Zinc-dependent effects of small molecules on the insulin-sensitive transcription factor FOXO1a and gluconeogenic genes
Amy R.
Cameron
,
Siji
Anil
,
Emma
Sutherland
,
Jean
Harthill
and
Graham
Rena
*
Centre for Neuroscience, Ninewells Hospital and Medical School, University of Dundee, Dundee, Scotland. E-mail: g.rena@dundee.ac.uk; Fax: +44 (0)1382 667120; Tel: +44 (0)1382 660111 ext 33126
First published on 19th November 2009
Abstract
Metal-binding compounds have recently been reported to have anti-hyperglycaemic properties in vivo. In the current study, we have investigated the ability of these compounds and related structures to induce insulin-like signal transduction to downstream effectors such as the transcription factor FOXO1a and the key gluconeogenic regulatory enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase). Our results indicate that β-thujaplicin, diethyldithiocarbamate (DEDTC) and its clinically-used dimer disulfiram, induce insulin-like dose-dependent effects on signalling to FOXO1a in a manner that is strictly dependent on the presence of zinc ions, as other ions including aluminium, cobalt, copper, lithium and manganese cannot substitute. The most potent compound tested on gluconeogenesis is disulfiram, which in the presence of 10 μM zinc, inhibited both PEPCK and G6Pase with an IC50 of 4 μM. Our results demonstrate that metal-binding compounds with diverse structures can induce zinc-dependent insulin-like effects on signal transduction and gene expression.
Introduction
Our current studies focus on the identification and characterisation of compounds eliciting insulin-like signalling on downstream effectors of insulinsignal transduction, including the transcription factor FOXO1a1–8 and the gluconeogenic regulatory enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose 6-phosphatase (G6Pase).9–18 Characterisation of compounds with these signalling properties might be useful in the search for novel treatments of insulin resistance, where tissues become progressively resistant to insulin action. Originally attributed directly to dysfunction of metabolic enzymes,19 contemporary models of insulin resistance emphasise the importance of reversing defects in information processing by signal transduction pathways.20Recently we identified theaflavin polyphenols from black tea as novel mimics of insulin/IGF-1 signalling (IIS) to FOXO1a and PEPCK.21 Theaflavins contain a metal bindingtropolone -like motif22 and our evidence that this part of the molecule contributes to the insulin-like properties of theaflavins21 has prompted us to become interested in the effects of metals on IIS. There is growing evidence that dysregulation of metal homeostasis might have an impact on insulin action, with much of the attention focused on zinc.23 Zinc is known to be required for insulinpolymerisation and storage in many species24–27 and following zinc binding, the structure of insulin is altered28–30 and its duration of action is prolonged.31–33 Recently, a single nucleotide polymorphism for the zinc transporter SLC30A834 has been identified which is associated with increased risk of (insulin-resistant) type 2 c in humans,35 a finding which is replicated in the UK population36 providing genetic evidence that alterations in this transporter increases risk of T2D.
In cell models, zinc has insulin-like or sensitizing properties on key signalling and effector components of IIS, including tyrosinephosphorylation of the insulin and IGF-1 receptors,37,38 inhibition of the receptor phosphatase PTP1b,38phosphorylation and activation of mTOR,39phosphorylation of PKB/Akt,37,40–42 GSK-3,40,42 inhibition of FOXO transcription factors,40,41 enhanced glucose transport,37lipogenesis43 and antilipolysis.44In vivo, zinc supplementation by itself appears to have little effect on alleviation of metabolic dysfunction45 but recently, two structurally unrelated groups of zinc-binding compounds have been shown to alleviate hyperglycemia and improve glucose tolerance in obesity-induced diabetic rodents.46,47 Examples of these two groups include diethyldithiocarbamate (DEDTC)46 and β-thujaplicin,47 the second of which shares with theaflavins a metal-binding tropolone -like structural motif.21 These compounds are thought to work by increasing access of zinc to intracellular locations;46 however, the potential contribution of any metal-dependent alterations in IIS in their effects is unclear. In the current study, we have investigated the ability of DEDTC, β-thujaplicin and related compounds to induce IIS in the presence and absence of a variety of metals.
Experimental procedures
Materials and methods
(8-(4-Chlorophenylthio)-cAMP (8CPT-cAMP), 2,5-dihydroxy-3-oxo-1,4,6-cycloheptatriene-1-carboxylic acid, aluminium chloride, ammonium diethyldithiocarbamate, β-thujaplicin, copper sulfate, disulfiram, lithium chloride, manganese chloride, theaflavin extract, tropone , tropolone , wortmannin and zinc diethyldithiocarbamate all came from Sigma-Aldrich. The PKB inhibitor Akti-1/2, PI-103, PD98059 and rapamycin were from Calbiochem. Zinc acetate came from Riedel de Haen. The compounds used in this study were dissolved in DMSO, except for metal salts that were dissolved in water and β-thujaplicin which was dissolved in methanol. Compounds were stored aliquotted at −20 °C. Aliquots were discarded after one freeze-thaw cycle. All antibodies have been described previously by us4,48 except pFOXO1a Ser256, pPKB Ser 473 which came from CST and the actin antibody which came from Calbiochem/Merck.Cell culture and lysis
293 and HL1c cells were maintained essentially as described previously.4,49 293 cells were used for experiments four or five days after seeding and the DMEM/Fetal calf serum replaced the day before the experiment. HL1c cells were used for experiments on the second day after seeding and serum starved the evening before stimulation. Stimulation was performed in serum-free DMEM. The inhibitors PI-103 and wortmannin were added 1 h before stimulation, Akti was added 10 min before stimulation and both PD98059 and rapamycin were added 30 min before stimulation. Cells were lysed on ice using buffer A: (50 mM Tris acetate (pH 7.5), 1% (w/v) Triton X100, 1 mM EDTA, 1 mM EGTA, 50 mM NaF, 1 mM benzamidine, 0.2 mM phenylmethylsulfonyl fluoride and 0.1% (v/v) β-mercaptoethanol). The lysates were centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g and the supernatants removed and stored at −80 °C until use. In all experiments, cells were serum starved prior to stimulation for at least 30 min. Unless stated elsewhere, results are representative of at least three experiments.
000 g and the supernatants removed and stored at −80 °C until use. In all experiments, cells were serum starved prior to stimulation for at least 30 min. Unless stated elsewhere, results are representative of at least three experiments.
RT-PCR
Real-time polymerase chain reaction (RT-PCR ) assays of insulin-sensitive genes were carried out in HL1c cells, cultured in DMEM containing 10% FBS and 1 g l−1glucose. Serum starved cells were stimulated with agents for 4 h. RNA was obtained by using RNeasy ‘mini’ kit from Qiagen. RNA was reverse transcribed to produce first strand cDNA using SuperScript II reverse transcriptase (Invitrogen). Briefly, RNA, dNTPs and random primers were heated at 65 °C for 5 min and then chilled. Next, the reverse transcriptasebuffer and 0.1 M dithiothreitol were added, prior to incubation with reverse transcriptase at 25 °C for 10 min and 42 °C for 50 min. The reaction was terminated by heating at 70 °C for 15 min. RT-PCR was performed in a 96-well plate with sequence-specific primers and probes. In each experiment, results were normalized to Dex/cAMP where Dex/cAMP = 100 and in each sample, RNA levels were normalised to cyclophilin. Statistical significance was assessed by one-way ANOVA and Bonferroni post-hoc test. IC50s were calculated using GraphPad Prism.Results and discussion
Zinc-dependent FOXO1a phosphorylation elicited by compounds with a tropolone -like structure
Previously, we developed four antibodies that are capable of detecting endogenous FOXO1a in lysates to study FOXO1a phosphorylation in response to insulin4,48 and other regulators of the pathway.21,50 One antibody detects FOXO1a regardless of the phosphorylation state, while the others detect FOXO1a phosphorylated on Thr24, Ser256 and Ser325. PtdIns 3-kinase dependent phosphorylation of Ser325 follows phosphorylation at two priming sites, Ser319 and Ser322.2,48,51 In the current study, the ability of β-thujaplicin and related compounds to induce FOXO1a phosphorylation was investigated in 293 cells. Without added zinc, none of these compounds were able to induce FOXO1a phosphorylation but in contrast, when added together with 100 μM zinc acetate, β-thujaplicin and tropolone each induced FOXO1a phosphorylation (Fig. 1a). An extract containing gallated theaflavins TF2A, TF2B and TF3, which induce IIS in the absence of added extracellular zinc,21 is included here as a positive control (Fig. 1a). Next, we investigated derivatives of the tropolone structure (Table 1). Compared with β-thujaplicin, induction of FOXO1a phosphorylation was virtually abolished using tropone , which lacks the hydroxyl group found in tropolones (Fig. 1b). The hydroxyl group is thought to be involved in coordinating the zinc ions47 and therefore, loss of zinc binding is the most likely explanation for the lack of phosphorylating activity of this structure. Alteration of the tropolone structure by addition of a carboxyl group also eliminated IIS induction (Fig. 1b), presumably by changing the charge. Tropolone and β-thujaplicin are predicted to form uncharged complexes with zinc, whereas the carboxylated derivative will form charged complexes like EDTA does, which are likely to be membrane impermeant.52 Next, we tested for the presence of interaction between extracellular added zinc and tropolones in the context of the theaflavin structure. Since gallated theaflavins TF2A, TF2B and TF3 induce IIS without added extracellular zinc21 and bind metals not only through the tropolone ring but also through the gallate group,53,54 we exploited theaflavin TF1 (Table 1), which lacks a gallate group and does not induce IIS acutely in the absence of added extracellular zinc.21 Similar to the results with β-thujaplicin, we found that TF1 induced IIS in the presence of zinc (Fig. 1c)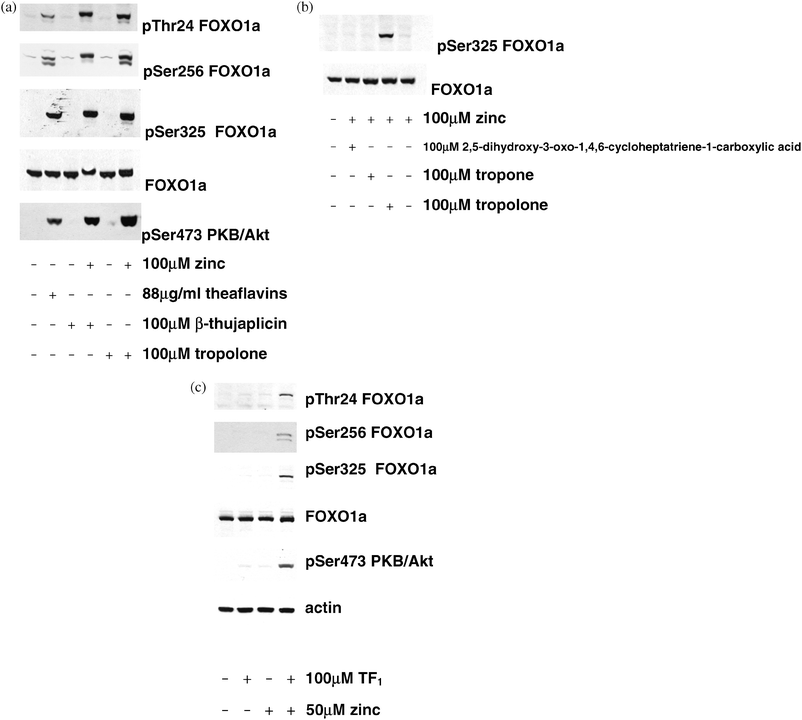 | ||
| Fig. 1 Effects of tropolone -containing molecules on IIS. (a) 293 cells were stimulated with the compounds shown for 30 min and lysed as described in the methods prior to SDS-PAGE and immunoblotting with the antibodies indicated. A theaflavin extract containing TF2A, TF2B and TF3 is present as a positive control. (b) Effect of compounds structurally related to the active tropolones . Cells were stimulated with the compounds shown for 30 min prior to lysis, SDS-PAGE and immunoblotting as in (a). (c) Effect of ungallated theaflavin TF1 in the presence and absence of zinc. Cells were stimulated with the compounds shown for 1 h prior to lysis, SDS-PAGE and immunoblotting as in (a). | ||
| Compound name | Structure | Zinc-dependent FOXO1a phosphorylation |
|---|---|---|
| β-Thujaplicin |

|
+ |
| Tropolone |

|
+ |
| Tropone |

|
— |
| 2,5-Dihydroxy-3-oxo-1,4,6-cycloheptatriene-1-carboxylic acid |

|
— |
| Theaflavin TF1 |

|
+ |
As β-thujaplicin complexed with zinc has been shown to have glucose-lowering properties in vivo,47 this structure was taken for further study. In dose response experiments, β-thujaplicin exerted insulin-like effects that were maximal between 50 μM and 100 μM in the presence of 30 μM zinc (Fig. 2a) and in time-course experiments, this combination of zinc and β-thujaplicin induced FOXO1a phosphorylation within 15 min (almost as quickly as IGF-1, Fig. 2b). The phosphorylation began to decline at 4 h (Fig. 2b). The extract containing TF2A, TF2B and TF3 is used again here simply as a positive control (Fig. 2a and b).
 | ||
| Fig. 2 Effect of β-thujaplicin and zinc on IIS (a) Dose-response of β-thujaplicin and zinc on IIS phosphorylation. 293 cells were stimulated with the concentrations of β-thujaplicin and zinc shown for 1 h prior to immunoblotting as in Fig. 1a. A theaflavin extract containing TF2A, TF2B and TF3 is present as a positive control. (b) Time-course. Cells were stimulated with 100 μM β-thujaplicin and 30 μM zinc for the times shown prior to immunoblotting as in Fig. 1a. (c) Ability of other metals to mimic the effect of zinc. Cells were stimulated with or without 100 μM β-thujaplicin and 30 μM metal ions and then incubated for 1 h prior to immunoblotting as in Fig. 1a. (d,e) effect of protein kinase inhibitors. Cells were stimulated as in Fig. 1a with or without pre-treatment with the kinase inhibitors indicated, prior to lysis, SDS-PAGE and immunoblotting as in Fig. 1a. Control experiments established inhibition of p70S6kinase phosphorylation by rapamycin and inhibition of p42MAP kinasephosphorylation by PD98059 (data not shown). | ||
Next, we investigated the ability of other metal ions to substitute for zinc. Cu2+ has previously been shown to induce IIS when added to cells incubated in a buffered saline solution;40,41,55 however, this effect of Cu2+ is more labile than zinc effects as it is valency-dependent55 and in addition, it is abolished by the presence of amino acids present in the plasma.40 In addition to Zn2+ and Cu2+ we tested Mn2+, which is present in the FOXO-regulated genemanganese superoxide dismutase,56 monovalent Li+, which is a well known inhibitor of the IIS downstream effector GSK3,57 and Al3+ (data not shown). In our experiments, carried out in the presence of amino acids, we found that none of the metals tested could substitute for zinc (Fig. 2c).
Residues Thr24, Ser256 and Ser319 on the FOXOs lie not only within consensus sequences for phosphorylation by PKB/Akt and SGK but also for phosphorylation by p70 S6 kinase and p90RSK. Previously we used kinase inhibitors to determine a common signalling pathway that insulin and IGF-1 use to induce FOXO1a phosphorylation. We repeated these experiments with β-thujaplicin/zinc. We found that FOXO1a phosphorylation was sensitive to two PtdIns 3-kinaseinhibitors, PI-103 and wortmannin (Fig. 2d). Additional experiments in which 293 cells were pre-incubated either with rapamycin (which prevents activation of p70 S6 kinase by inhibiting mTOR) or PD98059 (which prevents the activation of p90RSK) showed that neither of these drugs affected the β-thujaplicin/zinc-induced phosphorylation of FOXO1a, whereas a recently identified PKB-specific inhibitor15,58 did inhibit FOXO1a phosphorylation (Fig. 2e). We have shown previously that PtdIns 3-kinase dependent phosphorylation of Ser325 also indicates phosphorylation of Ser319 and Ser322 because it depends on prior phosphorylation of these residues.48 Taken together with this previous data, our results suggest that β-thujaplicin/zinc induce phosphorylation of FOXOs by PtdIns 3-kinase-sensitive and PKB-dependent phosphorylation of Thr24, Ser256 and Ser319, the latter residue then priming CK1 to phosphorylate Ser322 and Ser325. These observations of IIS induction may underlie the anti-hyperglycaemic effects of the β-thujaplicin/zinc complex in obese diabetic rodents47 and they further indicate that (in vitro at least) IIS can be induced by tropolones and zinc without the generation of preformed zinc/tropolone complexes.
Zinc-dependent IIS induction by diethyldithiocarbamate (DEDTC) and disulfiram
Having determined the strict zinc dependence of β-thujaplicin-mediated induction of IIS, we investigated additional zinc binding compounds structurally unrelated to the tropolones , in order to determine whether or not these properties are shared between them. One of the most commonly used zinc-binding compounds in biochemistry is diethyldithiocarbamate (DEDTC) (Table 2). Like β-thujaplicin, zinc/dithiocarbamate complexes have recently been found to alleviate hyperglycaemia in vivo.46 In dose response experiments, we found that a zinc/DEDTC complex induced FOXO1a phosphorylation with an effect close to maximal levels at a concentration of 10 μM (Fig. 3a). In time course experiments, the effect on IIS was maximal at 60 min (Fig. 3b). A similar response was achieved when ammoniumDEDTC and zinc acetate were added together, suggesting that addition of preformed zinc complexes does not improve potency (Fig. 3c).| Compound name | Structure | FOXO1a phosphorylation |
|---|---|---|
| Diethyldithiocarbamate |

|
+ |
| Disulfiram |

|
+ |
 | ||
| Fig. 3 Zinc-dependent IIS induction by zinc-binding compounds unrelated to tropolones . (a) Dose-response of zinc/DEDTC on IIS phosphorylation. 293 cells were stimulated with the concentrations of zinc/DEDTC shown for 1 h and lysed as described in the methods prior to SDS-PAGE and immunoblotting with the antibodies indicated. (b) Time-course of zinc/DEDTC on IIS phosphorylation. Cells were stimulated with 20 μM zinc/DEDTC for the times shown and lysed as described in the methods prior to SDS-PAGE and immunoblotting with the antibodies indicated. (c) Comparison of DEDTC and disulfiram on IIS phosphorylation. Cells were stimulated with 20 μM zinc DEDTC, ammonium DEDTC, disulfiram and zinc acetate for the times shown prior to immunoblotting as in (a). (d) Dose responses of disulfiram on IIS phosphorylation. Cells were stimulated with disulfiram and zinc for the times shown prior to immunoblotting as in (a). (e) Time course of disulfiram on IIS phosphorylation. Cells were stimulated with 20 μM zinc and 20 μM disulfiram for 1 h and lysed as described in the methods prior to SDS-PAGE and immunoblotting with the antibodies indicated. (f) Effect of protein kinase inhibitors. Cells were stimulated with 20 μM disulfiram plus 20 μM zinc with or without pre-treatment with the kinase inhibitors indicated, prior to lysis, SDS-PAGE and immunoblotting as in (a). (g) Ability of other metals to mimic the effect of zinc. Cells were stimulated with or without 20 μM disulfiram in the presence or absence of 20 μM metal ions shown and then incubated for 1 h prior to immunoblotting as in (a). | ||
DEDTC is not used clinically but it is produced stoichiometrically in the blood following administration of disulfiram (Table 2),59 which is used in alcohol aversion therapy and is at least 80% bioavailable.60Disulfiram is formed by the linkage of two molecules of DEDTC through two of their four metal-coordinating sulfhydryl groups but since we knew that the effect on IIS does not require preformation of zinc complexes (Fig. 1–3), we tested disulfiram in the presence of zinc acetate and found that it too induced FOXO1a phosphorylation (Fig. 3c). In dose-response experiments, disulfiram acted with a similar potency to DEDTC (Fig. 3a and d), producing IIS induction that was near maximal at 30 min (Fig. 3e) and was sensitive to inhibition of PtdIns 3-kinase and PKB/Akt (Fig. 3f). Next we investigated the ability of a variety of metal ions to substitute for zinc in the effect on FOXO1a phosphorylation. Similar to the results with β-thujaplicin we found that none of these ions could induce FOXO1a phosphorylation in the presence of the compound (Fig. 3g). This zinc-dependent induction of IIS is consistent with and might contribute to the earlier observations that administration of zinc/dithiocarbamate complexes reduces hyperglycaemia in obese diabetic rodents.46
Effect on expression of gluconeogenic genes
Next we examined the effect of β-thujaplicin/zinc and disulfiram/zinc on gene expression. Repression of hepatic gluconeogenesis by reduced expression of phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) is recognized as a key aspect of the anti-hyperglycaemic action of insulin. In the current studies we found that like insulin, β-thujaplicin/zinc and disulfiram/zinc inhibited PEPCK and G6Pase in the liver cell line HL1c (Fig. 4a and c). Consistent with the effects on IIS and in vivo, neither zinc, β-thujaplicin nor disulfiram were capable of repressing these genes on their own (Fig. 4a and c). In dose response experiments in the presence of 30 μM zinc, β-thujaplicin inhibited PEPCK with an IC50 of 25 μM and 11 μM for G6Pase (Fig. 4b) and in the presence of 10 μM zinc, disulfiram inhibited both PEPCK and G6Pase with an IC50 of 4 μM (Fig. 4d).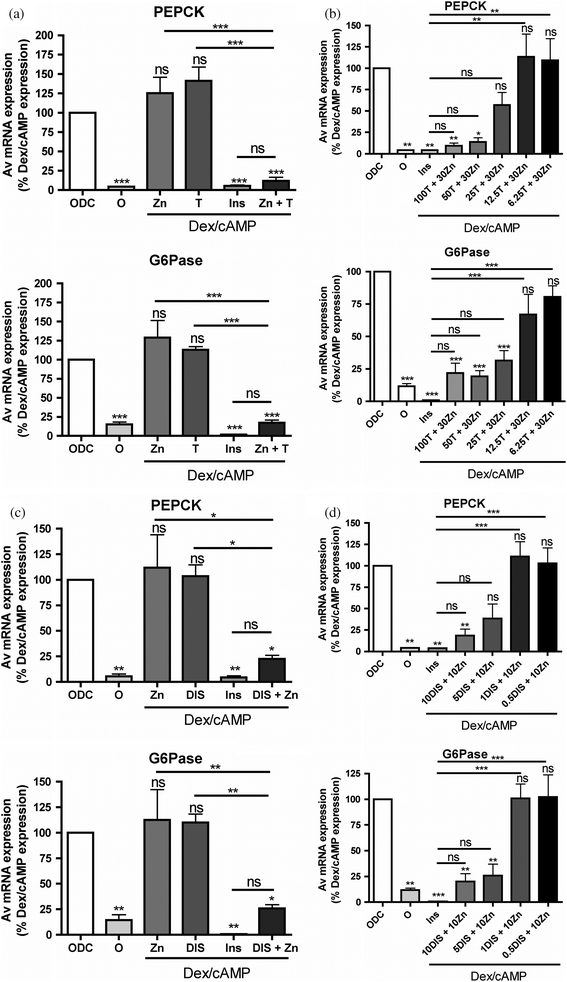 | ||
| Fig. 4 Zinc dependent regulation of gluconeogenic genes by zinc-binding compounds. (a) Serum starved HL1c cells were treated with (0DC) and without (0) 200 nM dexamethasone and 100 μM 8-CPT-cAMP for 4 h. The effects of 10 nM insulin (Ins) 20 μM zinc (Zn), 100 μM β-thujaplicin (T) or combinations of these agents on dexamethasone/cAMP-induced gene expression was determined in PEPCK expression (top panel) and G6Pase expression (bottom panel). (b) The dose response of PEPCK expression (top panel) and G6Pase expression (bottom panel) to β-thujaplicin treatment (c) same as (a) except that 10 μM disulfiram (DIS) is used in place of β-thujaplicin and 10 μM zinc acetate was used. (d) Same as (b) except that disulfiram replaces β-thujaplicin. Each bar consists of at least three separate determinations performed in triplicate. Errors are S.E.M. ‘ns’ above a column indicates not significant with respect to 0DC, asterisks above column indicates significant change with respect to 0DC (*** means p < 0.001, ** is p < 0.01, * is p < 0.05). The significance of other column to column differences are presented above a horizontal line that identifies the two columns. | ||
Conclusion
In conclusion, here we have shown that compounds from two groups of anti-hyperglycaemic small molecules induce IIS and repress gluconeogenesis in a strictly zinc-dependent manner. Our results indicate that the site of action is at or upstream of PtdIns 3-kinase. Previous work suggested that PTP1b is a direct target for inhibition by zinc61 and further work will be required to determine whether or not this is an important target for the compounds that we have investigated. Other possible targets in this part of the pathway include inhibition of PTEN, activation of the receptor kinases and enhanced recruitment of signalling proteins to the receptor.The IIS-inducing effects of the compounds that we have studied occur at concentrations of zinc that are in the region of 10–100 fold lower than those used to obtain effects with zinc alone,37,39–41,44 closer to the physiological low micromolar range of zinc in the plasma62,63 and consistent with the possibility that IIS induction contributes to the anti-hyperglycaemic properties of these and related compounds observed in vivo.46,47 The remarkable diversity of structures capable of producing zinc-dependent effects on IIS suggests that there may be a considerable degree of flexibility for chemical structures to be designed enabling zinc to be targeted for therapeutic effects on IIS. Identification of the molecular target(s) underlying these effects might in addition suggest zinc-independent pharmacological strategies capable of producing similar effects on IIS-regulated outputs.
Acknowledgements
The authors are grateful for funding from The Anonymous Trust, Tenovus Scotland for grants that supported this work and the Medical Research Council for studentship support for AC. We thank Professor Mike Ashford, Dr Geoff Lyles, Dr Paul Meakin and Dr Calum Sutherland for helpful discussions and comments on the manuscript.References
- L. P. van der Heide, M. F. M. Hoekman and M. P. Smidt, Biochem. J., 2004, 380, 297–309 CrossRef CAS.
- Y. L. Woods and G. Rena, Biochem. Soc. Trans., 2002, 30, 391–398 CAS.
- S. D. Guo, G. Rena, S. Cichy, X. W. He, P. Cohen and T. Unterman, J. Biol. Chem., 1999, 274, 17184–17192 CrossRef CAS.
- G. Rena, S. D. Guo, S. C. Cichy, T. G. Unterman and P. Cohen, J. Biol. Chem., 1999, 274, 17179–17183 CrossRef CAS.
- E. D. Tang, G. Nunez, F. G. Barr and K. L. Guan, J. Biol. Chem., 1999, 274, 16741–16746 CrossRef CAS.
- J. Nakae, B. C. Park and D. Accili, J. Biol. Chem., 1999, 274, 15982–15985 CrossRef CAS.
- W. H. Biggs, J. Meisenhelder, T. Hunter, W. K. Cavenee and K. C. Arden, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 7421–7426 CrossRef CAS.
- J. Nakae, V. Barr and D. Accili, EMBO J., 2000, 19, 989–996 CrossRef CAS.
- H. Onuma, B. T. Vander Kooi, J. N. Boustead, J. K. Oeser and R. M. O’Brien, Mol. Endocrinol., 2006, 20, 2831–2847 CrossRef CAS.
- B. T. Vander Kooi, R. S. Streeper, C. A. Svitek, J. K. Oeser, D. R. Powell and R. M. O’Brien, J. Biol. Chem., 2003, 278, 11782–11793 CrossRef CAS.
- C. Sutherland, R. M. O’Brien and D. K. Granner, J. Biol. Chem., 1995, 270, 15501–15506 CrossRef CAS.
- S. Patel, P. Lochhead, G. Rena and C. Sutherland, Biochem. J., 2001, 359, 611–619 CrossRef CAS.
- S. Patel, C. Lipina and C. Sutherland, FEBS Lett., 2003, 549, 72–76 CrossRef CAS.
- A. Mora, C. Lipina, F. Tronche, C. Sutherland and D. R. Alessi, Biochem. J., 2005, 385, 639–648 CrossRef CAS.
- L. Logie, A. J. Ruiz-Alcaraz, M. Keane, Y. L. Woods, J. Bain, R. Marquez, D. R. Alessi and C. Sutherland, Diabetes, 2007, 56, 2218–2227 CrossRef CAS.
- V. T. Samuel, C. S. Choi, T. G. Phillips, A. J. Romanelli, J. G. Geisler, S. Bhanot, R. M. McKay, B. Monia, J. R. Shutter, R. A. Lindberg, G. I. Shulman and M. M. Veniant, Diabetes, 2006, 55, 2042–2050 CrossRef CAS.
- W. Zhang, S. Patil, B. Chauhan, S. Guo, D. R. Powell, J. Le Angelos Klotsas, R. Matika, X. Xiao, K. A. Heidenrich, M. P. Sajan, R. V. Farese, D. B. Stolz, P. Tso, S.-H. Koo, M. Montminy and T. G. Unterman, J. Biol. Chem., 2006, 281, 10105–10117 CrossRef CAS.
- J. Nakae, W. H. Biggs III, T. Kitamura, W. K. Cavenee, C. V. E. Wright, K. C. Arden and D. Accili, Nat. Genet., 2002, 32, 245–253 CrossRef CAS.
- P. J. Randle, P. B. Garland, E. A. Newsholme and C. N. Hales, Ann. N. Y. Acad. Sci., 1965, 131, 324–333 CrossRef CAS.
- G. I. Shulman, J. Clin. Invest., 2000, 106, 171–176 CrossRef CAS.
- A. R. Cameron, S. Anton, L. Melville, N. P. Houston, S. Dayal, G. J. McDougall, D. Stewart and G. Rena, Aging Cell, 2008, 7, 69–77 CrossRef CAS.
- M. O’Coinceanainn, C. Astill and S. Schumm, Dalton Trans., 2003, 801–807 RSC.
- E. Mocchegiani, R. Giacconi and M. Malavolta, Trends Mol. Med., 2008, 14, 419–428 CrossRef CAS.
- D. A. Scott, Biochem. J., 1934, 28, 1592–1602 CAS.
- P. T. Grant, T. L. Coombs and B. H. Frank, Biochem. J., 1972, 126, 433–440 CAS.
- T. L. Blundell and S. P. Wood, Nature, 1975, 257, 197–203 CrossRef CAS.
- S. O. Emdin, G. G. Dodson, J. Cutfield and S. M. Cutfield, Diabetologia, 1980, 19, 174–182 CrossRef CAS.
- G. D. Smith, D. C. Swenson, E. J. Dodson, G. G. Dodson and C. D. Reynolds, Proc. Natl. Acad. Sci. U. S. A., 1984, 81, 7093–7097 CrossRef CAS.
- N. C. Kaarsholm, H-C. Ko and M. F. Dunn, Biochemistry, 1989, 28, 4427–4435 CrossRef CAS.
- E. R. Arquilla, P. Thiene, T. Brugman, W. Ruess and R. Sugiyama, Biochem. J., 1978, 175, 289–297 CAS.
- D. A. Scott and A. M. Fisher, J. Pharmacol. Exp. Ther., 1935, 55, 206–221 Search PubMed.
- D. A. Scott and A. M. Fisher, J. Pharmacol. Exp. Ther., 1936, 58, 78–92 Search PubMed.
- P. D. Home and K. G. M. M. Alberti, in International Textbook of Diabetes Mellitus, ed. K. G. M. M. Alberti, R. A. DeFronzo, H. Keen and P. Zimmet, John Wiley and Sons, Chichester, 1992, vol. 1 Search PubMed.
- E. S. Kang, M. S. Kim, Y. S. Kim, C. H. Kim, S. J. Han, S. W. Chun, K. Y. Hur, C. M. Nam, C. W. Ahn, B. S. Cha, S. I. Kim and H. C. Lee, Diabetes, 2008, 57, 1043–1047 CAS.
- R. Sladek, G. Rocheleau, J. Rung, C. Dina, L. Shen, D. Serre, P. Boutin, D. Vincent, A. Belisle, S. Hadjadj, B. Balkau, B. Heude, G. Charpentier, T. J. Hudson, A. Montpetit, A. V. Pshezhetsky, M. Prentki, B. I. Posner, D. J. Balding, D. Meyre, C. Polychronakos and P. Froguel, Nature, 2007, 445, 881–885 CrossRef CAS.
- E. Zeggini, M. N. Weedon, C. M. Lindgren, T. M. Frayling, K. S. Elliott, H. Lango, N. J. Timpson, J. R. B. Perry, N. W. Rayner, R. M. Freathy, J. C. Barrett, B. Shields, A. P. Morris, S. Ellard, C. J. Groves, L. W. Harries, J. L. Marchini, K. R. Owen, B. Knight, L. R. Cardon, M. Walker, G. A. Hitman, A. D. Morris, A. S. F. Doney, WTCCC, M. I. McCarthy and A. T. Hattersley, Science, 2007, 316, 1336–1341 CrossRef CAS.
- X-H. Tang and N. F. Shay, J. Nutrition, 2001, 131, 1414–1420 CAS.
- H. Haase and W. Maret, Exp. Cell Res., 2003, 291, 289–298 CrossRef CAS.
- C. J. Lynch, B. J. Patson, S. A. Goodman, D. Trapolsi and S. R. Kimball, Am. J. Physiol. Endocrinol. Metab., 2001, 281, E25–E34 CAS.
- P. L. Walter, A. Kampkotter, A. Eckers, A. Barthel, D. Schmoll, H. Sies and L-O. Klotz, Arch. Biochem. Biophys., 2006, 454, 107–113 CrossRef CAS.
- A. Barthel, E. A. Ostrakhovitch, P. L. Walter, A. Kampkotter and L-O. Klotz, Arch. Biochem. Biophys., 2007, 463, 175–182 CrossRef CAS.
- W. Basuki, H. Makoto and H. Sakurai, J. Inorg. Biochem., 2007, 101, 692–699 CrossRef CAS.
- L. Coulston and P. Dandona, Diabetes, 1980, 29, 665–667 CrossRef CAS.
- J. M. May and C. S. Contoreggi, J. Biol. Chem., 1982, 257, 4362–4368.
- V. Beletate, R. P. El Dib and A. N. Atallah, The Cochrane Collaboration, 2008.
- Y. Yoshikawa, Y. Adachi and H. Sakurai, Life Sci., 2007, 80, 759–766 CrossRef CAS.
- M. Yamane, Y. Adachi, Y. Yoshikawa and H. Sakurai, Chem. Lett., 2005, 34, 1694–1695 CrossRef CAS.
- G. Rena, Y. L. Woods, A. R. Prescott, M. Peggie, T. G. Unterman, M. R. Williams and P. Cohen, EMBO J., 2002, 21, 2263–2271 CrossRef CAS.
- G. Rena, A. R. Prescott, S. D. Guo, P. Cohen and T. G. Unterman, Biochem. J., 2001, 354, 605–612 CrossRef CAS.
- S. Anton, L. Melville and G. Rena, Cell. Signalling, 2007, 19, 378–383 CrossRef CAS.
- G. Rena, J. Bain, M. Elliott and P. Cohen, EMBO Rep., 2004, 5, 60–65 CrossRef CAS.
- A. R. Kay and K. Toth, Sci. Signaling, 2008, 1, re3 CrossRef.
- N. J. Miller, C. Castelluccio, L. Tijburg and C. Rice-Evans, FEBS Lett., 1996, 392, 40–44 CrossRef CAS.
- M. O’Coinceanainn, C. Astill and B. Baderschneider, J. Inorg. Biochem., 2003, 96, 463–468 CrossRef CAS.
- E. A. Ostrakhovitch, M. R. Lordnejad, F. Schliess, H. Sies and L-O. Klotz, Arch. Biochem. Biophys., 2002, 397, 232–239 CrossRef CAS.
- G. J. P. L. Kops, T. B. Dansen, P. E. Polderman, I. Saarloos, K. W. A. Wirtz, P. J. Coffer, T.-T. Huang, J. L. Bos, R. H. Medema and B. M. T. Burgering, Nature, 2002, 419, 316–320 CrossRef CAS.
- C. J. Phiel and P. S. Klein, Annu. Rev. Pharmacol. Toxicol., 2001, 41, 789–813 CrossRef CAS.
- C. J. Green, O. Goransson, G. S. Kular, N. R. Leslie, A. Gray, D. R. Alessi, K. Sakamoto and H. S. Hundal, J. Biol. Chem., 2008, 283, 27653–27667 CrossRef CAS.
- J. Cobby, M. Mayersohn and S. Selliah, J. Pharmacol. Exp. Ther., 1977, 202, 724–731 CAS.
- B. Johansson, Acta Psychiatr. Scand., 1992, 86, 15–26 CrossRef.
- H. Haase and W. Maret, BioMetals, 2005, 18, 333–338 Search PubMed.
- C. Hotz, J. M. Peerson and K. H. Brown, Am. J. Clin. Nutr., 2003, 78, 756–764 CAS.
- T. H. Hyun, E. Barrett-Connor and D. B. Milne, Am. J. Clin. Nutr., 2004, 80, 715–721 CAS.
| This journal is © The Royal Society of Chemistry 2010 |
