A colorimetric and fluorescent chemosensor for copper ions in aqueous media and its application in living cells†
Huan-Huan
Wang
ab,
Lin
Xue
ab,
Zhang-Jian
Fang
a,
Guo-Ping
Li
ab and
Hua
Jiang
*a
aBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P. R. China. E-mail: hjiang@iccas.ac.cn; Fax: +86 10-62553316; Tel: +86 10-62553316
bGraduate School of Chinese Academy of Sciences, Beijing, P. R. China
First published on 11th May 2010
Abstract
Merocyanine dye 1 was prepared as an effective colorimetric and fluorescent chemosensor for copper ions in aqueous media, and displays a significant color change from reddish-purple to yellow with a large blue shift of 94 nm in its absorption spectrum in the presence of one equivalent of copper. As an on–off fluorescent sensor, it is feasible to detect copper ions in living cells due to its good cell permeability and high solubility under physiological conditions.
Copper, a co-factor in various enzymes and copper-based pigments, is an essential element in the human body. Despite its important roles in organisms, the accumulation of excess amounts of copper ions or their misregulation can cause a series of severe diseases. It is believed that the disruption of copper homeostasis is implicated to some neurodegenerative disorders like Alzheimer's and Parkinson's diseases.1 There is therefore a need to develop chemosensors to monitor copper ions in living cells or tissues. To this end, much effort has been devoted to exploring chemosensors so as to improve our understanding of the biological roles of copper ions in many respects, for example, in neurophysiology and neuropathology. Among the reported sensors for copper ions, fluorescent sensors2 and colorimetric sensors3 have attracted much attention because of their advantageous features of low cost, rapid detection and analysis in situ using simple apparatus. In particular, sensors with colorimetric and fluorescent features for copper ions4 are appealing because they can monitor copper ions both in solution by the naked eye and in living cells by fluorescence microscopy. So far, only a few colorimetric and fluorescent sensors based on the rhodamine chromophore for copper ions have been reported.4a–f However, only few of them4b,e exhibit good performance in pure aqueous media, which is a very important factor for potential biological applications. Therefore, developing new colorimetric and fluorescent chemosensors for copper ions that behave well under physiological conditions is highly desirable.
To achieve good solubility in water and a suitable cellular permeability, we employed indolium as a prochromophore, due not only to its good water solubility but also to its wide use in cyanine dyes.5 In addition, indolium styryl dyes were prepared to probe various metal ions.6 On the other hand, the DPA (di-2-picolylamine) moiety was chosen as a metal ion receptor because it has been commonly employed to prepare a host of chemosensors for transition metal ions such as zinc, cadmium and copper, etc.1c,7 Herein, we have incorporated the DPA moiety into indolium via a stryl group to achieve a novel merocyanine dye, 1, which displays a significant color change from reddish-purple to yellow with a large blue shift of 94 nm in its absorption spectrum and a “turn-off” of its fluorescence spectrum after exposure to copper ions in an aqueous buffer (25 mM HEPES, 0.1 M NaClO4, pH 7.4, 25 °C). It turns out that merocyanine dye 1 is a cellular permeable sensor that is able to detect copper ions in living cells.
As shown in Scheme 1, merocyanine dye 1 was easily synthesized from DPA derivative 3 and indolium salt 2 in one step, and was characterized by 1H NMR, 13C NMR and TOF-MS (see the ESI†).
 | ||
| Scheme 1 Synthesis of merocyanine dye 1. | ||
The response of 1 to various metal ions was investigated in aqueous buffer. It displays a very distinctive selectivity for copper ions, as observed by the naked eye. When 1 was treated with various metal ions, such as Cd2+, Pb2+, Zn2+, Ag+, Hg2+, Cu2+, Mn2+, Fe2+, Cr3+, Ca2+, Mg2+, K+ and Co2+ in the buffer, only copper ions caused a distinct color change from reddish-purple to yellow. In contrast, the other metal ions didn't give rise to any evident color change (Fig. 1a). Furthermore, the color change could be observed in copper/other metal combined samples. All samples containing various other metal ions exhibited a very distinguished color change after being exposed to copper ions (Fig. 1b). It reveals that 1 can discriminate copper ions from other metal ions in a colorimetric manner. In addition, to determine the threshold of the color change by the naked eye, an increasing amount of copper ions was added to the aqueous buffer of 1. As depicted in Fig. 1c, the color of the buffer solution graded gradually from reddish-purple to yellow in the presence of 0–4 equiv. of copper ions. Accordingly, 1 equiv. of copper ions can be set as the colorimetric threshold for determination by the naked eye.
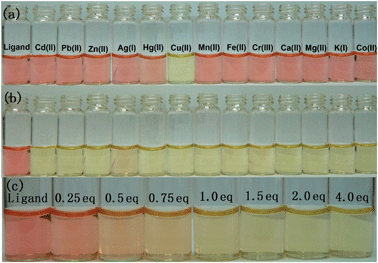 | ||
| Fig. 1 (a) The color change of 1 (12.5 μM) upon adding 1 equiv. of various metal ions. (b) The color change of 1 (12.5 μM) upon adding 1 equiv. of copper ions in the presence of various other metal ions. (c) The color change of 1 (12.5 μM) upon adding various amounts of copper ions to the aqueous buffer. | ||
Furthermore, we carried out UV-vis experiments to obtain the detailed absorption properties and binding constant. As shown in Fig. 2a, UV-vis experiments demonstrated that chemosensor 1 (12.5 μM) exhibited very strong absorption bands centered at 504 nm under identical conditions. As expected, the absorption band displayed a blue shift from 504 to 410 nm in the presence of 4 equiv. of copper ions. In addition, this spectral change could be reversed in the presence of either EDTA or TPEN (Fig. S1, see the ESI†), whereas Ag+, Fe2+, Co2+, Zn2+, Cd2+, Hg2+, Pb2+, Ca2+, Mg2+ and K+ didn't have any effect on the absorption spectrum of 1, which is consistent with the color change profile. Meanwhile, when various amounts of copper ions were added, an absorption band centered at 504 nm gradually decreased and, concomitantly, a new band at 410 nm appeared with a very clear isosbestic point at 444 nm, suggesting that the binding of copper ions disturbs the ground state charge transfer process in merocyanine (Fig. 2b).
 | ||
| Fig. 2 (a) UV-vis absorption spectra of 1 (12.5 μM) upon adding 4 equiv. of various metal ions. (b) The changes in the UV-vis absorption spectrum of 1 (25 μM) upon titrating 0–4 equiv. of copper ions. | ||
According to Job's plot, 1 and copper ions form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry complex (Fig. S2, see the ESI†). On the basis of a 1
1 stoichiometry complex (Fig. S2, see the ESI†). On the basis of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and a linear Benesi–Hildebrand expression, the measured 1/(A − A0) at 410 nm was plotted against 1/[Cu2+] to yield a binding constant of 15052 M−1 by a linear fit (R = 0.992) (Fig. S3, see the ESI†).
1 stoichiometry and a linear Benesi–Hildebrand expression, the measured 1/(A − A0) at 410 nm was plotted against 1/[Cu2+] to yield a binding constant of 15052 M−1 by a linear fit (R = 0.992) (Fig. S3, see the ESI†).
We next performed fluorescence titration experiments (Fig. 3). When a solution of 1 (2.5 μM) was excited at 444 nm, sensor 1 showed a strong fluorescence emission at 580 nm. Upon titration with various amounts of copper ions, the emission intensity at 580 nm was gradually quenched. The explanation for this phenomenon is partially due to the coordination of copper ions, which decreases the electron-donating ability of the nitrogen in the DPA moiety and thereby inhibits the ICT process,8 and partially due to the paramagnetic nature of copper ions. At the same time, we also evaluated the emission properties of the sensor in the presence of other metal ions. As expected, the fluorescence intensity of 1 remained unchanged upon adding 4 equiv. of other metal ions.
 | ||
| Fig. 3 (a) Fluorescence profiles of 1 (2.5 μM) upon titration with 0–4 equiv. of copper ions in an aqueous buffer (λex = 444 nm). (b) The fluorescence intensity enhanced index (F/F0) in the presence of 4 equiv. of various metal ions. | ||
With these findings at hand, we decided to rate the performance of 1 in living cells. Fibroblast NIH 3T3 cells (mouse embryonic fibroblast cell line) were incubated with 10 μM 1 in Dulbecco's modified Eagle's medium (DMEM, Hyclone) supplemented with 10% fetal calf serum (FCS, Hyclone), 1% penicillin (Hyclone) and 1% streptomycin (Hyclone) at 37 °C in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 CO2–air incubator. The cells were then washed with PBS to remove excess 1. A strong fluorescence emission was observed in the optical window at 560–610 nm, as shown in Fig. 4b. After it had been treated with 10 equiv. of copper ions for 20 min, intracellular fluorescence was almost completely suppressed, as shown in Fig. 4c. Subsequently, upon treating the cells with an excess amount of TPEN (N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine), the fluorescence intensity could be readily reversed. Thus, 1 is cell-permeable and can respond to copper ions within living cells.
95 CO2–air incubator. The cells were then washed with PBS to remove excess 1. A strong fluorescence emission was observed in the optical window at 560–610 nm, as shown in Fig. 4b. After it had been treated with 10 equiv. of copper ions for 20 min, intracellular fluorescence was almost completely suppressed, as shown in Fig. 4c. Subsequently, upon treating the cells with an excess amount of TPEN (N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine), the fluorescence intensity could be readily reversed. Thus, 1 is cell-permeable and can respond to copper ions within living cells.
 | ||
| Fig. 4 Confocal fluorescence images of intracellular Cu2+ in NIH 3T3 cells with 1: (a) Bright-field transmission image. (b) Confocal fluorescence image stained with 1. (c) When subsequently exposed to 10 equiv. of Cu2+ for 20 min. (d) Sequestration of intracellular Cu2+ by the addition of TPEN. | ||
In summary, chemosensor 1 behaves as a colorimetric and fluorescent chemosensor for copper ions under physiological conditions. Although its fluorescence emission was quenched while being exposed to copper ions, 1 is able to monitor the variation of copper ions in living cells. In addition, combining the indolium moiety with various receptors may generate many new colorimetric and fluorescent chemosensors for other specific metal ions in aqueous media.
We thank the Chinese Academy of Sciences “Hundred talents program”, the National Natural Science Foundation of China (90813002) and the State Key Laboratory of Fine Chemicals, Department of Chemical Engineering, Dalian University of Technology (KF0708) for financial support.
Experimental section
Synthesis of 1
2 (29 mg, 0.16 mmol) and 3 (50 mg, 0.165 mmol) were dissolved in ethanol with piperidine as the catalyst and the mixture refluxed for 2 h. The solvent was then evaporated and the residue purified by chromatography (silica gel, DCM/0–5% methanol) to give a dark red solid (37.51 mg, yield 40%). 1H NMR (400 MHz, CD3OD, ppm) δ 8.59 (2H, d, J = 4.0 Hz), 8.04 (3H, m), 7.66 (2H, m), 7.50 (5H, m), 7.21 (4H, m), 6.91 (2H, d, J = 8.0 Hz), 4.96 (4H, s), 4.25 (3H, s), 1.76 (6H, s). 13C NMR (100 MHz, CD3OD, ppm): δ 180.9, 157.1, 154.8, 153.8, 149.1, 142.7, 142.0, 137.6, 133.3, 128.8, 128.2, 123.7, 122.7, 122.3, 121.5, 113.3, 105.8, 56.5, 51.4, 25.5. Mass (MALDI-TOF): m/z 459.2 [M − I]+. HRMS (ESI) calc. for C31H31N4: 459.2543; found: 459.2537. mp: 135–137 °C.Cell culture
NIH 3T3 cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Hyclone) supplemented with 10% fetal calf serum (FCS, Hyclone), 1% penicillin (Hyclone) and 1% streptomycin (Hyclone) at 37 °C in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 CO2–air incubator. The cells were cultured for 3 d then loaded onto a 35 mm diameter glass-bottomed coverslip. The cells were incubated with 10 μM 1 for 30 min, washed with PBS three times and bathed in serum-free DMEM (2 mL) before imaging.
95 CO2–air incubator. The cells were cultured for 3 d then loaded onto a 35 mm diameter glass-bottomed coverslip. The cells were incubated with 10 μM 1 for 30 min, washed with PBS three times and bathed in serum-free DMEM (2 mL) before imaging.
Confocal fluorescence microscopy
An Olympus FV-1000 laser scanning microscopy system equipped with a 488 nm laser head was applied to confocal image NIH 3T3 cells stained with 1. The emission was collected at 560–610 nm. All of the images were gathered at the same confocal microscope settings and processed with Olympus FV10-ASWV0107A-1 software (Olympus, Japan).Notes and references
- (a) R. Krämer, Angew. Chem., Int. Ed., 1998, 37, 772 CrossRef CAS; (b) K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205 CrossRef CAS; (c) E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517 CrossRef CAS; (d) G. L. Millhauser, Acc. Chem. Res., 2004, 37, 79 CrossRef CAS; (e) E. Gaggelli, H. Kozlowski and G. Valensin, Chem. Rev., 2006, 106, 1995 CrossRef CAS; (f) G. Multhaup, A. Schlicksupp, L. Hess, D. Beher, T. Ruppert, C. L. Masters and K. Beyreuther, Science, 1996, 271, 1406 CAS; (g) R. A. Løvstad, BioMetals, 2004, 17, 111 Search PubMed.
- Fluorescence sensors for copper ions: (a) V. Dujols, F. Ford and A. W. Czarnik, J. Am. Chem. Soc., 1997, 119, 7386 CrossRef CAS; (b) J. S. Yang, C. S. Lin and C. Y. Hwang, Org. Lett., 2001, 3, 889 CrossRef CAS; (c) M. Royzen, Z. Dai and J. W. Canary, J. Am. Chem. Soc., 2005, 127, 1612 CrossRef CAS; (d) Z. C. Wen, R. Yang, H. He and Y. B. Jiang, Chem. Commun., 2006, 106 RSC; (e) J. Liu and Y. Lu, J. Am. Chem. Soc., 2007, 129, 9838 CrossRef CAS; (f) M. H. Kim, H. H. Jang, S. Yi, S. K. Chang and M. S. Han, Chem. Commun., 2009, 4838 RSC; (g) H. S. Jung, M. Park, D. Y. Han, E. Kim, C. Lee, S. Ham and J. S. Kim, Org. Lett., 2009, 11, 3378 CrossRef CAS; (h) Z. Xu, Y. Xiao, X. Qian, J. Cui and D. Cui, Org. Lett., 2005, 7, 889 CrossRef CAS; (i) H. S. Jung, P. S. Kwon, J. W. Lee, J. I. Kim, C. S. Hong, J. W. Kim, S. Yan, J. Y. Lee, J. H. Lee, T. Joo and J. S. Kim, J. Am. Chem. Soc., 2009, 131, 2008 CrossRef CAS; (j) Y.-Q. Weng, F. Yue, Y.-R. Zhong and B.-H. Ye, Inorg. Chem., 2007, 46, 7749 CrossRef; (k) V. S. Jisha, A. J. Thomas and D. Ramaiah, J. Org. Chem., 2009, 74, 6667 CrossRef CAS; (l) H. J. Kim, J. Hong, A. Hong, S. Ham, J. H. Lee and J. S. Kim, Org. Lett., 2008, 10, 1963 CrossRef CAS; (m) G.-K. Li, Z.-X. Xu, C.-F. Chen and Z.-T. Huang, Chem. Commun., 2008, 1774 RSC; (n) L. Zeng, E. W. Miller, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 10 CrossRef CAS.
- Colorimetric sensors for copper ions in organic or organic–aqueous mixed solutions: (a) S. H. Choi, K. Pang, K. Kim and D. G. Churchill, Inorg. Chem., 2007, 46, 10564 CrossRef CAS; (b) S. Basurto, O. Riant, D. Moreno, J. Rojo and T. Torroba, J. Org. Chem., 2007, 72, 4673 CrossRef CAS; (c) N. Shao, J. Y. Jin, H. Wang, Y. Zhang, R. H. Yang and W. H. Chan, Anal. Chem., 2008, 80, 3466 CrossRef CAS; (d) Y. Zhao, X.-B. Zhang, Z.-X. Han, L. Qiao, C.-Y. Li and L.-X. Jian, Anal. Chem., 2009, 81, 7022 CrossRef CAS; (e) M. S. Rodríguez-Morgade, M. Planells, T. Torres, P. Ballester and E. Palomares, J. Mater. Chem., 2008, 18, 176 RSC; (f) J. Huang, Y. Xu and X. Qian, Org. Biomol. Chem., 2009, 7, 1299 RSC; (g) S.-P. Wu, R.-Y. Huang and K.-J. Du, Dalton Trans., 2009, 4735 RSC; (h) N. Kaur and S. Kumar, Dalton Trans., 2006, 3766 RSC; (i) R. Sheng, P. Wang, Y. Gao, Y. Wu, W. Liu, J. Ma, H. Li and S. Wu, Org. Lett., 2008, 10, 5015 CrossRef CAS; (j) J. Huang, Y. Xu and X. Qian, Dalton Trans., 2009, 1761 RSC; (k) T. Gunnlaugsson, J. P. Leonard and N. S. Murray, Org. Lett., 2004, 6, 1557 CrossRef CAS.
- Selected examples: (a) Y. Xiang, A. Tong, P. Jin and Y. Ju, Org. Lett., 2006, 8, 2863 CrossRef CAS; (b) F. Yu, W. Zhang, P. Li, Y. Xing, L. Tong, J. Ma and B. Tang, Analyst, 2009, 134, 1826 RSC; (c) Y. Zhou, F. Wang, Y. Kim, S.-J. Kim and J. Yoon, Org. Lett., 2009, 11, 4442 CrossRef CAS; (d) X. Chen, M. J. Jou, H. Lee, S. Kou, J. Lim, S.-W. Nam, S. Park, K.-M. Kim and J. Yoon, Sens. Actuators, B, 2009, 137, 597 CrossRef; (e) K. M. K. Swamy, S.-K. Ko, S. K. Kwon, H. N. Lee, C. Mao, J.-M. Kim, K.-H. Lee, J. Kim, I. Shin and J. Yoon, Chem. Commun., 2008, 5915 RSC; (f) M. Yu, M. Shi, Z. Chen, F. Li, X. Li, Y. Gao, J. Xu, H. Yang, Z. Zhou, T. Yi and C. Huang, Chem.–Eur. J., 2008, 14, 6892 CrossRef CAS; (g) R. Martínez, F. Zapata, A. Caballero, A. Espinosa, A. Tárraga and P. Molina, Org. Lett., 2006, 8, 3235 CrossRef CAS.
- A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Chem. Rev., 2000, 100, 1973 CrossRef CAS.
- Selected examples of indolium styryl dyes: (a) I. K. Lednev, R. E. Hester and J. N. Moore, J. Chem. Soc., Faraday Trans., 1997, 93, 1551 RSC; (b) J. L. Bricks, J. L. Slominskii, M. A. Kudinova, A. I. Tolmachev, K. Rurack, U. R. Genger and W. Rettig, J. Photochem. Photobiol., A, 2000, 132, 193 CrossRef CAS; (c) O. A. Fedorova, Y. V. Fedorov, A. I. Vedernikov, S. P. Gromov, O. V. Yescheulova and M. V. Alfimov, J. Phys. Chem. A, 2002, 106, 6213 CrossRef CAS.
- Selected examples of fluorescence sensors containing a DPA receptor: (a) E. M. Nolan and S. J. Lippard, Acc. Chem. Res., 2009, 42, 193 CrossRef CAS; (b) X. Peng, J. Du, J. Fan, J. Wang, Y. Wu, J. Zhao, S. Sun and T. Xu, J. Am. Chem. Soc., 2007, 129, 1500 CrossRef CAS; (c) E. Ballesteros, D. Moreno, T. Gómez, T. Rodríguez, J. Rojo, G.-M. Valverde and T. Torroba, Org. Lett., 2009, 11, 1269 CrossRef CAS; (d) L. Xue, C. Liu and H. Jiang, Org. Lett., 2009, 11, 1655 CrossRef CAS.
- B. Valeur, Molecular Fluorescence: Principles and Applications, Wiley-VCH, Weinheim, 2002 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, characterization of 1 and additional spectroscopic data. See DOI: 10.1039/c0nj00168f |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2010 |
