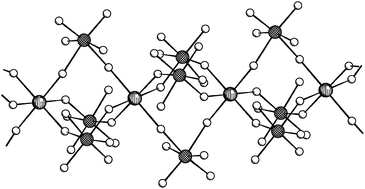
Reactions between strong Lewis acids (i.e. AsF5, SbF5) and MF3 (M = Al, Ga, In, Tl) in anhydrous hydrogen fluoride at ambient temperature proceeded only in three cases, yielding InF3·3SbF5, TlF3·3SbF5, TlF3·AsF5·2HF and TlF3·AsF5. Crystal structure of InF3·3SbF5 consists from infinite chains of In atoms connected by three SbF6 units, with two bridging fluorine atoms (Fb) in cis-position. Due to the strong interaction of SbF6 units with In3+, the Sb–Fb bonds are significantly elongated (200.7(4) pm). Such long Sb–Fb bonds have been observed in the crystal structure of SbF5. The crystal structure of TlF3·3SbF5 is built from slabs where thallium atoms are connected by SbF6 units. The thallium is nine-fold coordinated by fluorine atoms in the shape of a tricapped trigonal prism. In the crystal structure of TlF3·AsF5·2HF there are puckered layers, composed of rectangular rings made of Tl and F atoms. The seven-fold coordination around each Tl is completed by three axial fluorine atoms, provided by one HF molecule and two AsF6 units arranged below and above the puckered layers forming infinite slabs on that way. The second molecule of HF is placed between the slabs. Vibrational spectra of isolated InF3·3SbF5, TlF3·3SbF5, and TlF3·AsF5 are consistent with the presence of highly deformed SbF6/AsF6 octahedra.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...