There is plenty of room in the middle: crystal clear opportunities abound for coordination polymers
“I can hardly doubt that when we have some control of the arrangement of things on a small scale we will get an enormously greater range of possible properties that substances can have, and of different things that we can do.”(Richard P. Feynman, December 29, 1959).
Feynman's vision succinctly describes the rationale for crystal engineering, which was defined by Desiraju as
“…the understanding of intermolecular interactions in the context of crystal packing and utilization of such understanding in the design of new solids with desired physical and chemical properties.” 1
Crystal engineering was first coined by R. Pepinsky2 in 1955 and brought to practice by G. M. J. Schmidt in the context of topochemical reactions.3 Crystal engineering has blossomed into a paradigm for the understanding of existing crystalline solids and the design of new compounds with a customized composition and physical properties. However, this does not mean that crystal engineering and crystal structure prediction are synonymous, despite great advances in the latter in recent years.4 Rather, crystal engineering has been effective because one preselects molecular or ionic components and that are amenable to self-assembly.5 In this context, there is no class of compound that exemplifies the potential of crystal engineering better than coordination polymers. Indeed, one can assert that coordination polymers address both Feynman's vision and Desiraju's definition, partly as a result of their inherently modular nature and also because they can exhibit such extraordinary properties. Coordination polymers are a class of materials composed of metals or metal clusters (the “node”) coordinated to multi-functional organic ligands (the “linker”).6,7 It would be an understatement to assert that coordination polymers are now at the forefront of contemporary solid-state chemistry. A Web of Science® analysis of papers on the topic of coordination polymers is presented in Fig. 1, and it reveals that it was not until the early 1990s that systematic approaches were adopted for the design of coordination polymers. However, it was soon realized that coordination polymers could exhibit permanent porosity, thereby spawning a plethora of new jargon, such as porous coordination polymers (PCPs), metal–organic frameworks (MOFs) and porous coordination networks (PCNs). Today, we know the rest of the story. In 1999, it was discovered that coordination polymers could exhibit unprecedented levels of permanent porosity in the context of porous materials, far exceeding the maximum surface areas of existing porous materials such zeolites, carbon nanotubes, mesoporous aluminosilicates, aerogels and activated carbon. Indeed, even within the past year, new records have been set, with coordination polymers now exhibiting surface areas beyond 6000 m2 g−1.8 It is therefore unsurprising that since 2000, we have witnessed an exponential growth in the number of papers and the impact of those papers. This can be attributed to potential applications associated with extra-large surface area materials and also to major contributions to the field from research groups in China, which today contributes ca. 80% of the publications reporting new coordination polymers. The situation today is that the “genie is out of the bottle” with regard to coordination polymers; it is evident that they offer exciting opportunities to solve problems related to drug delivery, and energy and environmental sustainability.
 | ||
| Fig. 1 A Web of Science® analysis of publications and citations associated with “coordination polymers” since 1991 reveals a steady growth in activity since 1991 and an acceleration since ca. 2000 (search conducted 09/2010). | ||
The foundation for today's activity in coordination polymers resides in the seminal work of A. F. Wells,9 who introduced the “node and linker” interpretation of inorganic crystal structures. Inorganic crystal structures were thereby described as networks defined by metal ions (nodes) linked together via bonds (linkers or edges). An important aspect of this approach is that it is inherently modular, meaning that any existing network structure is in principle prototypal and might serve as a blueprint for many compounds with the same topology but with different chemical composition. Furthermore, even simple, ubiquitous molecular building blocks such as the dimetaltetracarboxylate or “paddlewheel” moieties can exhibit structural diversity, form multiple secondary building units (SBUs) and thereby afford multiple blueprints (Fig. 2). As the nascent field of coordination polymers advanced, the level of complexity and scale increased, and researchers began to address the functionality of this emerging class of materials. In the late 1990s, the research groups of S. Kitagawa10 and O. M. Yaghi11 reported the first examples of coordination polymers that exhibit permanent porosity. Remarkably, the modest porosity seen in these first examples of porous coordination polymers, i.e. surface areas of hundreds of m2 g−1, was quickly superseded by two coordination polymers that exhibit extra-large surface areas of ca. 1900 and 3000 m2 g−1, respectively. These compounds, which are called HKUST-112 and MOF-5,13 respectively, are illustrated in Fig. 3, and are largely responsible for the explosion of activity of the past decade. Specifically, they represent platforms, since they are prototypal of two classes of structure that are inherently predictable and fine-tunable. HKUST-1 is an example of a coordination polymer that is based upon a net formed by fused polyhedra, and it therefore has relatively large cavities and relatively small windows. It is related to zeolites but it does not exhibit a zeolitic topology. MOF-5 is an “isoreticular net” because it is based upon the linking of an octahedral node, and its cavities and windows are directly controlled by the length and width of the linker. The use of SBUs to generate porous coordination polymers can therefore be viewed as a landmark event in terms of design and utility because the greater relative size of SBUs vs. metal ions affords increased pore and cavity sizes, which in turn leads to extra-large surface areas.
 | ||
| Fig. 2 The versatility of the dimetaltetracarboxylate “molecular square” building block means that it can form at least three “blueprints” for coordination polymers based upon SBUs (colored purple): (i) square grid based upon square SBUs only; (ii) Kagomé lattice nets based upon triangular SBUs only; (iii) molecular faceted polyhedra based upon combinations of square and triangular SBUs. | ||
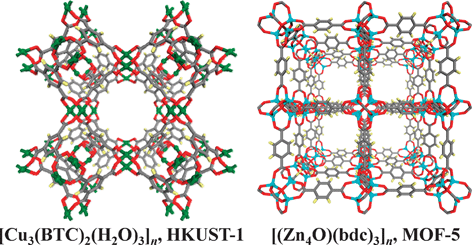 | ||
| Fig. 3 The prototypal extra-large surface area coordination polymers HKUST-1 (BTC = 1,3,5-benzenetricarboxylateand) and MOF-5 (bdc = 1,4-benzenedicarboxylate); both exhibit surface areas greater than any other class of porous materials. | ||
To summarize, the inclusion of SBUs into a chemist’s toolbox has facilitated the rapid development of new classes of coordination polymers, with properties and structures that can be readily understood and exploited from a crystal engineering perspective. Today, it is fair to assert that we are at the “end of the beginning”, since there is a general realization that a plethora of coordination polymer platforms exist, and that they offer nanoscale pores and cavities, i.e. a regime of nanospace that was hitherto unreachable in the context of crystalline solids. However, this does not mean that there is no longer any room for new design principles that offer even greater control over structure and/or scale or for systematic approaches to control bulk properties. This themed issue is a microcosm of the overall situation, since the papers published herein exemplify the diversity and practical relevance of coordination polymers as follows: by reinforcing the wide range of metal ions that can be exploited, by emphasizing how there is still a need for new ligand design, and by demonstrating how the issues of topology, polymorphism and interpenetration remain relevant.
Michael J. Zaworotko
(Department of Chemistry, University of South Florida, USA)
References
- G. R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier, Amsterdam, 1989.
- R. Pepinsky, Phys. Rev., 1955, 100, 971.
- G. M. J. Schmidt, Pure Appl. Chem., 1971, 27, 647–678.
- S. L. Price, Int. Rev. Phys. Chem., 2008, 27, 541–568.
- B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629–1658.
- B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546.
- S. R. Batten, S. M. Neville and D. R. Turner, Coordination Polymers: Design, Analysis and Application, Royal Society of Chemistry, Cambridge, 2009.
- H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. O. Yazaydin, R. Q. Snurr, M. O'Keeffe, J. Kim and O. M. Yaghi, Science, 2010, 329, 424.
- (a) A. F. Wells, in Three-Dimensional Nets and Polyhedra, Wiley, New York, 1977; (b) A. F. Wells, in Structural Inorganic Chemistry, Oxford University Press, London, 5th edn, 1984.
- M. Kondo, T. Yoshitomi, K. Seki, H. Matsuzaka and S. Kitagawa, Angew. Chem., Int. Ed. Engl., 1997, 36, 1725–1727.
- H. Li, M. Eddaoudi, T. L. Groy and O. M. Yaghi, J. Am. Chem. Soc., 1998, 120, 8571–8572.
- S. S.-Y. Chui, S. M.-F. Lo, J. P. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2010 |
