Nanoneedle: A multifunctional tool for biological studies in living cells
Kyungsuk
Yum
,
Ning
Wang
and
Min-Feng
Yu
*
Department of Mechanical Science and Engineering, University of Illinois at Urbana-Champaign, 1206 West Green Street, Urbana, Illinois 61801, USA. E-mail: mfyu@illinois.edu
First published on 9th December 2009
Abstract
Studying biology in living cells is methodologically challenging but highly beneficial. Recent advances in nanobiotechnology offer exciting new opportunities to address this challenge. The nanoneedle technology, as an emerging technology that uses a cell membrane-penetrating nanoneedle to probe and manipulate biological processes in living cells, is expected to play an important role in this endeavor. Here we review the recent development and future direction of the nanoneedle technology for biological studies in living cells. The nanoneedle technology is shown to be powerful and versatile, and can offer numerous new ways to explore biological processes and biophysical properties of living cells with high spatial and temporal precision potentially reaching molecular resolution.
 Kyungsuk Yum | Kyungsuk Yum is a postdoctoral research associate in the Department of Mechanical Science and Engineering at the University of Illinois at Urbana-Champaign (UIUC). He received his PhD in mechanical engineering from UIUC in 2009, an MSc in physics from UIUC in 2006, an MSc in mechanical engineering from the University of Texas at Austin in 2002, and a BSc in mechanical and aerospace engineering from Seoul National University in 1999. He was a researcher at the Center for Nanoscale Chemical-Electrical-Mechanical Manufacturing Systems (Nano-CEMMS) at UIUC from 2004 to 2008. His research interests are in nanobiotechnology, nanoscale mechanics and physics, and cellular and molecular biomechanics. |
 Ning Wang | Ning Wang is a professor in Department of Mechanical Science and Engineering at University of Illinois at Urbana-Champaign (UIUC). After receiving his ScD degree in Physiology from Harvard University in 1990, he completed his postdoctoral training at Harvard Medical School and Children's Hospital Boston from 1991 to 1994. He was a faculty member in Physiology Program at Harvard School of Public Health from 1994 to 2006 before moving his laboratory to UIUC. His laboratory's interests are in cell mechanics, mechanotransduction, stem cell biology, nanobiotechnology, and molecular mechanomedicine. |
 Min-Feng Yu | Min-Feng Yu received his BSc, MSc and PhD degrees in Physics from the University of Science and Technology of China in 1992, Fudan University in 1997, and Washington University in St. Louis in 2000, respectively. He is currently an associate professor in the Department of Mechanical Science and Engineering at the University of Illinois at Urbana-Champaign. He has authored ∼60 journal publications and holds several technology patents. He pioneered the development of key technologies that accelerated the experimental study of nanomaterials and enabled the practical nanofabrication of 3-D nanostructures. His main research interests are in nanomechanics, biomechanics, and nanomanufacturing. |
Introduction
The interface of nanotechnology and biology, or nanobiotechnology, has brought in new opportunities and generated exciting development recently. Nanotechnology provides new tools for studying biology; biology provides the ultimate examples of functional nanomachines for nanotechnology.1,2 From the point view of the former, the materials and tools developed in nanotechnology can provide new ways to study biological mechanisms, especially at the cellular and molecular levels, because of the similar length scales that nanomaterials and biomolecules share and the similar physical scales (e.g., nanometre-scale displacement and pico-Newton scale force) between what nanotechnology tools can provide/measure and what biologists want to observe in living cells.Studying biology in living cells is one of the most challenging tasks in cell and molecular biology.3–5 Traditional approaches of studying cell and molecular biology outside living cells (e.g., by using biomolecules purified from cells), while being highly productive, often lose valuable information on cellular mechanisms that can only be observed in their natural environment of living cells. It is recognized that the in vitro test solutions are fundamentally different from the cellular environment.4 Scientists are therefore increasingly interested in conducting experiments in living cells, which are highly beneficial but methodologically challenging.3–5
Recent advances in nanobiotechnology may offer new solutions for this challenge by providing new ways to visualize, manipulate, and characterize living cells and sub-cellular components inside cells.1 For example, with their bright and stable fluorescence, fluorescent quantum dots6,7 have been used to visualize dynamic processes in living cells, including the dynamic behavior of single membrane receptors,8–12 molecular motors,13 nerve growth factors,14 and synaptic vesicles;15,16 magnetic nanoparticles have been used to physically manipulate single membrane receptors to activate signal transduction of living cells.17
With their high-aspect ratio, nanoscale geometry and excellent mechanical, electrical, and chemical properties, one-dimensional nanostructures, such as nanotubes and nanowires, play an important role in biology. For instance, carbon nanotubes have been explored as biosensors , membrane-penetrating delivery systems, and bio-imaging agents.18–25 In addition, such needle-like nanostructures can be mechanically manipulated to penetrate into living cells with minimal invasiveness, physically interconnecting intracellular environments to the outside of the cells.26–31 Conventional tools for manipulating cells and biomolecules, including optical tweezers, magnetic tweezers, atomic force microscopes, and micropipettes, are mostly limited to use for studies on cell membranes or outside of cells32,33 because of the difficulty in accessing the interior of cells with such tools. The unique ability of nanoneedles to access the interior of living cells can open up new opportunities to probe and manipulate biological processes and biophysical properties in living cells. For example, a nanoneedle functionalized with biomolecular species via cleavable linker molecules can be used to directly deliver the biomolecular species into a living cell over the cell membrane.28,31 A conductive nanoneedle electrically insulated with a thin layer of polymers, except the very end of its tip that serves as a nanoelectrode, can be used as a nanoscale electrochemical probe to measure electrochemical reactions and signaling processes occurring inside living cells or in the cellular network.34 Additionally, the small diameter and high-aspect-ratio structure of the nanoneedle provides a nanoscale surface accessible for surface functionalization with numerous surface chemistry methods and a nanoscale carrier that can be physically manipulated with nanopositioning tools. This unique combination can enable targeted delivery of chemically unmodified species into microenvironments and even organelles in living cells and thus realize many new strategies to investigate biological mechanisms of living cells that are otherwise technically challenging or impossible to achieve with existing techniques.
Here we discuss the recent progress in the development and application of one-dimensional needle-like nanostructures, or nanoneedles, for biological studies in living cells. We focus on the use of individual nanoneedles as nanoprobes for single-cell experiments,26–29,31,35 rather than the use of an array of nanoneedles fixed on the substrate.30,36–40 Several research groups have been developing the basic platform of nanoneedle technology for living cell experiments;26–29,31 however, the full potential of this emerging technology for studying fundamental biological problems remains to be realized.
Nanoneedle systems: Fabrication and manipulation of nanoneedles
The nanoneedle system consists of three main components: a nanoscale needle, a manipulator, and an optical microscope. The most critical component is the nanoneedle. The nanoneedle is a straight, rigid and high-aspect-ratio solid structure with a diameter of ∼1–100 nm and a length of ∼1–20 μm (Fig. 1). The nanoneedle is attached to a macroscopic supporting structure, such as an atomic force microscope (AFM) tip, for easy handling (Fig. 1). The ideal nanoneedle for biological studies in living cells must meet the following criteria: (i) the nanoneedle must have a high-aspect-ratio geometry with nanoscale diameters (typically, less than ∼100 nm) and microscale lengths, comparable to the height of cells (∼1–20 μm), required for penetrating cell membranes and reaching areas of interest inside cells without damaging cells, (ii) the nanoneedle must have mechanical robustness, required for its stable operation in aqueous environments26,29 and (iii) the surface of the nanoneedle must be easily functionalized with various molecules to add functionalities to the nanoneedle.29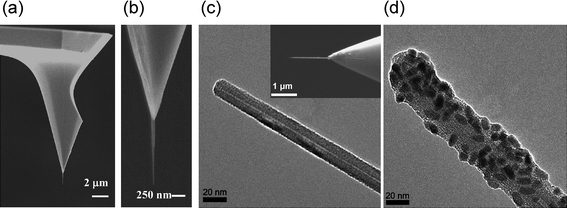 | ||
| Fig. 1 Various types of nanoneedles. (a) Scanning electron microscope (SEM) image of a carbon nanotube (CNT) nanoneedle attached to a standard atomic force microscope (AFM) tip. (b) Enlarged image of the CNT nanoneedle in (a). (c) Transmission electron microscope (TEM) image of the tip region of the CNT nanoneedle shown in the inset. Inset: SEM image of a CNT nanoneedle attached to an AFM tip. (d) TEM image of a CNT nanoneedle conjugated with nanoparticles. (a) and (b) reproduced with permission from ref. 29. Copyright 2007 American Chemical Society. (c) and (d) reproduced with permission from ref. 28. Copyright 2007 National Academy of Sciences, USA. | ||
Fabrication of nanoneedles
Advances in nanotechnology have offered many ways to construct nanoneedle structures. A straightforward method is the direct use of chemically-synthesized one-dimensional nanostructures, such as nanotubes and nanowires. In particular, nanotubes, such as carbon nanotubes (CNTs) and boron nitride nanotubes (BNNTs), have attractive physical properties ideal as nanoneedles,28,31 including (i) needle-like geometry with small diameter (∼1 to 100 nm), (ii) large elastic modulus (∼1 TPa) and high tensile strength,41–44 and (iii) remarkable flexibility and resilience: nanotubes elastically buckle rather than fracture under a large load.41,42,45,46Although significant progress has been made in synthesizing one-dimensional nanostructures, it is still challenging to precisely align and reliably assemble such nanostructures into a needle-like probe configuration (Fig. 1).26 Several approaches have been explored, mostly in the effort of developing nanotube-tipped AFM probes,45,47 including (i) a direct mechanical attachment of nanotubes (e.g., by manipulating individual nanotubes with a nanomanipulator inside a scanning electron microscope or under an optical microscope),28,29,45,46,48,49 (ii) catalyst-patterning and subsequent growth of nanotubes by chemical vapor deposition,50–52 (iii) assembly of nanotubes by dielectrophoresis,26,53–58 (iv) alignment and manipulation of nanotubes by magnetic fields,35,59 and (v) transplanting an assembly of individual nanotubes encapsulated in microscale polymer blocks.60,61 These methods have their advantages and disadvantages, but a common limitation is that they either do not reproducibly produce nanoneedles in large quantities or do not produce “water-stable” nanoneedles having a high aspect ratio (and length of several micrometres).26,29,48 Another practical concern is to fabricate high-aspect-ratio nanoneedles that can withstand capillary forces during their immersion into aqueous solutions; as the length of nanoneedles increases, the force required to bend or buckle the nanoneedles decreases.29,62 A strategy to improve the mechanical integrity of nanoneedles is to coat them with a thin layer of polymers or carbon.29,34,63,64
Alternatively, nanoneedles can be fabricated by nanofabrication techniques, including focused-ion-beam (FIB) machining,27,65–71 electrochemical fountain pen nanofabrication,72,73 and direct-write nanofabrication techniques.74 For example, Nakamura and colleagues fabricated nanoneedles of 200–300 nm in diameters and 6–8 μm in length by carving pyramidal Si AFM tips with FIB machining and demonstrated the capability of the nanoneedles to penetrate into living cells (Fig. 2).27,65–71 Yu and colleagues developed micropipette-based electrochemical and direct-write nanofabrication techniques that can directly grow nanowires of various materials (diameter of ∼100 nm).72–74 However, compared with chemically-synthesized nanotubes or nanowires (1–100 nm in diameter), the nanoneedles produced by these nanofabrication methods27,65–74 have relatively large diameters (typically, larger than 100 nm).
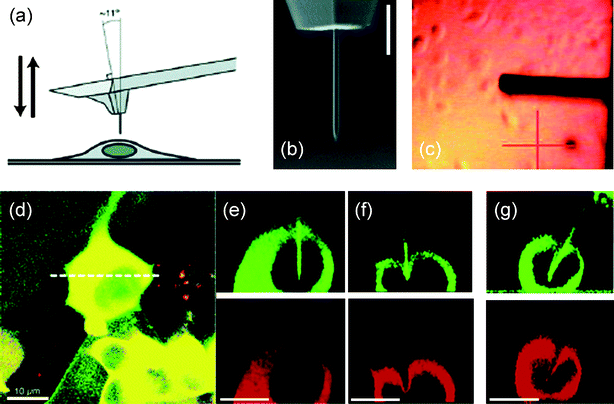 | ||
Fig. 2 Nanoneedle manipulation with an AFM. (a) Schematic of the operation of a nanoneedle on an AFM tip over a living cell. (b) SEM image of a Si nanoneedle fabricated by FIB machining. (c) Image of an AFM tip with a nanoneedle over the cell culture as observed by the AFM optical system during the nanoneedle positioning. (d) Stack of confocal images of a human embryonic kidney (HEK293) cell expressing red-fluorescent protein (DsRed2-NES) and a Si nanoneedle conjugated with fluorescein isothiocyanate (FITC) with green emission when the nanoneedle was inserted into the cell. (e) Cross-section images for green and red emission at the position indicated by the white dotted line in (d) processed from the confocal images. (f) Images taken when a nanoneedle failed to penetrate the cell membrane. (g) Images taken when a pyramidal tip with a high aspect ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was indenting the cell membrane, causing significant deformation of the cell membrane and the nucleus. Scale bars, 3 μm in (b) and 10 μm in (d)–(g). (a), (b), and (d)–(g) reproduced with permission from ref. 27. Copyright 2005 American Chemical Society. (c) reproduced with permission from ref. 29. Copyright 2007 American Chemical Society. 1 was indenting the cell membrane, causing significant deformation of the cell membrane and the nucleus. Scale bars, 3 μm in (b) and 10 μm in (d)–(g). (a), (b), and (d)–(g) reproduced with permission from ref. 27. Copyright 2005 American Chemical Society. (c) reproduced with permission from ref. 29. Copyright 2007 American Chemical Society. | ||
Manipulation of nanoneedles
To visualize the nanoneedle operation and target cells, the nanoneedle system integrated with an optical microscope has often been used as a platform to manipulate the nanoneedle. Examples include the integration of an AFM system or a standard micromanipulator with an inverted optical microscope (Fig. 2 and 3). The AFM-based nanoneedle system integrated with an inverted optical microscope has the advantage that the displacement of the nanoneedle can be controlled with nanometre-scale resolution, while in the meantime the force exerted through the nanoneedle can be monitored with pico-Newton force resolution (Fig. 2).27,28 Thus, the manipulation of the nanoneedle is not limited by the resolution of an optical microscope. However, the AFM-based nanoneedle system restricts the motion of the nanoneedle to along the vertical direction. Moreover, the AFM probe cantilever blocks the optical view of the nanoneedle attached onto the AFM tip [Fig. 2(a–c)] and the direct visualization of the exact cell penetration process of the nanoneedle. Alternatively, an AFM-based nanoneedle system combined with a confocal microscope can be used. This configuration enables the visualization of the nanoneedle and the cell in three-dimensions [Fig. 2(d–g)];27,75 but, the timescale of the confocal imaging is generally larger than that of nanoneedle operation or dynamic cellular processes, restricting its use for real-time monitoring of such processes.76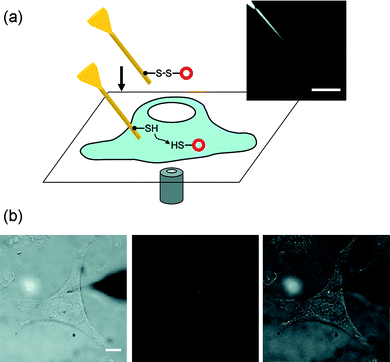 | ||
| Fig. 3 Nanoneedle manipulation with a micromanipulation technique: the nanoscale mechanochemical delivery of fluorescent quantum dots into living cells. (a) Schematic of the mechanochemical delivery of nanoparticles into living cells. Inset, optical microscope image of a nanoneedle. (b) Optical microscope images of a nanoneedle functionalized with quantum dots during the quantum dot delivery into the cytoplasm of a living HeLa cell. The nanoneedle could be precisely located at the targeted release site in the three-dimensional cellular environment by focusing the tip of the nanoneedle. The whole process was monitored in situ in the bright field (left), the fluorescence (middle), or the combined bright-field and fluorescence (right) image mode. The quantum dots attached on the nanoneedle are shown in red (middle) and bright white (right). The unfocused dark shade on the right side is the tip of the macroscopic needle on which the nanoneedle is attached. Scale bars, 5 μm in (a) and 10 μm in (b). Reproduced with permission from ref. 31. Copyright 2009 American Chemical Society. | ||
The micromanipulator-based nanoneedle system has the advantage that (i) the nanoneedle can be manipulated without technically demanding and expensive equipment, such as an AFM, and (ii) the whole operation can be visually monitored in situ, not possible with the AFM-based nanoneedle system (Fig. 3). The second feature also facilitates the direct integration of the nanoneedle technique with well-established optical microscopy techniques in biology.31 A drawback of the micromanipulator-based technique is that the operation of nanoneedles is limited by the optical resolution of an optical microscope; the manipulation of a nanoneedle with a diameter less than ∼30 nm or a length less than ∼3 μm is typically difficult. A way to circumvent this limitation is to use nanoscale optical beacons: for instance, quantum dots attached to nanoneedles can aid the visualization and hence the manipulation of nanoneedles, potentially allowing the use of nanoneedles with small diameters (<30 nm) beyond the optical resolution (Fig. 3).31 Additionally, in case an electrically conductive nanoneedle (e.g., electrochemical nanoneedle nanoprobes) is used, the cell penetration process can be monitored electrically by measuring the electrical potential (i.e., the membrane potential) or current (as in scanning electrochemical microscopy).77,78 As the nanoneedle approaches the cell membrane, one may detect the change in ionic current of specific electrochemically active species;77 and as the nanoneedle penetrates into the cell, one can measure the change in potential due to the cell membrane potential (∼70 mV).78
It would be desirable to have a nanoneedle system capable of simultaneously controlling nanoneedles at the nanoscale resolution, monitoring the force involved in the process, and directly visualizing nanoneedles and target cells. Recent efforts toward this direction include the development of an in-plane scanning probe system, specifically designed for the use of nanotube nanoneedles for biological applications79 and the development of an AFM combined with a side-view optical imaging capability.76
Surface functionalization of nanoneedles
Many surface chemistry and bioconjugation techniques, developed in chemistry, biology, and materials science, render numerous strategies for surface functionalization. Significant progress has also been made in surface functionalization and chemical modification of nanomaterials (e.g., CNTs and BNNTs), especially in the effort of interfacing nanomaterials with biomolecules and cells for their use in biology and medicine.18–21,23–25,80 There are two main strategies to functionalize nanoneedles: (i) direct functionalization of the pristine surface of nanoneedles (e.g., the graphitic surface of CNTs)28,58 [Fig. 4(a)] and (ii) functionalization of the surface of nanoneedles coated with other materials (e.g., Au) [Fig. 4(b)].29,31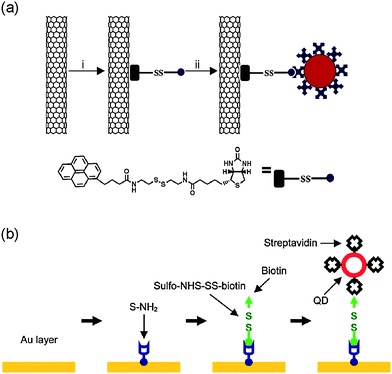 | ||
| Fig. 4 Surface functionalization of nanoneedles. (a) Surface functionalization of CNT nanoneedles with streptavidin-conjugated quantum dots using a linker molecule synthesized to contain a pyrene moiety that binds on CNT surfaces, a biotin moiety, and a disulfide bond in its spacer. (b) Surface functionalization of Au-coated nanoneedles with streptavidin-conjugated quantum dots using a general procedure that consists of four steps: (i) coating nanoneedles with a thin layer of Au, (ii) forming a NH2-terminated SAM, (iii) conjugating a linker molecule that contains a disulfide bond, and (iv) attaching streptavidin-conjugated nanoparticles by the specific binding of streptavidin and biotin. (a) reproduced with permission from ref. 28. Copyright 2007 National Academy of Sciences, USA. (b) reproduced with permission from ref. 31. Copyright 2009 American Chemical Society. | ||
CNT nanoneedles can be functionalized by both covalent and noncovalent methods. Covalent methods use chemical reactions that form chemical groups on the surface of CNTs,58 whereas noncovalent methods use favorable interactions between the hydrophobic domain of functional molecules and the surface of CNTs.24,25,28,80 A common covalent method is to oxidize CNTs, forming carboxyl groups on their surface, and use the carboxyl groups for further functionalization.24,25,58,80 Noncovalent methods can also be used with various functional molecules, including surfactants, polymers, polynuclear aromatic compounds, and biomolecules.46–48 Common noncovalent methods include the use of hydrophobic and π–π interactions. In particular, many types of pyrene derivatives have been used for functionalization of CNTs through π–π stacking. For example, Chen et al.28 used a linker molecule containing the pyrene moiety that binds to CNT surfaces and the biotin moiety that binds to streptavidin to conjugate streptavidin-coated quantum dots on their CNT nanoneedles [Fig. 4(a)].28
A common strategy for functionalizing Si-based nanoneedles is forming self-assembled monolayers (SAMs) of alkylsilanes through the silane coupling reaction. Nakamura and colleagues demonstrated the immobilization of proteins,68DNA,67,70,71 and polymers69 on their Si nanoneedles. For example, they used (3-aminopropyl)triethoxysilane27 and (3-mercaptopropyl)trimethoxysilane67,68,70,71 to form amine and thiol groups on Si nanoneedles. They then attached His-tagged proteins by using nickel-chelating nitrilotriaceticacid68 or DNA by adsorbing DNA onto the positively charged surface of the nanoneedle functionalized with poly(L-lysine) through electrostatic interactions.67,70,71 They also showed that the modification of the surface of a Si nanoneedle with a copolymer of (2-methacryloyloxyethyl)phosphorylcholine (MPC) and (3-methacryloxypropyl)triethoxysilane (MTES) could reduce the nonspecific adsorption of cytosolic proteins on the nanoneedle surface inside a living cell. The MPC unit blocked nonspecific adsorption of proteins on the nanoneedle and the MTES unit functioned as an anchor to the surface of the Si nanoneedle through the silane coupling reaction.69
An alternative approach to functionalize nanoneedles, nonspecific to the types of nanoneedles, is to first coat nanoneedles with other materials (e.g., Au) and subsequently functionalize the coated nanoneedles [Fig. 4(b)].29,31 Thus, instead of synthesizing functional molecules for different nanoneedles, “standard” surface chemistry can be used for functionalization of various types of nanoneedles. The surface coating not only facilitates the surface chemistry but also improves the mechanical integrity of nanoneedle structures.29,31,63 A straightforward scheme is to coat nanoneedles with a thin layer of Au (∼10–20 nm in thickness) and form a self-assembled monolayer (SAM) on the Au surface through the chemisorption of thiol groups on Au.29,31 For instance, Yu and colleagues31 developed a general procedure to functionalize Au-coated nanoneedles with nanoparticles by forming an amine-terminated SAM on the Au surface [Fig. 4(b)]. Moudgil and colleagues29 also conjugated gold nanoparticles on Au-coated CNT nanoneedles in a similar manner, by forming an amine-terminated SAM of 4-aminothiophenol and subsequently attaching Au nanoparticles by electrostatic interactions between the negatively charged Au nanoparticles and the amine-terminated nanoneedle.
Mechanics of cell membrane penetration of nanoneedles
For the rational design and operation of nanoneedles, understanding how nanoneedles penetrate cells is important. The AFM-based nanoneedle system (shown in Fig. 2) has, in this regard, provided insight into the cell membrane penetration process (Fig. 5).27,29,65 Force–distance curves obtained from the AFM studies show that as the nanoneedle contacts and penetrates into a cell, the force (i) initially increases, (ii) then suddenly drops, and (iii) remains constant with little fluctuation [Fig. 5(a)]. The initial increase in force indicates that when it first contacts the cell membrane, the nanoneedle only bends and stretches the cell membrane without penetrating into the cell; the sudden drop in force indicates the instant when the nanoneedle actually penetrates through the cell membrane. The two important parameters in the process are the force and the indentation depth required for penetrating the cell membrane, closely related to the potential effects of the nanoneedle on cellular processes and functions. The AFM studies also show that as the diameter of the nanoneedle decreases, the required indentation depth and penetration force also decrease. For example, nanoneedles of 30–40 nm in diameter required indentation depths of 0.1–0.2 μm and penetration forces of 0.1–0.2 nN (for human pleural mesotheilial cells),29 while nanoneedles of 200–300 nm in diameter required indentation depths of 0.5–2 μm and penetration forces of 0.5–2.0 nN (for human epidermal melanocytes).27,65 Certainly the specific values in particular cases depend also on other factors, such as the cell type, the location of the penetration, the shape of the nanoneedle, and the approaching direction and speed of the nanoneedle. The indentation depth, or the deformation of the cell, in the range of 100–200 nm when using nanoneedles of 30–40 nm in diameter, is comparable to the amplitude of native cell membrane oscillations,29,81 and the penetration force of 0.1–0.2 nN is ten times smaller than the wetting force (∼1 nN) that water exerts on nanotubes with a similar diameter.82,83 These results suggest that nanoneedles with small diameters (<100 nm) may penetrate cell membranes without transmitting significant forces to the cells; therefore, the related mechanotransduction processes are less likely to occur. The AFM studies also showed that the success rate of the cell membrane penetration depends on the diameter and shape of the nanoneedle; for example, the success rate of nanoneedles with 200 and 800 nm in diameter was ∼70–90% and ∼20–60% (depending on the shape of the nanoneedle), respectively.65 In contrast, a standard pyramidal AFM tip, even with its nanoscale tip radius, can occasionally penetrate into cells only when the penetration force is larger than 10 nN, deforming cells significantly [Fig. 5(b)].27,29,65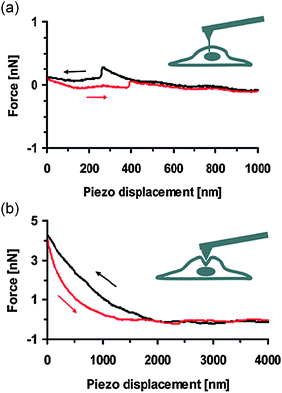 | ||
| Fig. 5 Force–distance curves obtained from the AFM system, using (a) a nanoneedle attached to an AFM tip and (b) an unmodified standard AFM tip as indicated in the inset schematic (see also Fig. 2). The AFM tip approached (black curves) and then retracted from the cell (red curves). Reproduced with permission from ref. 29. Copyright 2007 American Chemical Society. | ||
Nanoneedles in cell biology
Nanoneedles for intracellular delivery
Delivering macromolecules into living cells is important for experiments in cell and molecular biology. A major challenge of intracellular delivery is to introduce a “cargo” into a living cell over the barrier of the cell membrane without causing damage.28,31,84 The unique ability of nanoneedles to access the interior of living cells with minimal invasiveness has led several research groups to explore the use of nanoneedles for intracellular delivery (Fig. 6).26,28,31,66–68,70,71Intracellular delivery using nanoneedles can be achieved by using either (i) the inner channels of hollow nanoneedles (e.g., nanotubes and nanopipes) as a nanoscale counterpart of microinjection35,78,85–88 or (ii) the outer surface of nanoneedles by loading cargo on the surface (Fig. 4).26,28,31,66–68,70,71 The first approach can enable continuous delivery of molecules, but several technical difficulties at the nanoscale, including clogging of the needles and the high pressure required for injection, have hindered its realization,89 except a few systems using nanopipes or nanopipettes with relative large diameters (around several hundred nanometres) and tapered shapes.78,85–88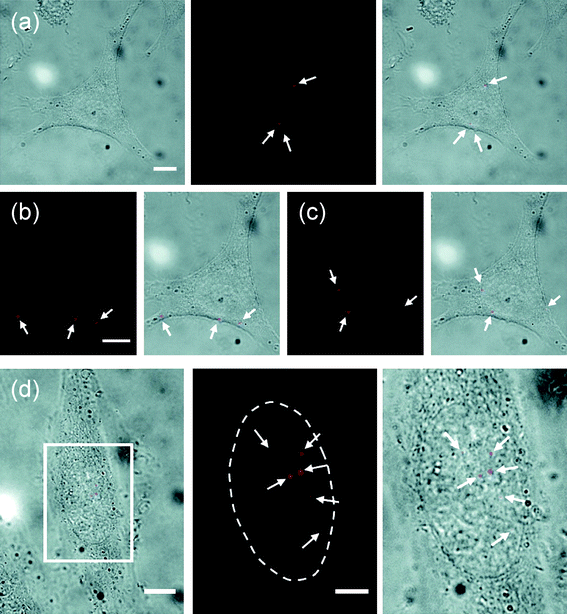 | ||
| Fig. 6 Delivery of quantum dots into the cytoplasm and the nucleus of living HeLa cells using the nanoneedle-based mechanochemical delivery method (shown in Fig. 3). (a–c) Delivery into the cytoplasm: (a) bright field (left), fluorescence (middle) images of the cell after delivery and overlay of the bright field and fluorescence images (right) and (b, c) fluorescence and overlay images acquired on two different focal planes. The cell is the same cell shown in Fig. 3(b). (d) Delivery into the nucleus: overlay of bright-field and fluorescence images of a cell after delivery (left) and enlarged fluorescence image (middle) and overlay of bright-field and fluorescence images (right) of the marked region in the left image. The arrows indicate quantum dots (red). The dotted line locates the boundary of the nucleus. Scale bars, 10 μm in (a–c; d, left) and 5 μm in (d, middle and right). Reproduced with permission from ref. 31. Copyright 2009 American Chemical Society. | ||
Several research groups reported successful intracellular deliveries by loading cargo onto the surface of nanoneedles (Figs. 3(a), 4, and 6).28,31,67,70,71 Beyond the capability to access specific regions inside living cells and the numerous surface functionalization schemes available for attaching cargo to the nanoneedle surface, a major remaining challenge for the nanoneedle system is to controllably release cargo from nanoneedles inside living cells. One approach is to simply use the passive desorption of cargo from the nanoneedle.26,67,70,71 For example, Nakamura and colleagues67,70,71 demonstrated the direct delivery of green fluorescent protein (GFP) DNA into the nucleus of individual human mesenchymal stem cells (MSCs), human embryonic kidney cells (HEK293), and breast cancer cells (MCF-7), using Si nanoneedles of 200 nm diameter operated by an AFM system. They also achieved a high efficient gene expression rate (e.g., ∼70% for human MSCs), compared with microinjection (∼10% for human MSCs).70 The low expression rate of the microinjection was attributed to the low rate of the successful injection: because of its large diameter and conical shape, it was particularly difficult to insert a microinjection pipette into a flat cell, such as a human MSC (∼5 μm in thickness). In their study, however, the cargo was nonspecifically adsorbed on the nanoneedle by electrostatic interactions, and the cargo was unselectively desorbed from the nanoneedle both in the extracellular media and inside the cell: about 25% of DNA was desorbed from the nanoneedle in the extracellular media in two minutes after the immersion of the nanoneedle into the medium. Thus, this approach has the limitation that the nanoneedle penetration process must be completed within several minutes after the immersion of the nanoneedle into the cell media (i.e., before the complete desorption of the cargo from the nanoneedle into the cell medium).
Zettl, Bertozzi and colleagues28 and Yu and colleagues31 demonstrated a more sophisticated scheme for the controlled release of cargo specifically inside a live cell; the scheme uses the reductive cleavage of the disulfide bond in the reducing environment of the cell interior (Fig. 3). They exploited the regulatory mechanism of a cell that maintains the redox equilibrium of the intracellular environment, in which the disulfide bond is reduced into two thiol groups. By attaching cargo to the nanoneedle via a linker molecule that contains a disulfide bond, they could release the cargo specifically inside a cell (by maintaining the nanoneedle inside a cell for ∼15–30 min, a typical time required for the reductive cleavage of the disulfide bond) (Figs. 3(a), 4, and 6).28,31 Both groups demonstrated this approach by delivering a discrete, small number of protein-coated quantum dots into living HeLa cells (with the height of only ∼5 μm), using either a carbon nanotube nanoneedle manipulated by an AFM28 or a gold-coated nanotube nanoneedle manipulated by a micromanipulator.31 In particular, Yu and colleagues31 demonstrated that this method can deliver well-dispersed single quantum dots into the cell, potentially enabling the use of the delivered QDs for molecular imaging inside living cells. (Despite their excellent photophysical properties for single-molecule imaging, the difficulty of delivering dispersed single QDs into the cytoplasm of living cells has so far hindered the routine use of QDs for molecular imaging inside living cells.) Furthermore, they demonstrated the tracking of the delivered single QDs inside the cells: because of the significant reduction of background signal from out-of-focus QDs, achieved by delivering only a small number of QDs, they could detect single QDs inside living cells, even with a simple fluorescent microscope.31 The diffusion coefficient D of the QDs introduced into the cytoplasm ranged from ∼0.1 to 4 μm2 s−1 (the D value of the QDs in aqueous solutions is ∼17 μm2 s−1), indicating the molecular crowding and the high physical heterogeneity of the intracellular environment. Additionally, the ability of nanoneedles to reach target areas inside living cells has enabled the direct delivery of cargo into the target areas inside living cells, not readily achievable with conventional delivery methods. For example, Yu and colleagues31 showed this capability by specifically delivering QDs into the nucleus of living HeLa cells (Fig. 6).
The nanoneedle system offers unique ways to deliver a small number of molecules into target areas inside living cells without the need of a carrier solvent and without inducing apparent cell damage.28,31 The spatially-resolved release of the cargo may potentially enable spatially-resolved experiments inside living cells (including inside the nucleus).31 The spatial resolution of the delivery is mostly determined by the size of the nanoneedle penetrated into a cell (<100 nm in diameter and ∼1–2 μm in length) and the manipulation precision of the nanoneedle (<1 nm achievable with a piezo-driven manipulator). If the loading or release of cargo can be limited to the tip region of the nanoneedle, the spatial resolution can be further improved. Additionally, the delivery can be done repeatedly at desirable times through the cell cycle (with a temporal resolution of ca. 15–30 min if the reductive cleavage of the disulfide bond is used as the cargo release mechanism). An obvious limitation of the nanoneedle-based delivery method is its low throughput: one nanoneedle can only be used to deliver cargo into a limited number of cells (if the cargo is loaded on the surface of nanoneedles). Certainly, the same concept exploited in the single nanoneedle-based delivery can be extended to an array of nanoneedles30,36–40 or the delivery process can be automated to increase the delivery throughput, but at the cost of losing the spatial and temporal precision of the single nanoneedle-based delivery.
Nanoneedles for electrophysiology
The ability to monitor electrophysiological processes of living cells or cellular networks has enhanced our understanding of cellular signaling processes. Although they are widely used in various electrophysiological measurements, conventional probes, such as glass micropipettes (used in patch-clamp and microelectrode techniques) and microscale and nanoscale solid electrodes (used in scanning electrochemical microscopy), are not appropriate for use in intracellular measurements of animal cells, because their conical shape, thus a relatively large structure except for the very end of the probe, can cause cell damage and significant cell deformation when they penetrate through cell membranes.Since Crooks and colleagues90 first demonstrated individual nanotube-based nanoelectrodes for electrochemistry (∼100 nm in diameter), several research groups have explored the use of a nanoneedle as an electrochemical nanoprobe,34,91,92 including the use of nanoneedle probes in scanning electrochemical microscopy.63,64,93,94 Such nanoneedle nanoprobes not only possess the inherent qualities of small electrodes (e.g., enhanced mass transport rate at the electrode and the high spatial and temporal resolution) but also have the unique capability of sensing local microenvironments (potentially, inside living cells) with nanometre-scale resolution, determined by the size of the nanoelectrode (<100 nm).34,95 For example, Yu and colleagues34 demonstrated the use of nanotube-based nanoneedle probes for electrochemical sensing in picolitre microenvironments (the size of a typical living cell) by using another metal nanoneedle as a reference electrode. They also revealed a unique electrochemical mechanism in confined microenvironments: because of the close proximity of the electrodes, the redox-active molecules can be regenerated and dominate the electrochemical reaction at the reference electrode in confined microenvironments, establishing a stable reference potential.34 The redox cycling mechanism may potentially be important for the use of such nanoneedle nanoprobes for electrochemical measurements in the microenvironment of cells and intracellular organelles, where only a small number of redox-active molecules exist.
However, despite significant current interest in developing nanoneedle nanoprobes for single-cell experiments, the full potential of nanoneedle nanoprobes has not been realized. A major challenge in the development of nanoneedle nanoprobes is forming a pinhole-free insulation layer with nanoscale thickness and maintaining the high-aspect-ratio nanoscale geometry of nanoneedles. Nanoneedles must be electrically insulated except the electrochemically active area at the very end of the nanoneedle for spatially resolved measurements.34,64 Many different strategies to electrically insulate nanoneedles have been investigated, including electropolymerization of poly(phenol) (∼10 nm in thickness),34,90 a three-stage insulation process (the total thickness of ∼100–200 nm) consisting of an oxide layer, a conformal insulating film of poly(oxyphenylene) (∼40–80 nm in thickness), and a silicon nitride layer (∼100 nm in thickness),64 and a conformal coating of parylene,63fluorocarbon,93silicon dioxide,94 or hafnium dioxide (HfO2).92 However, these approaches generally require a trade-off between the thickness and the effectiveness and long-term reliability of the insulation layers. Another practical concern is to ensure good electrical contact between the nanoneedle part (the nanotube or the nanowire) and the macroscale supporting structure for the nanoneedle, especially in their operation in aqueous solutions.93,94
Currently, only a few types of probes based on glass and carbon pipettes, fabricated by pulling methods and thus having a conical shape, have been exploited for cell electrophysiology and electrochemical measurements inside living cells.77,78,88 For example, Mirkin and colleagues measured the charge transfer rate across the cell membrane, the membrane potential, and the intracellular redox properties of the human breast epithelial cell line.77 Bau and colleagues measured the resting membrane potential of the mouse hippocampal cell line and the transient membrane potential response to extracellularγ-aminobutyric acid (GABA), a principle inhibitory neurotransmitter in the mammalian central nervous system.78 However, the conical shape of these nanoprobes, even with their relatively sharp nanoscale tip (∼200–400 nm in outer diameter), may cause cell damage and deformation when used for intracellular measurements [Fig. 5(b)].
Cell viability
Cell viability or more generally how the nanoneedle interferes with cellular functions is a critical issue in the use of nanoneedles for living cell experiments. Cell viability studies using the trypan blue exclusion assay , the Calcein AM assay , and the Annexin V-FITC/propidium iodideassay and monitoring the proliferation of cells showed that the penetration of nanoneedles (with diameters less than ∼100 nm) did not cause any distinct effect on the viability or membrane integrity of mammalian cells, such as HeLa cells, mouse embryonic stem cells and human embryonic kidney cells (HEK 293), over the time span of the experiments (several minutes to several days).28,30,31 Another cell viability study using the 4,6-diamido-2-phenylindole (DAPI) exclusion assay also suggests that the nanoneedle with diameter less than 400 nm can be maintained inside cells for at least one hour without affecting the viability of human epidermal melanocytes, HEK293 cells, and breast cancer cells (MCF-7).67 However, how and to what extent the penetration of nanoneedles affects cellular processes, such as mechanotransduction, are not clear yet.30 Although nanoneedles do not directly affect cell viability, as we can expect from the dependence of the penetration force and the deformation of cells on the diameter of nanoneedles (from the AFM studies),27,29,65 the effects of nanoneedles on cellular processes and functions are also likely to depend on the diameter and shape of the nanoneedles. For example, Yang and colleagues quantitatively measured the viability of mouse embryonic stem cells and human embryonic kidney cells cultured on vertically aligned Si nanoneedles with diameters of ∼30, 90, and 400 nm via the proliferation of the cells and propidium iodide staining.30 They showed that the cells cultured on 30 nm nanoneedles survived and proliferated for more than 5 days without showing any distinct difference from the cells cultured on glass surfaces and the cells cultured on 90 nm nanoneedles were alive for up to 3 days, while the cells cultured on 400 nm nanoneedles were dead within a day.30Perspectives
The unique ability of nanoneedles to probe and manipulate living cells can allow numerous new strategies for studying biological processes in living cells with high spatial and temporal precision, potentially with molecular resolution. For example, together with molecular targeting strategies, the ability of the nanoneedle to precisely deliver monodispered nanoparticles into target areas of living cells may enable simultaneous observation and manipulation of single biomolecules inside living cells (e.g., by using quantum dots and magnetic nanoparticles as molecular probes), allowing new ways to investigate biological processes at the single-molecule level inside living cells and thus overcoming one of the major challenges in single-molecule studies.31,33 As cellular probes using glass micropipettes have revolutionized cellular electrophysiology, the nanoneedle nanoprobe capable of spatially-resolved electrochemical measurements can further enhance our understanding of related mechanisms, including cellular signaling and intracellular redox states.77,78 Beyond intracellular delivery and electrophysiology, the use of nanoneedle technology is wide open. For example, nanoneedles immobilized with molecular biosensors (e.g., enzymes and quantum dots) can serve as intracellularbiosensors that can monitor local cellular processes and environments inside living cells, potentially at nanoscale resolution.58,96 Importantly, the recent discovery that a soft embryonic stem cell (ESC) is very sensitive to externally applied forces in force-induced spreading and differentiation97 makes nanoneedle-based technology very attractive for manipulating the cytoplasm and the nucleus of ESCs mechanically and electrochemically.Although the basic technical platform of nanoneedle technology has been established over the past several years, we are just beginning to use this emerging technology to tackle biologically important problems. To realize its full potential, the nanoneedle technology requires further research and development: for example, it is required to make this emerging technology more accessible to biological scientists (as glass micropipettes are) and more compatible with other techniques established in biology. A major challenge is the development of methods to produce nanoneedles in large quantities. Additionally, the multidisciplinary nature of nanoneedle technology requires that physical and biological scientists and engineers work together for the synergetic development and use of the nanoneedle technology. As it continues to develop, nanoneedle technology is expected to bring us enormous new opportunities to address more challenging problems in biology.
References
- G. M. Whitesides, Nat. Biotechnol., 2003, 21, 1161 CrossRef CAS.
- M. G. L. van den Heuvel and C. Dekker, Science, 2007, 317, 333 CrossRef.
- S. Weiss, Science, 1999, 283, 1676 CrossRef CAS.
- X. S. Xie, J. Yu and W. Y. Yang, Science, 2006, 312, 228 CrossRef CAS.
- D. Evanko, Nat. Methods, 2008, 5, 25 CrossRef CAS.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538 CrossRef CAS.
- A. P. Alivisatos, W. Gu and C. Larabell, Annu. Rev. Biomed. Eng., 2005, 7, 55 CrossRef CAS.
- M. Dahan, S. Levi, C. Luccardini, P. Rostaing, B. Riveau and A. Triller, Science, 2003, 302, 442 CrossRef CAS.
- M. Howarth, K. Takao, Y. Hayashi and A. Y. Ting, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 7583 CrossRef CAS.
- H. Bannai, S. Levi, C. Schweizer, M. Dahan and A. Triller, Nat. Protoc., 2007, 1, 2628 Search PubMed.
- M. Howarth, W. Liu, S. Puthenveetil, Y. Zheng, L. F. Marshall, M. M. Schmidt, K. D. Wittrup, M. G. Bawendi and A. Y. Ting, Nat. Methods, 2008, 5, 397 CrossRef CAS.
- V. Roullier, S. Clarke, C. You, F. Pinaud, G. r. Gouzer, D. Schaible, V. r. Marchi-Artzner, J. Piehler and M. Dahan, Nano Lett., 2009, 9, 1228 CrossRef CAS.
- S. Courty, C. Luccardini, Y. Bellaiche, G. Cappello and M. Dahan, Nano Lett., 2006, 6, 1491 CrossRef CAS.
- B. Cui, C. Wu, L. Chen, A. Ramirez, E. L. Bearer, W.-P. Li, W. C. Mobley and S. Chu, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 13666 CrossRef CAS.
- Q. Zhang, Y.-Q. Cao and R. W. Tsien, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17843 CrossRef CAS.
- Q. Zhang, Y. Li and R. W. Tsien, Science, 2009, 323, 1448 CrossRef CAS.
- R. J. Mannix, S. Kumar, F. Cassiola, M. Montoya-Zavala, E. Feinstein, M. Prentiss and D. E. Ingber, Nat. Nanotechnol., 2008, 3, 36 CrossRef CAS.
- D. Pantarotto, R. Singh, D. McCarthy, M. Erhardt, J.-P. Briand, M. Prato, K. Kostarelos and A. Bianco, Angew. Chem., Int. Ed., 2004, 43, 5242 CrossRef CAS.
- D. Cai, J. M. Mataraza, Z.-H. Qin, Z. Huang, J. Huang, T. C. Chiles, D. Carnahan, K. Kempa and Z. Ren, Nat. Methods, 2005, 2, 449 CrossRef CAS.
- N. W. Shi Kam, M. O'Connell, J. A. Wisdom and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11600 CrossRef CAS.
- X. Chen, U. C. Tam, J. L. Czlapinski, G. S. Lee, D. Rabuka, A. Zettl and C. R. Bertozzi, J. Am. Chem. Soc., 2006, 128, 6292 CrossRef CAS.
- K. Kostarelos, L. Lacerda, G. Pastorin, W. Wu, Sebastien Wieckowski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J.-P. Briand, S. Muller, M. Prato and A. Bianco, Nat. Nanotechnol., 2007, 2, 108 CrossRef.
- X. Chen, P. Wu, M. Rousseas, D. Okawa, Z. Gartner, A. Zettl and C. R. Bertozzi, J. Am. Chem. Soc., 2009, 131, 890 CrossRef CAS.
- F. Lu, L. Gu, M. J. Meziani, X. Wang, P. G. Luo, L. M. Veca, L. Cao and Y.-P. Sun, Adv. Mater., 2009, 21, 139 CrossRef CAS.
- Z. Liu, S. Tabakman, K. Welsher and H. Dai, Nano Res., 2009, 2, 85 Search PubMed.
- N. A. Kouklin, W. E. Kim, A. D. Lazareck and J. M. Xu, Appl. Phys. Lett., 2005, 87, 173901 CrossRef.
- I. Obataya, C. Nakamura, Han, N. Nakamura and J. Miyake, Nano Lett., 2005, 5, 27 CrossRef CAS.
- X. Chen, A. Kis, A. Zettl and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8218 CrossRef CAS.
- I. U. Vakarelski, S. C. Brown, K. Higashitani and B. M. Moudgil, Langmuir, 2007, 23, 10893 CrossRef CAS.
- W. Kim, J. K. Ng, M. E. Kunitake, B. R. Conklin and P. Yang, J. Am. Chem. Soc., 2007, 129, 7228 CrossRef CAS.
- K. Yum, S. Na, Y. Xiang, N. Wang and M.-F. Yu, Nano Lett., 2009, 9, 2193 CrossRef CAS.
- D. Weihs, T. G. Mason and M. A. Teitell, Biophys. J., 2006, 91, 4296 CrossRef CAS.
- K. C. Neuman and A. Nagy, Nat. Methods, 2008, 5, 491 CrossRef CAS.
- K. Yum, H. N. Cho, J. Hu and M.-F. Yu, ACS Nano, 2007, 1, 440 CrossRef CAS.
- J. R. Freedman, D. Mattia, G. Korneva, Y. Gogotsi, G. Friedman and A. K. Fontecchio, Appl. Phys. Lett., 2007, 90, 103108 CrossRef.
- T. E. McKnight, A. V. Melechko, G. D. Griffin, M. A. Guillorn, V. I. Merkulov, F. Serna, D. K. Hensley, M. J. Doktycz, D. H. Lowndes and M. L. Simpson, Nanotechnology, 2003, 14, 551 CrossRef CAS.
- T. E. McKnight, A. V. Melechko, D. K. Hensley, D. G. J. Mann, G. D. Griffin and M. L. Simpson, Nano Lett., 2004, 4, 1213 CrossRef CAS.
- D. G. J. Mann, T. E. McKnight, J. T. McPherson, P. R. Hoyt, A. V. Melechko, M. L. Simpson and G. S. Sayler, ACS Nano, 2008, 2, 69 CrossRef CAS.
- D. B. Peckys, N. d. Jonge, M. L. Simpson and T. E. McKnight, Nanotechnology, 2008, 19, 435301 CrossRef.
- S. Park, Y.-S. Kim, W. B. Kim and S. Jon, Nano Lett., 2009, 9, 1325 CrossRef CAS.
- M. R. Falvo, G. J. Clary, R. M. Taylor, V. Chi, F. P. Brooks, S. Washburn and R. Superfine, Nature, 1997, 389, 582 CrossRef CAS.
- E. W. Wong, P. E. Sheehan and C. M. Lieber, Science, 1997, 277, 1971 CrossRef CAS.
- M.-F. Yu, O. Lourie, M. J. Dyer, K. Moloni, T. F. Kelly and R. S. Ruoff, Science, 2000, 287, 637 CrossRef CAS.
- M.-F. Yu, B. S. Files, S. Arepalli and R. S. Ruoff, Phys. Rev. Lett., 2000, 84, 5552 CrossRef CAS.
- H. Dai, J. H. Hafner, A. G. Rinzler, D. T. Colbert and R. E. Smalley, Nature, 1996, 384, 147 CrossRef CAS.
- J. H. Hafner, C.-L. Cheung, T. H. Oosterkamp and C. M. Lieber, J. Phys. Chem. B, 2001, 105, 743 CrossRef CAS.
- N. R. Wilson and J. V. Macpherson, Nat. Nanotechnol., 2009, 4, 483 CrossRef CAS.
- S. Akita, H. Nishijima, Y. Nakayama, F. Tokumasu and K. Takeyasu, J. Phys. D: Appl. Phys., 1999, 32, 1044 CrossRef CAS.
- J. Martinez, T. D. Yuzvinsky, A. M. Fennimore, A. Zettl, R. Garcia and C. Bustamante, Nanotechnology, 2005, 16, 2493 CrossRef CAS.
- J. H. Hafner, C. L. Cheung and C. M. Lieber, Nature, 1999, 398, 761 CrossRef CAS.
- C. L. Cheung, J. H. Hafner and C. M. Lieber, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 3809 CrossRef CAS.
- E. Yenilmez, Q. Wang, R. J. Chen, D. Wang and H. Dai, Appl. Phys. Lett., 2002, 80, 2225 CrossRef CAS.
- J. Tang, B. Gao, H. Geng, O. D. Velev, L. C. Qin and O. Zhou, Adv. Mater., 2003, 15, 1352 CrossRef CAS.
- J. Tang, G. Yang, Q. Zhang, A. Parhat, B. Maynor, J. Liu, L.-C. Qin and O. Zhou, Nano Lett., 2005, 5, 11 CrossRef CAS.
- J. Zhang, J. Tang, G. Yang, Q. Qiu, L. C. Qin and O. Zhou, Adv. Mater., 2004, 16, 1219 CrossRef CAS.
- H. Wei, A. Craig, B. D. Huey, F. Papadimitrakopoulos and H. L. Marcus, Nanotechnology, 2008, 19, 455303 CrossRef.
- H. Wei, S. N. Kim, M. Zhao, S.-Y. Ju, B. D. Huey, H. L. Marcus and F. Papadimitrakopoulos, Chem. Mater., 2008, 20, 2793 CrossRef CAS.
- M. Jouzi, M. B. Kerby, A. Tripathi and J. Xu, Langmuir, 2008, 24, 10786 CrossRef CAS.
- A. Hall, W. G. Matthews, R. Superfine, M. R. Falvo and S. Washburn, Appl. Phys. Lett., 2003, 82, 2506 CrossRef CAS.
- T. A. El-Aguizy, J.-h. Jeong, Y.-B. Jeon, W. Z. Li, Z. F. Ren and S.-G. Kim, Appl. Phys. Lett., 2004, 85, 5995 CrossRef CAS.
- S. Kim, H. W. Lee and S.-G. Kim, Appl. Phys. Lett., 2009, 94, 193102 CrossRef.
- J. E. D. de Asis, Y. Li, R. Ohta, A. Austin, J. Leung and C. V. Nguyen, Appl. Phys. Lett., 2008, 93, 023129 CrossRef.
- A. Patil, J. Sippel, G. W. Martin and A. G. Rinzler, Nano Lett., 2004, 4, 303 CrossRef CAS.
- D. P. Burt, N. R. Wilson, J. M. R. Weaver, P. S. Dobson and J. V. Macpherson, Nano Lett., 2005, 5, 639 CrossRef CAS.
- I. Obataya, C. Nakamura, S. Han, N. Nakamura and J. Miyake, Biosens. Bioelectron., 2005, 20, 1652 CrossRef CAS.
- S. W. Han, C. Nakamura, I. Obataya, N. Nakamura and J. Miyake, Biosens. Bioelectron., 2005, 20, 2120 CrossRef.
- S. Han, C. Nakamura, I. Obataya, N. Nakamura and J. Miyake, Biochem. Biophys. Res. Commun., 2005, 332, 633 CrossRef CAS.
- I. Obataya, C. Nakamura, S. Han, N. Nakamura and J. Miyake, NanoBiotechnology, 2005, 1, 347 CrossRef CAS.
- T. Kihara, N. Yoshida, S. Mieda, K. Fukazawa, C. Nakamura, K. Ishihara and J. Miyake, NanoBiotechnology, 2007, 3, 127 CrossRef CAS.
- S.-W. Han, C. Nakamura, N. Kotobuki, I. Obataya, H. Ohgushi, T. Nagamune and J. Miyake, Nanomedicine, 2008, 4, 215.
- S.-W. Han, C. Nakamura, Y. Imai, N. Nakamura and J. Miyake, Biosens. Bioelectron., 2009, 24, 1219 CrossRef CAS.
- A. P. Suryavanshi and M.-F. Yu, Appl. Phys. Lett., 2006, 88, 083103 CrossRef.
- A. P. Suryavanshi and M.-F. Yu, Nanotechnology, 2007, 18, 105305 CrossRef.
- A. P. Suryavanshi, J. Hu and M. F. Yu, Adv. Mater., 2008, 20, 793 CrossRef CAS.
- G. T. Charras and M. A. Horton, Biophys. J., 2002, 82, 2970 CrossRef CAS.
- O. Chaudhuri, S. H. Parekh, W. A. Lam and D. A. Fletcher, Nat. Methods, 2009, 6, 383 CrossRef CAS.
- P. Sun, F. O. Laforge, T. P. Abeyweera, S. A. Rotenberg, J. Carpino and M. V. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 443 CrossRef CAS.
- M. G. Schrlau, N. J. Dun and H. H. Bau, ACS Nano, 2009, 3, 563 CrossRef CAS.
- C. Mueller-Falcke, S. D. Gouda, S. Kim and S.-G. Kim, Nanotechnology, 2006, 17, S69 CrossRef.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef CAS.
- A. Y. Krol, M. G. Grinfeldt, S. V. Levin and A. D. Smilgavichus, Eur. Biophys. J., 1990, 19, 93.
- A. H. Barber, S. R. Cohen and H. D. Wagner, Phys. Rev. Lett., 2004, 92, 186103 CrossRef.
- K. Yum and M.-F. Yu, Nano Lett., 2006, 6, 329 CrossRef CAS.
- D. J. Stephens and R. Pepperkok, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 4295 CrossRef CAS.
- F. O. Laforge, J. Carpino, S. A. Rotenberg and M. V. Mirkin, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 11895 CrossRef CAS.
- M. G. Schrlau, E. M. Falls, B. L. Ziober and H. H. Bau, Nanotechnology, 2008, 19, 015101 CrossRef.
- M. G. Schrlau, E. Brailoiu, S. Patel, Y. Gogotsi, N. J. Dun and H. H. Bau, Nanotechnology, 2008, 19, 325102 CrossRef.
- M. Schrlau and H. Bau, Microfluid. Nanofluid., in press Search PubMed.
- E. J. Wallace and M. S. P. Sansom, Nano Lett., 2008, 8, 2751 CrossRef CAS.
- J. K. Campbell, L. Sun and R. M. Crooks, J. Am. Chem. Soc., 1999, 121, 3779 CrossRef CAS.
- H. Boo, R.-A. Jeong, S. Park, K. S. Kim, K. H. An, Y. H. Lee, J. H. Han, H. C. Kim and T. D. Chung, Anal. Chem., 2006, 78, 617 CrossRef CAS.
- J. Shen, W. Wang, Q. Chen, M. Wang, S. Xu, Y. Zhou and X.-X. Zhang, Nanotechnology, 2009, 20, 245307 CrossRef.
- M. J. Esplandiu, V. G. Bittner, K. P. Giapis and C. P. Collier, Nano Lett., 2004, 4, 1873 CrossRef.
- Y. Narui, D. M. Ceres, J. Chen, K. P. Giapis and C. P. Collier, J. Phys. Chem. C, 2009, 113, 6815 CrossRef CAS.
- R. W. Murray, Chem. Rev., 2008, 108, 2688 CrossRef CAS.
- S. J. Clarke, C. A. Hollmann, Z. Zhang, D. Suffern, S. E. Bradforth, N. M. Dimitrijevic, W. G. Minarik and J. L. Nadeau, Nat. Mater., 2006, 5, 409 CrossRef CAS.
- F. Chowdhury, S. Na, D. Li, T. S. Tanake, F. Wang and N. Wang, Nat. Mater., 2009 DOI:10.1038/nmat2563.
| This journal is © The Royal Society of Chemistry 2010 |
