Bioimaging of metals in brain tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) and metallomics
J.
Sabine Becker
a,
Andreas
Matusch
b,
Christoph
Palm
b,
Dagmar
Salber
b,
Kathryn A.
Morton
c and
J.
Susanne Becker
d
aCentral Division of Analytical Chemistry, Forschungszentrum Jülich, D-52425, Jülich, Germany. E-mail: s.becker@fz-juelich.de
bInstitute of Neuroscience and Medicine (INM-1, INM-2, INM-4), Forschungszentrum Jülich, D-52425, Jülich, Germany
cDepartment of Radiology, University of Utah, Salt Lake City, UT, USA
dAeropharm GmbH, Rudolstadt, Germany
First published on 28th October 2009
Abstract
Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) has been developed and established as an emerging technique in the generation of quantitative images of metal distributions in thin tissue sections of brain samples (such as human, rat and mouse brain), with applications in research related to neurodegenerative disorders. A new analytical protocol is described which includes sample preparation by cryo-cutting of thin tissue sections and matrix-matched laboratory standards, mass spectrometric measurements, data acquisition, and quantitative analysis . Specific examples of the bioimaging of metal distributions in normal rodent brains are provided. Differences to the normal were assessed in a Parkinson’s disease and a stroke brain model. Furthermore, changes during normal aging were studied. Powerful analytical techniques are also required for the determination and characterization of metal-containing proteins within a large pool of proteins, e.g., after denaturing or non-denaturing electrophoretic separation of proteins in one-dimensional and two-dimensional gels . LA-ICP-MS can be employed to detect metalloproteins in protein bands or spots separated after gel electrophoresis. MALDI-MS can then be used to identify specific metal-containing proteins in these bands or spots. The combination of these techniques is described in the second section.
 J. Sabine Becker | Dr habil. J. Sabine Becker is head of the mass spectrometric group of the Central Division of Analytical Chemistry, Research Centre Jülich, Germany. Her present research activities are focused on development and application of highly sensitive methods for trace, surface and isotope analysis in life science studies by ICP-MS and LA-ICPMS. She has pioneered imaging LA-ICP-MS for micro- and nanolocal analysis of metals for brain research combined to metallomics. She is the author of the comprehensive handbook: Inorganic Mass Spectrometry: Principles and Applications, Wiley, 2007 and of 300 scientific publications, and is a member of several Advisory Boards e.g., the Int. J. Mass Spectrom. and J. Anal. At. Spectrom. |
 Andreas Matusch, Dagmar Salber and Christoph Palm | Dr Andreas Matusch studied medicine and physics at the universities of Marburg, Munich, Poitiers and Paris-VI. After obtaining his MD in medicine 2000 he received clinical education in Neurology in Paris and since 2003 he has worked at the Research Center Jülich in the field of molecular neuroimaging using mass spectrometric and radiotracer techniques at the junction of medicine, analytical and nuclear chemistry. |
Dr Christoph Palm received his PhD in computer science from the Aachen University of Technology (Aachen, Germany) in 2003. He is now with the Instiute of Neuroscience and Medicine (INM-1) at the Research Centre Jülich (Jülich, Germany). His research focuses include software solutions for LA-ICP-MS data and their fusion with other medical images resulting from several imaging techniques (i.e., autoradiography, immunostaining, MRI, and PET). |
Dr Dagmar Salber studied at RWTH Aachen (Aachen, Germany) and received her PhD in biology science from the Ludwig Maximilian University of Munich (Munich, Germany) in 2000. She is now with the Institute of Neuroscience and Medicine (INM-4) at the Forschungszentrum Jülich (Jülich, Germany). Her research focuses on the molecular background of brain lesions including cell-, protein- and metal accumulations as well as diagnostic prospects like PET and MRI to improve the understanding of pathological brain processes and their diagnostics. |
 Kathryn A. Morton | Dr Kathryn A. Morton, MD, is a tenured professor of radiology at the University of Utah (Salt Lake City, Utah, USA), where she completed her bachelor of science degree in biology in 1978, her medical doctorate degree in 1982, and residencies in both nuclear medicine and diagnostic radiology in 1987. She is the author of 108 publications and two books. Her areas of research emphasis have included the use of radioactive metals in neurology and oncology, neurofunctional imaging of pancreatic beta cell function, and imaging strategies in inflammation, infection and cancer-associated thrombosis. |
 J. Susanne Becker | Dr J. Susanne Becker is working in the pharmaceutical industry as an analytical method developer. She received her PhD at the University of Konstanz, Konstanz, Germany in the field of Alzheimer research using 1D/2D gel electrophoresis (SDS-PAGE) and high resolution mass spectrometry MALDI-/ESI-FTICR-MS for protein identification and characterization. She combined LA-ICP-MS and biomolecular MS methods for metallomics studies for the identification and quantification of metallo- and phosphoproteins. Afterwards she did her PostDoc at the CNRS in Pau, France, where she developed 2D BN-PAGE for the separation of metalloproteins. |
Introduction
There is a growing interest in the application of imaging techniques to map the distribution of elements (metals and non-metals) and molecules in biological tissues. Biomolecular imaging techniques are applicable to many areas of life sciences, including studies of the role of metals in neurodegenerative diseases.1–3 In oncology, the mapping of metal distribution may show different regional properties within the tumors,4,5 relevant to the growth6 and therapeutic response of the tumor.7 Bioimaging of metals and biomolecules has been performed both in animal tissue samples8–10 and in plants for investigation of nutrient uptake.11,12One of the most challenging topics in life science studies is the analysis of the distribution of essential and toxic metals in brain tissues. Essential trace metals such as iron, copper, zinc, manganese, and others are required for normal brain function and play a functional role in signalling, metabolism, as gene expression regulators, and as co-factors for enzymes that protect the brain from reactive oxide species (ROS). Trace metals are involved in a number of metabolic and physiological processes in the human body. Thereby, an excess or deficiency, either nutritional, environmental or genetically induced13 may contribute to neurological disorders. Neurodegenerative disorders such a Parkinson’s (PD) or Wilson’s disease, may be characterized by abnormal metal deposition, and metals may contribute to the formation of beta amyloid plaques in Alzheimer’s disease (AD).14–16 Therefore, total metal concentration as well as the regional spatial distribution of trace metals in diseased brain tissues compared to normal brain is of increasing importance for understanding the pathogenesis and potential treatment of neurodegenerative diseases.
In recent years due to the availability of emerging MALDI-MS (matrix assisted laser desorption/ionization mass spectrometry) instrumentation—including a powerful laser system, imaging software and sample preparation techniques—MALDI-MS has been established in many laboratories to study neurodegenerative disorders.17,18 However, there is a deficit in validated metal imaging instrumentation, quantitative analytical techniques for routine measurements and especially in imaging software packages for a fast evaluation of analytical data. Of the different imaging techniques for metals in life science studies, such as secondary ion mass spectrometry (SIMS)19 and X-ray spectroscopic techniques,20laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) plays an increasingly important role as a sensitive and isotope-specific, microanalytical technique to study the distribution and content of metals, with microscale resolution at trace and ultratrace concentrations. LA-ICP-MS is becoming the method of choice for the analysis of metals and non-metals in material and environmental research, in geological studies, and in biological and clinical samples to investigate detailed regionally specific element distributions in thin tissue sections of different sizes.21LA-ICP-MS is suitable for the imaging of metals (especially transition metals) in thin cross sections of soft and hard biological tissues.1,6,22–26 The detection limits in brain sections have been improved to the sub μg g−1 level and are in general lower than in SIMS. The advantages of LA-ICP-MS are high sample throughput, high sensitivity, accuracy and precision of the analytical data. In addition, no charging phenomena (required to compensate for the surface charge) occur, which is a limitation of SIMS, and fewer matrix effects simplify quantification of analytical data. Since no suitable certified standard reference materials for quantification procedures are available, different calibration strategies have been created in the author’s lab.
As the method of choice for quantification of the ion intensity images, matrix-matched laboratory standards were prepared and measured in the same run together with the biological samples.1,27 Solution-based calibration has also been created as an alternative quantification procedure by inserting a micronebulizer into the laser ablation chamber.1,27
LA-ICP-MS has been employed as a fast screening technique for analysis of metals, metalloids and non-metals in protein bands or protein spots separated by one- and two-dimensional denaturing (SDS-PAGE) or non-denaturing (Blue native PAGE) gel electrophoresis.28–33 This powerful analytical technique allows identification of metal-containing proteins within a large pool of proteins.33 Following elemental detection by LA-ICP-MS, MALDI-MS can be used as a complementary technique to identify the specific metal-containing proteins.
The aim of this paper is to describe the progress and selected applications of advanced imaging mass spectrometric techniques using quadrupole-based LA-ICP-MS in order to analyze the elemental distribution in brain samples. Different applications of imaging mass spectrometry by LA-ICP-MS focus on the quantitative distribution analysis in tissue sections. A strategy is discussed that combines LA-ICP-MS imaging with metal speciation using MALDI-MS, thus constituting a comprehensive metallomics approach in order to decipher the interplay of metals, metal-binding proteins and metal-containing precipitates.
Analytical techniques
All imaging LA-ICP-MS measurements were performed by a quadrupole-based mass spectrometer (ICP-MS Agilent 7500ce) coupled to a laser ablation system (New Wave UP 266). Slide-mounted slices of brain tissue were ablated in raster modus (line-by-line) with a focused laser beam (wavelength: 266 nm, laser pulse duration: 20 ns, repetition frequency: 20 Hz and laser power density: 1 × 109 W cm−2) with a laser spot size at 120 μm. The measurement time for one brain tissue section by imaging LA-ICP-MS (at the laser scan speed applied of 80 μm s−1) was about 3–4 h. All mass spectrometric measurements were performed under optimized experimental conditions: rf power, 1500 W and carrier gas flow rate, 1.2 L min−1.At present, no commercial software is available for the evaluation of the analytical LA-ICP-MS data. New software packages were developed in order to correlate the LA-ICP-MS imaging data with MRI (magnetic resonance images), immuno-stained, autoradiographic and histochemical images of the same rat brain at Forschungszentrum Jülich.34 The software package allows image reconstruction, a correct selection of specific brain regions such as cortical layers or deep nuclei, averaging of the measured ion intensities therein and calibration to finally obtain maps of elemental concentrations.
Quantification strategies in imaging LA-ICP-MS
LA-ICP-MS allows easy quantification of the measured metal ion distribution in biological sections. Quantitative images of metals were produced using matrix-matched homogenized laboratory standards. Control mouse (or rat) brain samples spiked with standard solutions of defined concentrations were used as the in-house laboratory standard for quantification of the LA-ICP-MS analytical data. The matrix (tissue) matching of lab standards is strongly recommended. In our experience, the quantified results could be biased if the matrix composition of laboratory standards differs from that of the sample tissue sections. These laboratory standards with defined matrix and trace metal composition were prepared as described previously,1 mounted on the glass substrate together with the sample tissue and employed for the quantification of measured LA-ICP-MS data in imaging brain tissues in routine mode. Because of the importance of an accurate calibration procedure for imaging LA-ICP-MS of human and rodent brain,35 we recommend a series of matrix-matched laboratory standards covering the biologically relevant range of analyte concentrations, prepared by spiking homogenized samples of human, rat or mouse brain tissue with standard solutions containing all the analytes of interest. After careful homogenization, the mixtures were frozen and cut into 20 μm sections by a cryostat microtome. The final concentrations of these standards were validated by ICP-MS after microwave induced digestion with HNO3 and H2O2. Measurements of a series of laboratory standards and the brain tissue under exactly the same experimental conditions in a single run provide ideal matrix matching for calibration purposes and correction for possible signal drifts.21 The homogeneity of prepared brain standards was studied by imaging LA-ICP-MS.During each second, one complete mass spectrum was obtained. Due to multielemental capability of LA-ICP-MS a multitude of metals, metalloids and non-metal images can be analyzed quasi-simultaneously.
To validate the metal ion images, two isotopes of the same element (e.g., 63Cu+ and 65Cu+) were analyzed, whenever possible. The validation of metal images was also performed by measuring the elemental distribution in neighboring tissue slices under the same experimental parameters. Possible isobaric interferences of single-charged analyte ions with polyatomic ions or double-charged ions were studied carefully. Fewer interference problems were observed in the LA-ICP-MS analysis of tissues in dry plasma compared to ICP-MS measurements of aqueous solutions.21 The detection limits for the metals in thin tissue sections were observed at trace concentration level (μg g−1 and below).
Bioimaging workflow and generation of ion images
The entire procedure of bioimaging LA-ICP-MS, including sample preparation by cryo-cutting of tissue sections, mass spectrometric measurement by scanning the samples line-by-line, acquisition and evaluation of analytical data, construction of metal ion images and quantification of analytical data is illustrated in Fig. 1. | ||
| Fig. 1 Workflow of bioimaging mass spectrometry from sample preparation of thin tissue sections by cryo-cutting, via the measurement procedure by scanning of thin tissue section (line by line), acquisition and evaluation of analytical data including quantification using matrix-matched laboratory standards. | ||
Calibration strategies
The calibration curves measured by LA-ICP-MS using prepared laboratory standards doped with defined concentrations of analyte (from 0–12 μg g−1 for Cu and Zn and to 120 μg g−1 for Fe) are summarized in Fig. 2. Correlation coefficients were between 0.991 (for Cu) and 0.997 (for Zn) with good linearity. This calibration strategy was applied for quantitative determination of copper, zinc and iron distribution in normal rat brain sections. Images of these three essential metals compared to potassium, manganese, magnesium and the nonmetals carbon, phosphorus and sulfur are illustrated in Fig. 3. Maximum metal concentrations in analyzed brain section were determined for Zn with 15 μg g−1, Cu with 6 μg g−1 and Fe with 29 μg g−1. For each element a specific and anatomically highly informative distribution pattern was measured. Non-metals were enriched in white matter (e.g., corpus callosum), zinc in the CA3 part of the hippocampus, the amygdala and cortex layers (grey matter) and copper in periventricular regions. A multi-layered structure of the cortex was visible in the iron and zinc images. The shape and structure of these LA-ICP-MS images are in good agreement with photographs of corresponding sections stained with cresyl violet (not shown in this figure). The highest ion intensities were observed for potassium, followed by sodium, phosphorus and iron.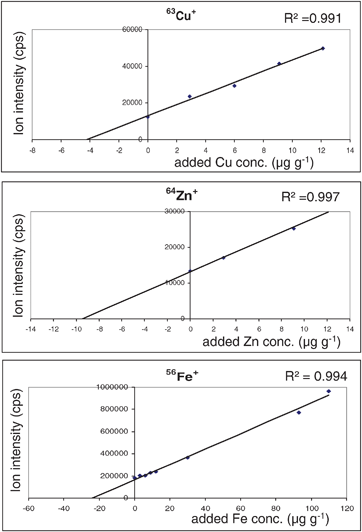 | ||
| Fig. 2 Calibration curves for Cu, Zn and Fe using matrix matched laboratory standards. The LA-ICP-MS measurements were performed using the ICP-MS, Agilent 7500ce coupled with the laser ablation system, New Wave UP 266. | ||
 | ||
| Fig. 3 Images of selected elements (Zn, Cu, Fe, K, Mn, Mg, C, P and S) in a rat brain section. For Zn, Cu and Fe the maximum of the concentration scale bar is indicated, the minimum being zero. | ||
Now the question is whether calibration not of the ion intensity, but of the ratio of the ion intensities, of an element of interest and a reference element can improve accuracy and the yield of biological information. Necessarily, such a reference element needs homogenous distribution. Unfortunately, this does not apply to cryo-sections of brain tissue. In a cryo-section the constant is the wet tissue weight per square centimetre. The water content is highly inhomogeneous and thus is any element concentration. Grey matter preferentially consists of cell bodies and synapses, its water content is 80%. White matter is predominantly composed of lipid rich fibres, and has a water content of 70%. In images obtained by calibration of the ratio of ion intensities of an element of interest and 13C, it is not clear whether a high signal results from a high element or high water content. We give an example of the interrelation of metal, 13C and quotient images using our own data obtained from a mouse brain section in Fig. 4. In white matter with low water and therefore high carbon content, element concentrations are underestimated as in the corpus callosum (cc) and the cerebral peduncle (cp). In grey matter structures with high water and low carbon contents, element concentrations are overestimated as in cortical layer II. These data originate from our recent systematic studies on brain sections (on 39 slices from 19 animals) of subchronically 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridin (MPTP; daily 30 mg kg−1, five days) intoxicated mice as a model of Parkinson’s disease and are discussed in a forthcoming paper.36
 | ||
| Fig. 4 Comparison of either calibrating the ion intensity of one element or the ratio of ion intensities of an element of interest (here 56Fe+) and a reference element (here 13C+). Upper row: Fe concentration image obtained by calibrating 56Fe+ and 13C+ images normalised to the average ion intensity in the corpus callosum. Lower row: Fe concentration image with an identical average concentration obtained by calibrating the ratio of ion intensities 56Fe+/13C+ for each pixel. Here, background intensity of the glass was set to zero. In white matter with low water and therefore high carbon content, element concentrations are underestimated as in the corpus callosum (cc), the cerebral peduncle (cp) and the corpus mammillare (cm). In grey matter, structures with high water and low carbon contents, element concentrations are overestimated as in cortical layer II. A photomicrograph of a cresyl-violet stained adjacent section is given for anatomical orientation. | ||
Applications of bioimaging of metals and metallomics
Photoinduced thrombosis—stroke model
In further studies at Research Centre Jülich (Institute of Neurosciences and Medicine) we investigated brain sections of rats with thrombosis,37 which serves as a model for stroke and was induced by the application of intense light according to Watson et al.38 The iron distribution in a section through such a cortical is illustrated in Fig. 5. The stroke region, here in the upper margin of the left hemisphere, was demarcated by an iron enriched zone. Further details of these investigations are described in a forthcoming paper.39 | ||
| Fig. 5 Iron distribution in rat brain with photothrombosis. | ||
Aging studies on mouse brain
A relevant research topic is studying changes of the cerebral metal distribution with aging. Fig. 6 exemplifies the different iron and zinc distribution in the brain of a young (2 months) and an old (14 months) mouse. These studies, performed with colleagues from the University of Utah, Salt Lake City, Utah, demonstrated increased Fe in structures such as the substantia nigra, the thalamus and the CA1 region of the hippocampus. Iron catalyzes formation of reactive oxygen species (ROS).14 Increased cerebral Fe-levels may contribute to age-related neurodegeneration. Zn concentrations largely appeared constant. The prominent Zn-enrichment in the CA3 was already detected in young mice, in line with the functionally essential role of Zn as a neuro-co-transmitter that is stored within vesicles. | ||
| Fig. 6 Comparison of the iron and zinc distribution in young and old mouse brain measured by LA-ICP-MS (left: photograph of mouse brain hippocampus). The LA-ICP-MS measurements were performed using the ICP-MS, Agilent 7500ce coupled with the laser ablation system, New Wave UP 266. | ||
Combination of bioimaging LA-ICP-MS in tissue sections and detection of metal-containing proteins in gels
The emerging field of metallomics includes studies of the role, uptake, transport and storage of trace metals essential for, or toxic to, protein functions, and provides information on the identity and quantity of metalloproteins. Complementary information is provided by the combination of element imaging mass spectrometric techniques, including LA-ICP-MS and biomolecular mass spectrometry such as ESI-MS (electrospray ionization mass spectrometry) or MALDI-MS (see Fig. 7). These techniques have been applied in different labs worldwide to elucidate the structure and sequence of metal- or phosphorus-containing proteins.28,30,33,40 Recently, Becker and Jakubowski40 reviewed the synergy of elemental and biomolecular mass spectrometry as a new analytical strategy in life sciences. | ||
| Fig. 7 Combination of bioimaging LA-ICP-MS on rat brain section (with photothrombosis) and metallomics studies using LA-ICP-MS to detect copper-binding proteins and MALDI-/ESI-MS for identification. | ||
LA-ICP-MS has been used to identify the metals bound to a protein and MALDI-/ESI-MS to elucidate identity, structure, dynamics and function of metal-protein complexes. As information resulting from sequence homology screens has been included into protein databases the metal binding status of a protein can now be used as additional search criterion.41
The combination of imaging mass spectrometry by LA-ICP-MS and proteome analysis is shown schematically at the example of a rodent brain in Fig. 7. This new analytical strategy begins with biometal imaging of thin sections of tissues: in this example, a rat brain was analyzed by LA-ICP-MS in routine mode with respect to iron. In the second step, the proteins extracted from the selected region of interest were separated by one-dimensional or two-dimensional gel electrophoresis. Using LA-ICP-MS, metalloproteins (and also phosphoproteins) were detected in gels by screening of the gels . A second gel was created under the same conditions, candidate spots containing metals and/or phosphorus were cut out, and after a tryptic digestion, the proteins were identified and the sequence determined by MALDI- or ESI-MS.
In order to study the binding of Cu and Zn in bovine serum proteins, isotopic tracer experiments were used in conjunction with LA-ICP-MS measurements.28 Bovine serum proteins were separated by 1D BN-PAGE and after gel electrophoresis the gels were doped with an enriched isotope copper tracer (65Cu) as a function of time (from 30 s up to 24 h). In the control 1D gel (without tracer) metal ions (mostly Zn) were found in several protein bands by LA-ICP-MS, but after experiments using enriched 65Cu a fast exchange of Zn bound to bovine serum albumin by copper was observed. This experimental finding demonstrates the formation of copper-binding proteins during the tracer experiments in the 1D gel . Furthermore, proteins of bovine serum were separated by two-dimensional (2D) blue native gel electrophoresis.28 The transfer of the imaging technique developed for tissues onto gels while studying metal distributions in protein spots allows the detection of metal containing spots. In addition, several protein spots that appeared as a single spot upon optical inspection could be subdivided for further metallomic studies. Also, LA-ICP-MS identified additional spots that were not visible in the stained gel , thus increasing the overall sensitivity of 2D-BN-PAGE.
Future applications of imaging LA-ICP-MS in neuroscience will likely include improvements of lateral resolution using nano-LA-ICP-MS.42–44 Imaging LA-ICP-MS will likely be increasingly combined with biomolecular mass spectrometric techniques such as MALDI- or ESI-MS, for the molecular identification and quantification of proteinphosphorylation and metal concentrations. This will enable the study of many important physiological and pathological processes, such as the post-translational modifications of proteins.21
Conclusion
Novel imaging mass spectrometric techniques using LA-ICP-MS have been established for quantitative distribution analysis of elements in clinical specimens at routine scale. New analytical tools were applied for sensitive and quantitative imaging of metals and non-metals in brain sections. The detection limits for metals in brain tissues were in the low μg g−1 range and below.Bioimaging analysis of essential and toxic elements in tissues by LA-ICP-MS enables novel studies of element distribution, transport processes and bioavailability. Combination of bioimaging of metals and proteome analysis (MALDI-MS) allows identification of metal-containing proteins and metal-dependent changes to the proteome morphologically guided by the quantitative metallo-anatomy of a sample specimen. Combination of bioimaging LA-ICP-MS techniques, established biomedical imaging techniques such as immunohistochemistry, autoradiography, magnetic resonance imaging (MRI) and metallomics provides opportunities to identity, quantify and establish the function of metalloproteins important in health and in the pathogenesis of neurodegenerative disorders within a unique spatial frame.
Acknowledgements
The authors would like to thank A. Zimmermann for technical support of LA-ICP-MS measurements.References
- J. S. Becker, M. Zoriy, C. Pickhardt, N. Palomero-Gallagher and K. Zilles, Anal. Chem., 2005, 77, 3208–3216 CrossRef CAS.
- R. W. Hutchinson, A. G. Cox, C. W. McLeod, P. S. Marshall, A. Harper, E. L. Dawson and D. R. Howlett, Anal. Biochem., 2005, 346, 225 CrossRef CAS.
- J. S. Becker, M. Zoriy, J. S. Becker, J. Dobrowolska and A. Matusch, J. Anal. At. Spectrom., 2007, 22, 736–744 RSC.
- J. S. Becker, M. Zoriy, M. Dehnhardt, C. Pickhardt and K. Zilles, J. Anal. At. Spectrom., 2005, 20, 912–917 RSC.
- M. Dehnhardt, M. Zoriy, Z. Khan, G. Reifenberger, T. J. Ekstrom, J. S. Becker, K. Zilles and A. Bauer, J. Trace Elem. Med. Biol., 2008, 22, 17–23 CrossRef CAS.
- M. Zoriy, M. Dehnhardt, A. Matusch and J. S. Becker, Spectrochim. Acta, Part B, 2008, 63, 375–382 CrossRef.
- M. Zoriy, A. Matusch, T. Spruss and J. S. Becker, Int. J. Mass Spectrom., 2007, 260, 102–106 Search PubMed.
- J. S. Becker, A. Matusch, C. Depboylu, J. Dobrowolska and M. Zoriy, Anal. Chem., 2007, 79, 6074–6080 CrossRef CAS.
- R. M. Caprioli, T. B. Farmer and J. Gile, Anal. Chem., 1997, 69, 4751–4760 CrossRef CAS.
- A. Kidness, N. Sekaran and J. Feldmann, Clin. Chem., 2003, 49, 1916–1923 CrossRef CAS.
- B. Wu, M. Zoriy, Y. Chen and J. S. Becker, Talanta, 2009, 78, 132–137 CrossRef CAS.
- J. S. Becker, R. C. Dietrich, A. Matusch, D. Pozebon and V. L. Dressler, Spectrochim. Acta, Part B, 2008, 63, 1248–1252 CrossRef.
- P. Zatta and A. Frank, Brain Res. Rev., 2007, 54, 19–33 CrossRef CAS.
- A. Sigel, H. Sigel and R. K. O. Sigel, Neurodegenerative Diseases and Metal Ions, John Wiley & Sons Ltd., Chichester, 2006 Search PubMed.
- A. I. Bush and R. E. Tanzi, Neurotherapeutics, 2008, 5, 421–432 CrossRef CAS.
- G. J. Liu, W. D. Huang, R. D. Moir, C. R. Vanderburg, B. Lai, Z. C. Peng, R. E. Tanzi, J. T. Rogers and X. D. Huang, J. Struct. Biol., 2006, 155, 45–51 CrossRef CAS.
- D. S. Cornett, S. L. Frappier and R. M. Caprioli, Anal. Chem., 2008, 80, 5648–5653 CrossRef CAS.
- E. H. Seeley, S. R. Oppenheimer, D. Mi, P. Chaurand and R. M. Caprioli, J. Am. Soc. Mass Spectrom., 2008, 19, 1069–1077 CrossRef CAS.
- F. D. Mai, B. J. Chen, L. C. Wu, F. Y. Li and W. K. Chen, Appl. Surf. Sci., 2006, 252, 6809–6812 CrossRef CAS.
- T. Punshon, B. P. Jackson, A. Lanzirotti, W. A. Hopkins, P. M. Bertsch and J. Burger, Spectrosc. Lett., 2005, 38, 343–363 CrossRef CAS.
- J. S. Becker, Inorganic Mass Spectrometry: Principles and Applications, John Wiley and Sons, Chichester, 2007 Search PubMed.
- A. M. Ghazi, J. C. Wataha, N. L. O'Dell, B. B. Singh, R. Simmons and S. Shuttleworth, J. Anal. At. Spectrom., 2002, 17, 1295–1299 RSC.
- B. Wu, M. Zoriy, Y. Chen and J. S. Becker, Talanta, 2009, 78, 132–137 CrossRef CAS.
- J. Feldmann, A. Kindness and P. Ek, J. Anal. At. Spectrom., 2002, 17, 813–818 RSC.
- C. Austin, D. Hare, A. L. Rozelle, W. H. Robinson, R. Grimm and P. Doble, Metallomics, 2009, 1, 142–147 RSC.
- M. C. Santos, M. Wagner, B. Wu, J. Scheider, J. Oehlmann, S. Cadore and J. S. Becker, Talanta, 2009, 80, 423–430.
- J. Dobrowolska, M. Dehnhardt, A. Matusch, M. Zoriy, P. Koscielniak, K. Zilles and J. S. Becker, Talanta, 2008, 74, 717–723 CrossRef CAS.
- J. S. Becker, D. Pozebon, V. L. Dressler, R. Lobinski and J. S. Becker, J. Anal. At. Spectrom., 2008, 23, 1076–1082 RSC.
- J. S. Becker, M. Zoriy, J. S. Becker, C. Pickhardt and M. Przybylski, J. Anal. At. Spectrom., 2004, 19, 149–152 RSC.
- N. Jakubowski, L. Waentig, H. Hayen, A. Venkatachalam, A. von Bohlen, P. H. Roos and A. Manz, J. Anal. At. Spectrom., 2008, 23, 1497–1507 RSC.
- P. H. Roos, A. Venkatachalam, A. Manz, L. Waentig, C. U. Koehler and N. Jakubowski, Anal. Bioanal. Chem., 2008, 392, 1135–1147 CrossRef CAS.
- J. S. Becker, M. Zoriy, M. Przybylski and J. S. Becker, J. Anal. At. Spectrom., 2007, 22, 63–68 RSC.
- J. S. Becker, R. Lobinski and J. S. Becker, Metallomics, 2009, 1, 312–316 RSC.
- C. Palm, A. Vieten, D. Salber and U. Pietrzyk, Phys. Med. Biol., 2009, 54, 3269–3289 CrossRef.
- J. S. Becker, M. Zoriy, A. Matusch, B. Wu, D. Salber, C. Palm and J. S. Becker, Mass Spectrom. Rev., 2009 DOI:10.1002/mas.20239 , published on-line.
- A. Matusch, C. Depboylu, C. Palm, B. Wu, G. U. Höglinger, M. K.-H. Schäfer and J. S. Becker, J. Amer. Soc. Mass Spectrom., 2009 DOI:10.1016/j.jasms.2009.09.022 , published on-line.
- D. Salber, G. Stoffels, D. Pauleit, A. M. Oros-Peusquens, N. J. Shah, P. Klauth, K. Hamacher, H. H. Coenen and K. J. Langen, J. Nucl. Med., 2007, 48, 2056–2062 CrossRef CAS.
- B. D. Watson, W. D. Dietrich, R. Busto, M. S. Wachtel and M. D. Ginsberg, Ann. Neurol., 1985, 17, 497–504 CrossRef CAS.
- D. Salber, B. Wu, C. Palm and J. S. Becker , 2009, in preparation.
- J. S. Becker and N. Jakubowski, Chem. Soc. Rev., 2009, 38, 1969–1983 RSC.
- W. Shi and M. R. Chance, Cell. Mol. Life Sci., 2008, 65, 3040–3048 CrossRef CAS.
- M. Zoriy and J. S. Becker, Rapid Commun. Mass Spectrom., 2009, 23, 23–30 CrossRef CAS.
- M. Zoriy, D. Mayer and J. S. Becker, J. Am. Soc. Mass Spectrom., 2009, 20, 883–890 CrossRef CAS.
- M. Zoriy, M. Kayser and J. S. Becker, Int. J. Mass Spectrom., 2008, 273, 151–155 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
