggregate with macromolecules of similar Mw,35 and furthermore (if we consider the above situation as possible) the starting polymer displays a monomodal Mw distribution. Therefore, this behaviour could be attributed to two favourable assembly modes of the block-copolymer, each one corresponding to different number of polymeric chains participating in the formation of one micelle/aggregate.
Turning again to the MagC18/PLA-PEG derivative, the incorporation of the magnetic nanoparticles in the PLA organophilic core improves the polydispersity of the system (Table 1), without any observable increase in the maximum hydrodynamic diameter (Fig. 4b). At loadings from 4.2 to 15.3% (w/w), the Dh of the hybrid nanospheres is constrained in the region of 102 nm, while the polydispersity indices vary from 0.2 ± 0.02, for the lower loadings, down to 0.1 ± 0.01 for higher loadings. The systematic PdI improvement, as the loading in MNPs increases, is probably the result of the decrease in the fraction of the very small ∼30 nm micelles (which was the dominant one in the plain PLA-PEG sample). Therefore, the incorporation of MNPs probably selectively favours the formation of micelles of 100 nm.
Salt and temperature effects. As human blood contains NaCl at a concentration of about 0.135 M, it is crucial to evaluate the behaviour of the colloid under such conditions. Therefore, the stability of the hybrid nanospheres in aqueous media containing different amounts of NaCl was assessed with DLS. The unmodified, charge-stabilized maghemite aqueous nanocolloids are very sensitive to the presence of electrolytes and start to aggregate, as detected by the increase in the recorded Dh, and finally precipitate at a very low salt content of ∼0.004 M.36 Such response is prohibitive for applications involving intravenous administration of the nanocolloid, since the formulation may coagulate posing the risk of clot growth in the blood stream. In Fig. 5a the dependence of the Dh of the two formulations (MagC18/PLA and MagC18/PLA-PEG) is available upon increasing NaCl concentrations. In the same plot, the response from plain MNPs (Mag) dispersed in H2O is also provided for comparison. The results unequivocally point to the significant improvement of the colloidal stability of the magnetic nanoparticles by their encapsulation in the cholate-stabilized PLA nanospheres, while in the PEG-stabilized analogues the improvement is dramatic. It appears that the neutral amphiphilic PEG chains effectively antagonize the Van der Waals and hydrophobic attractive forces between the MagC18-loaded PLA cores without being affected by the presence of electrolytes, since the origins of the stabilization in this case are not electrostatic.
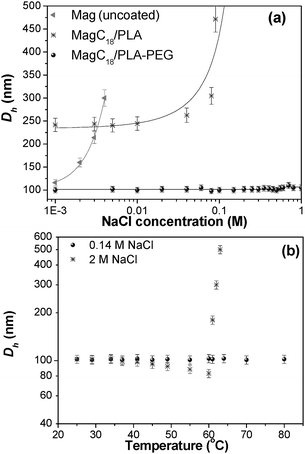 | ||
| Fig. 5 D h change (a) upon NaCl addition at 25 °C for Mag (i.e. uncoated iron oxide MNPs) and the two studied hybrids (the lines are guides to the eye) and (b) upon temperature increase at 0.14 and 2 M NaCl concentrations for the MagC18/PLA-PEG hybrid. | ||
PEG modified nanoparticles exhibit prolonged blood residence following intravenous administration due to their antifouling properties and therefore their ability not to be recognized from the free or cell-surface proteins of the immune system.20,37 Nevertheless, complement activation in response to PEG presence has been observed.38 Despite the fact that it is generally accepted that the smaller the size the higher the blood residence time, of interest are the investigations according to which there is a lower and an upper size threshold, below or beyond which rapid clearance from blood-circulation takes place. Stolnik et al. reported extended circulation times when the Dh of PLA-PEG micelles increased from 42 to 76 nm.24 Moghimi et al. also noted that “a lower size window in the design of long-circulating particles must also be considered”20 and it has been demonstrated that liposomes with sizes below ca. 100 nm have a lower systemic circulation time.39 It is also generally accepted that carriers over 200 nm are prone to fast opsonization and clearance by the reticule endothelial system (RES). Therefore, the size (∼100 nm) of MagC18/PLA-PEG hybrids, prepared here, could be beneficial with regard to their blood residence time following intravenous injection.
Temperature is another parameter that controls the hydration and solubility of PEG polymers, especially in salted media.40 Therefore, temperature dependent studies are of a high interest if such materials are to be transferred within body fluids. In the presence of water-structuring ions (such as Na+ and Cl−),41 the salting-out effect and polymer's precipitation is pronounced, with intensity which follows the Hofmeister series. As a result, the recorded hydrodynamic diameter of PEG-stabilized particles should start decreasing, as it is actually observed for the present system (vide infra). Upon further temperature increase, the solvent turns to a poor solvent for the PEG segments and the suspended particles aggregate and finally precipitate. The temperature-dependent DLS measurements were performed at 0.14 M NaCl and at low MagC18/PLA-PEG concentration (∼0.05% w/v), in an attempt to roughly simulate the concentration of the nanospheres after injection in the bloodstream (Fig. 5b). The results showed that there were no structural changes in the system and Dh values remained constant. Only at a very high [NaCl] the thermal sensitivity of the system and salting-out were detectable, as evidenced by the initial brush layer thickness decrease and eventually by the sharp Dh increase and precipitation at 60 °C. It was found that the brush layer thickness variation was reversible if the temperature did not exceed 60 °C.
The thermal response of the hybrids can therefore be precisely tuned by adjusting the salinity of the medium or by changing the concentration of the hybrids. The thermoresponsive properties of such systems are of great technological importance, and thus stimuli-responsive polymers and nano-assemblies are under extensive investigation.42 The combined thermoresponsive and magnetic properties significantly increase the range of applications of the MagC18/PLA-PEG system.43 Furthermore, this particular behaviour of the system is another indirect evidence of the non-dissociating character of the micelles, considering the following rational. All micellar systems tend to decrease their surface area, and thus their free surface energy, by fusion when the interactions with the solvent become less favourable, resulting in the Dh increase of the observed assemblies. On the contrary, in the present case, as the solvency power of water for PEG segments is reduced upon temperature increase, the Dh decreases due to PEG contraction. This behaviour indicates the strong binding of the block-copolymers in the hydrophobic core which does not allow dissociation and re-association of the polymeric micelles into larger ones.
Interactions with human blood plasma. In order to further elucidate the stability of the products in physiological media, the DLS signal from the hybrids was recorded before and after incubation with human blood plasma (Fig. 6). For comparison uncoated MNPs were also tested, while the response from the MagC18/PLA colloids is available in the ESI.† Precipitation was not macroscopically observed, but the DLS footprint from plain MNPs and MagC18/PLA was utterly changed after incubation with plasma, indicating the formation of micron-sized entities. The results probably point to a protein adsorption related destabilization of plain MNPs and MagC18/PLA. On the other hand, incubation with plasma did not cause significant changes in the DLS signal of MagC18/PLA-PEG (only a minor Dh increase from ∼100 to ∼110 nm was observed). Protein adsorption on therapeutic or diagnostic nanoparticles must be suppressed since it initiates the immune system's response and the clearance of the nanoparticles from systemic circulation.44 In the MagC18/PLA-PEG colloid, the PEG corona apparently confers to the system the necessary anti-biofouling properties.
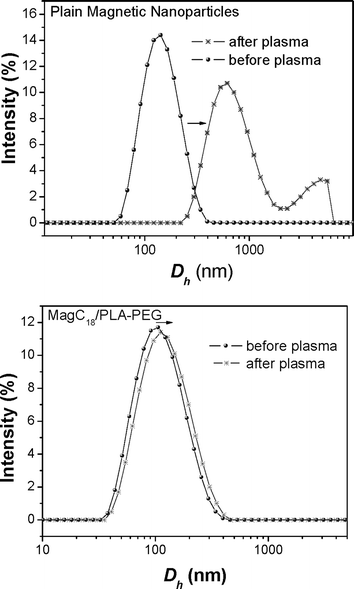 | ||
| Fig. 6 D h distribution before and after incubation of the magnetic colloids with human blood plasma. | ||
Magnetic properties
The static magnetic properties of the magnetic colloids were monitored by measuring the hysteresis loops at 2 and 300 K, shown in Fig. 7. At 2 K, the field-dependent magnetization curve exhibits hysteresis (Fig. 7a and d), indicating that the system is in a magnetically blocked state. The values of coercivities for both systems (HC = 250–340 Oe) correspond well to the values reported for maghemite nanosystems,45 where their coercivity is predominantly driven by the magnetocrystalline anisotropy (other contributions to the overall magnetic anisotropy are negligible). At 300 K, all magnetic nanoparticles behave in a superparamagnetic manner (from the viewpoint of the timescale of the magnetization measurements) since no hysteresis is observed (Fig. 7b and e). The observed values of the saturation magnetization (Ms) at room temperature are 4.8 and 8.4 emu g−1 for MagC18/PLA and MagC18/PLA-PEG, respectively, which is considered sufficient for their pertinent biomedical applications.46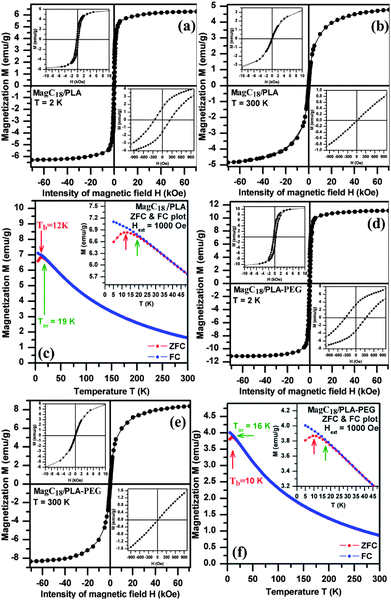 | ||
| Fig. 7 Hysteresis loops and FC-ZFC curves recorded for the MagC18/PLA (top) and MagC18/PLA-PEG (bottom) systems. The blocking temperature (Tb) and irreversibility temperature (Tirr) are indicated by the arrows. All insets show detailed representations of areas of particular interest in the corresponding diagrams. | ||
Taking into account the weight content of MNPs in the polymeric micelles, the Ms for the MNPs amounts to 64 and 54.3 emu g−1, respectively, which is somewhat lower than the value reported for bulk maghemite (≈ 80 emu g−1).47 This reduction of the Ms is often observed in nanoparticulate systems and is merely a consequence of the size confinement of the magnetic material (high surface/core volume ratio).48
In order to study the magnetic dynamics of the assembly of MNPs, the ZFC and FC magnetization curves were obtained (Fig. 7c and f). MagC18/PLA and MagC18/PLA-PEG nanoparticle systems behave in a similar manner. The general results from the curves are the superparamagnetic nature of the particles above 10 K, and the low size distribution based on the small difference between the values of temperature of irreversibility (Tirr) and blocking temperature (Tb) (the full description of the measurement results is available in the ESI).† The superparamagnetic behaviour and the non-interacting nature of the studied systems represent valuable features for colloidal magnetic carriers which withstand aggregation arising from magnetic coupling49 (magnetic dipole–dipole interactions), while, at the same time, respond when external magnetic fields are applied, without displaying remnant magnetization after removal of the field.
Regarding related materials reported in the literature, Ms of 6.7 emu g−1 has been reported for 12% w/w MNPs loaded in PLA-PEG particles with a final size above 3 μm,50 which precludes their use as targeted therapeutic or diagnostic carriers for systemic administration. In another work,51 hysteresis loops were recorded for similar systems verifying the superparamagnetic nature of the colloids, but quantitative Ms values were not provided and the MNPs were not well protected inside the hydrophobic PLA core (i.e. MNPs were found absorbed in the periphery of the particles). Structurally, the present MagC18/PLA-PEG colloid resembles those reported by the group of Dr Gao et al.23,52 In these studies, the magnetic properties of the hybrid particles were not determined but their efficiency as MRI contrast agents was evaluated with very promising results. The magnetic results obtained in the present work, further add to the value of the products, since apart from effective MRI agents (as found before23,52), they also display magnetic properties appropriate for magnetically targeted nanocarriers. Another work by Yang et al. should be referenced,53 where structurally similar products were reported as well, along with part of the magnetic properties (hysteresis loops). Very high Ms values were obtained, however, the values for the hybrids were higher than those of the plain organophilic MNPs used for the hybrids preparation, without explaining how this was possible. Furthermore, considering the very high reported value of Ms = 83.5 emu g−1 for the hybrid with MNP-loading of 71% w/w, then an Ms of 117.6 emu g−1 would be attained for the plain magnetic material. This, once more, is very surprising since the bulk magnetic iron-oxide phases (maghemite or magnetite) have Ms of 80–90 emu g−1, while nanophase oxides certainly display even lower values.
Cytocompatibility results
The MagC18/PLA and MagC18/PLA-PEG hybrids exhibited low toxicity to HUVEC cells since even at concentrations of nanospheres as high as 1000 μg cm−3 the viability of cells was higher than 60% for both types of hybrids (Fig. 8). Although it is recognized that more tests are required in order to characterize the biocompatibility of these materials, the cytocompatibility results obtained here, considered together with the established biocompatibility of the PLA polymers, suggest that the biocompatibility of the MagC18/PLA and MagC18/PLA-PEG hybrids should not be an issue with regard to their biomedical application.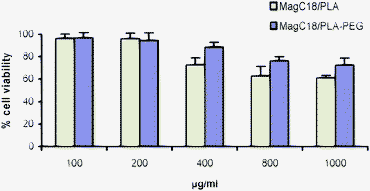 | ||
| Fig. 8 Viability of HUVEC cells in the presence of increasing concentrations of MagC18/PLA and MagC18/PLA-PEG nanospheres. Error bars indicate the standard deviation. | ||
Conclusions
Hybrid organic–inorganic colloids were synthesized in this work. Hydrophobic iron oxide magnetic nanoparticles were organized in superclusters inside the hydrophobic core of cholate-stabilized PLA nanospheres or PLA-PEG micelles. In the first case, assemblies of Dh ∼250 nm were obtained, whereas in the second case the Dh was constrained at ∼100 nm. PLA-PEG magnetic hybrids displayed very high colloidal stability at high ionic strength, temperature, and in human blood plasma. Their unique attributes were further substantiated by the low in vitro cytotoxicity and by the very low critical micelle concentration of 2 × 10−5 mM (0.3 mg L−1), indicating the stability (low probability of dissociation) of the micelles in the blood compartment. The PLA-PEG micelles exhibited relatively high loading efficiency with MNPs, which depended on the feed amount of MNPs, imparting them with a Ms value of 8.4 emu g−1 for a 15.3% w/w iron oxide loading. The MNP-loaded PLA-PEG micelles also displayed magnetic properties of a non-interacting assembly of magnetic particles and a very low blocking temperature, both of which are important prerequisites for magnetic targeted drug delivery and MRI. The results obtained clearly underpin the potential of the hybrid magnetic/PLA-PEG nanocolloid for biomedical applications, stimulating their further in vivo study.Acknowledgements
The State Scholarships Foundation of Greece is acknowledged by A.B. for financial support. We thank Dr Jancik (Palacky University in Olomouc) for TEM measurements. Partial financial support from the projects of Ministry of Education (MSM6198959218) and Grant Agency of the Academy of Sciences of Czech Republic (KAN115600801, KAN200380801) are also gratefully acknowledged. Dr V. Nikolakis and Dr V. Drakopoulos (Institute of Chemical Engineering and High Temperature Chemical Processes) are acknowledged for the provision of Malvern Zeta Sizer and for the SEM measurements, respectively.References
- S. Förster and T. Plantenberg, Angew. Chem., Int. Ed., 2002, 41, 688–714 CrossRef CAS.
- O. C. Farokhzad and R. Langer, ACS Nano, 2009, 3, 16–20 CrossRef CAS.
- R. Savic, L. Luo, A. Eisenberg and D. Maysinger, Science, 2003, 300, 615–618 CrossRef CAS.
- N. Nishiyama, Nat. Nanotechnol., 2007, 2, 203–204 CrossRef CAS.
- K. Kataoka, A. Harada and Y. Nagasaki, Adv. Drug Delivery Rev., 2001, 47, 113–131 CrossRef CAS.
- D. Attwood, J. H. Collett and C. J. Tait, Int. J. Pharm., 1985, 26, 25–33 CrossRef CAS and references there in.
- C. J. H. Porter, S. M. Moghimi, L. Illum and S. S. Davis, FEBS Lett., 1992, 305, 62–66 CrossRef CAS.
- V. Yu Alakhov, E. Yu. Moskaleva, E. V. Batrakova and A. V. Kabanov, Bioconjugate Chem., 1996, 7, 209–216 CrossRef CAS.
- K. Park, S. Lee, E. Kang, K. Kim, K. Choi and I. C. Kwon, Adv. Funct. Mater., 2009, 19, 1553–1566 CrossRef CAS.
- H. Younes and D. Cohn, J. Biomed. Mater. Res., 1987, 21, 1301–1316 CrossRef CAS.
- R. Gref, Y. Manitake, M. T. Perachia, V. Trubetskoy, V. Torhcilin and R. Langer, Science, 1994, 263, 1600–1603 CrossRef CAS.
- J. H. Bader, H. Ringsdorf and B. Schmidt, Angew. Makromol. Chem., 1984, 123, 457–485 CrossRef.
- M. Yokoyama, M. Miyauchi, N. Yamada, T. Okano, Y. Sakurai, K. Kataoka and S. Inoue, Cancer Res., 1990, 50, 1693–1700 CAS.
- S. Takae, K. Miyata, M. Oba, T. Ishii, N. Nishiyama, K. Itaka, Y. Yamasaki, H. Koyama and K. Kataoka, J. Am. Chem. Soc., 2008, 130, 6001–6009 CrossRef CAS.
- A. S. Lübbe, C. Alexiou and C. Bergrmann, J. Surg. Res., 2001, 95, 200–206 CrossRef CAS.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and P. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS.
- A. Jordan, R. Scholz, P. Wust, H. Schirra, T. Schiestel, H. Schmidt and R. Felix, J. Magn. Magn. Mater., 1999, 194, 185–196 CrossRef CAS.
- S.-H. Hu, D.-M. Liu, W.-L. Tung, C.-F. Liao and S.-Y. Chen, Adv. Funct. Mater., 2008, 18, 2946–2955 CrossRef CAS.
- S. A. Gomez-Lopera, R. C. Plaza and A. V. Delgado, J. Colloid Interface Sci., 2001, 240, 40–47 CrossRef CAS.
- S. M. Moghimi, A. C. Hunter and J. C. Murray, Pharm. Rev., 2001, 53, 283–318 Search PubMed.
- S. Stolnik, L. Illum and S. S. Davis, Adv. Drug Delivery Rev., 1995, 16, 195–214 CrossRef CAS.
- Z. Li, L. Wei, M. Gao and H. Lei, Adv. Mater., 2005, 17, 1001–1005 CrossRef CAS.
- N. Nasongkla, E. Bey, J. Ren, H. Ai, C. Khemtong, J. S. Guthi, S.-F. Chin, A. D. Sherry, D. A. Boothman and J. Gao, Nano Lett., 2006, 6, 2427–2430 CrossRef CAS.
- S. Stolnik, C. R. Heald, J. Neal, M. C. Garnett, S. S. Davis, L. Illum, S. C. Purkis, R. J. Barlow and P. R. Gellert, J. Drug Target., 2001, 9, 361–378 CAS.
- J. Ren, H. Hong, T. Ren and X. Teng, React. Funct. Polym., 2006, 66, 944–951 CrossRef CAS.
- X. Liu, V. Novosad, E. A. Rozhkova, H. Chen, V. Yefremenko, J. Pearson, M. Torno, S. D. Bader and A. G. Rosengart, IEEE Trans. Magn., 2007, 43, 6 CrossRef.
- A. B. Bourlinos, A. Simopoulos and D. Petridis, Chem. Mater., 2002, 14, 89–903 CrossRef.
- J. Park, K. An, Y. Hwang, J. E.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891–895 CrossRef CAS.
- Y. J. Du, P. J. Lemstra, A. J. Nijenhuis, H. A. M. van Aert and C. Bastiaansen, Macromolecules, 1995, 28, 2124–2132 CrossRef CAS.
- E. Pierri and K. Avgoustakis, J. Biomed. Mater. Res., Part A, 2005, 75a, 639–647 CrossRef CAS.
- T. Riley, C. R. Heald, S. Stolnik, M. C. Garmett, L. Illum, S. S. Davis, S. M. King, R. K. Heeman, S. C. Purkiss, R. J. Barlow, P. R. Gellert and C. Washington, Langmuir, 2003, 19, 8428–8435 CrossRef CAS.
- M. Wilhelm, C.-L. Zhao, Y. Wang, R. Xu, M. A. Winnik, J.-L. Mura, G. Riess and M. D. Croucher, Macromolecules, 1991, 24, 1033–1040 CrossRef CAS.
- E. C. Gryparis, M. Hatziapostolou, E. Papadimitriou and K. Avgoustakis, Eur. J. Pharm. Biopharm., 2007, 67, 1–8 CrossRef CAS.
- H. P. Klug, L. E. Alexander, in X-ray Diffraction Procedures for Polycrystaline and Amorphous Materials, Wiley, New York, 1974, pp 687–690 Search PubMed.
- Such a behaviour has only been observed for oppositely charged block copolymers: A. Harada and K. Kataoka, Science, 1999, 283, 65–67 Search PubMed.
- A. Bakandritsos, G. C. Psarras and N. Boukos, Langmuir, 2008, 24, 11489–11496 CrossRef CAS.
- S. M. Moghimi, I. S. Muir, L. Illum, S. S. Davis and V. Kolb-Bachofen, Biochim. Biophys. Acta, Mol. Cell Res., 1993, 1179, 157–165 CrossRef CAS.
- I. Hamad, A. C. Hunter, J. Szebeni and S. M. Moghimi, Mol. Immunol., 2008, 46, 225–232 CrossRef CAS.
- D. C. Litzinger, A. M. Buiting, N. van Rooijen and L. Huang, Biochim. Biophys. Acta, Biomembr., 1994, 1190, 99–107 CrossRef CAS.
- R. Ganguly and V. K. Aswal, J. Phys. Chem. B, 2008, 112, 7726–7731 CrossRef CAS; K. Pandya, K. Lad and P. Bahadur, J. Macromol. Sci., Pure Appl. Chem., 1993, A30, 1–18 CrossRef CAS.
- J. P. Jolivet, in Metal Oxide Chemistry and Synthesis: From Solution to Solid State, Wiley-VCH, Weinheim, 2000, pp 9–12 Search PubMed.
- D. M. Jones, J. R. Smith, W. T. S. Huck and C. Alexander, Adv. Mater., 2002, 14, 1130–1134 CrossRef CAS; S.-I. Yamamoto, J. Pietrasik and K. Matyjaszewski, Macromolecules, 2008, 41, 7013–7020 CrossRef CAS.
- A. M. Schmidt, Colloid Polym. Sci., 2007, 285, 953–966 CrossRef CAS.
- W. Bae and K. Kataoka, Adv. Drug Delivery Rev., 2009, 61, 768 CrossRef.
- D. H. Han, J. P. Wang and H. L. Luo, J. Magn. Magn. Mater., 1994, 136, 176–182 CrossRef CAS.
- Z.-Q. Tian, Z.-L. Zhang, J. Gao, B.-H. Huang, H.-Y. Xie, M. Xie, H. D. Abruna and D.-W. Pang, Chem. Commun., 2009, 4025–427 RSC.
- R. M. Cornell, U. Schwertmann, The Iron Oxides, 2nd ed., Wiley-VCH, Weinheim, 2003, p 123 Search PubMed.
- R. Zboril, A. Bakandritsos, M. Mashlan, V. Tzitzios, P. Dallas, Ch. Trapalis and D. Petridis, Nanotechnology, 2008, 19, 095602 CrossRef.
- T. Tartaj, M. P. Morales, S. Veintemillas-Verdaguer, T. Gonzalez- Carreno, C. J. Serna, in Handbook of Magnetic Materials: Synthesis, Properties and Biomedical Applications of Magnetic Nanoparticles, ed. K. H. J. Buschow, Elsevier, Amsterdam, 2006, vol. 16, p. 431 Search PubMed.
- M. D. Kaminski, Y. Xie, C. J. Mertz, M. R. Finck, H. Chen and A. J. Rosengart, Eur. J. Pharm. Sci., 2008, 35, 96 CrossRef CAS.
- J. Ren, H.-Y. Hong, T.-B. Ren and X.-R. Teng, Mater. Lett., 2005, 59, 2655 CrossRef CAS.
- H. Ai, C. Flask, B. Weinberg, X. Shuai, M. D. Pagel, D. Farell, J. Duerk and J. Gao, Adv. Mater., 2005, 17, 1949 CrossRef CAS.
- X. Wang, Y. Chen, R. Yuan, E. Blanco, J. Gao and X. Shuai, Polymer, 2008, 49, 3477 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Electronic supplementary information (ESI) available: FT-IR characterization of the parent organophilic Fe2O3 nanocrystallites, SEM from plain PLA-PEG micelles, thermogravimetric results from the hybrids, data from the measurements regarding the critical micelle concentration, DLS from MagC18/PLA after interaction with plasma, graph from electrokinetic measurements, spectrophotometric FeII determination protocol. See DOI: 10.1039/b9nr00253g |
| This journal is © The Royal Society of Chemistry 2010 |
