Complexes of hydroxy(thio)pyrone and hydroxy(thio)pyridinone with Zn(II) and Mo(VI). Thermodynamic stability and insulin-mimetic activity†
Sílvia
Chaves
a,
Ratomir
Jelic
ab,
Catarina
Mendonça
a,
Marta
Carrasco
a,
Yutaka
Yoshikawa
c,
Hiromu
Sakurai
d and
M. Amélia
Santos
*a
aCentro de Química Estrutural, Instituto Superior Técnico, Av. Rovisco Pais 1, 1049-001 Lisboa, Portugal. E-mail: masantos@ist.utl.pt; Fax: +351-21-8464455; Tel: +351-21-8419273
bFaculty of Science, Chemistry Department, Kragujevac, Serbia and Montenegro
cDepartment of Analytical and Bioinorganic Chemistry, Kyoto Pharmaceutical University, 5 Nakauchy-cho, Misasagi, Yamashina-ku, Kyoto 607-8414, Japan
dFaculty of Pharmaceutical Sciences, Suzuka University of Medical Science, 3500-3 Minami-Tamagaki-cho, Suzuka, Mie 513-8670, Japan
First published on 9th November 2009
Abstract
The development of metal-containing pharmaceuticals as insulin-mimetics has been the object of recent worldwide research. We have examined a series of zinc(II) and molybdenum(VI) complexes with model O,S-donor ligands (thiomaltol and 1,2-dimethyl-3-hydroxypyridine-4-thione (DMHTP)) and the corresponding O,O-analogues (maltol and DMHP) for their insulin-mimetic activity. Aimed at getting structure-activity relationships, some physical-chemical properties were also studied, such as metal-complex formation, speciation at different pH conditions and ligand lipophilicity. The Zn-complexes exhibit considerably higher insulin-mimetic activity than the corresponding Mo-analogues. Particularly, the bis(thiomaltolato)zinc(II) complex reveals a very high activity, ascribed to the effect of the thione π character and to the soft nature of the sulfur donor atom enhancing the Zn(II)–ligand affinity and the ligand/complex lipophilicity, two determinant parameters for delivering the metal-drug into the cells. Hence, these preliminary studies indicate that the Zn(thiomaltol)2 complex can be considered a potential drug candidate for treatment of diabetes mellitus, upon in vivo evaluations.
1. Introduction
Diabetes mellitus (DM) is one of the most widespread diseases in the world. Type 1 DM can be controlled only by daily injections of insulin, while type 2 is treated by several types of synthetic therapeutics. Due to drawbacks associated with currently available treatments, DM is an important target in drug development and several metal-containing drug candidates have been recently studied.1,2Vanadium-containing complexes have by far been the metal complexes mostly tested both in vitro and in vivo as potential therapeutic agents for oral treatment of DM.1–4 However, due to the non-established essentiality and also to some toxicity of vanadium (UL – tolerable upper intake level – VO2+ 1800 μg/day for humans),5 a range of other metal (e.g. zinc, chromium, molybdenum, cobalt) complexes has been also tested as potential alternatives or coadjuvants of anti-hyperglycemic drugs for treating DM and associated metabolic syndromes.1,3 Among those metal-containing drug candidates, a special emphasize can be given to the Zn(II) and Mo(VI) compounds. In fact, zinc is an essential element (UL 40 mg/day)5 with structural and functional roles in proteins and life processes and zinc complexes have been the mostly recently tested compounds for their anti-diabetic properties.1,2 On the other hand, molybdenum and vanadium oxo-ions present quite a number of similarities, namely in terms of electronic configuration and ability for the inactivation of glycogen synthase,1 besides displaying synergistic stimulation of glucose uptake in rat adipocytes in the presence of H2O2;6,7 however, as compared with vanadium, molybdenum is an essential element with some toxicity (UL MoO22+ 2000 μg/day)5 that is present in the active site of several human enzymes (oxidases), thus possessing a considerably high bio-relevance.8,9 To guarantee the bioavailability and the non-toxicity of metallodrugs, they should be administered as metal-complexes with low molecular weight, neutral charge and adequate lipo/hydrophilic and thermodynamic properties. In fact, to deliver a metal-complex to a specific site of action, besides the above referred properties to ensure bioavailability and efficient membrane-crossing ability, the thermodynamic stability of the complex is also an important factor because the ligand carrier is in contact with endogenous ligands (e.g.albumin and transferrin) which can compete for the metal ion.10
The 3-hydroxy-4-pyrone and –pyridinone compounds, in particular maltol (3-hydroxy-2-methyl-4-pyrone) and its N-derivative analogue deferriprone (3-hydroxy-2-methyl-4-pyridinone), are heterocyclic bidentate chelators, which have been particularly attractive for pharmaceutical purposes, mostly for iron and aluminium decorporation.11–13 Due to their small molecular weight, non-toxicity, commercial availability or easy feasibility, as well as their ability to form stable neutral complexes, they have also been considered suitable to deliver zinc into cells,14 and their complexes with vanadium,15 zinc16 and molybdenum17 have proved insulin mimetic activity. Comparison of the oral activity of several bis(maltolato)-metal complexes in animal models has shown that the most effective compounds, involving non-essential and essential metal ions, were the complexes with oxovanadium(IV) and dioxomolybdenum(VI), respectively.1
Despite the considerable number of solution and in vivo studies related with insulin mimetic properties of several metal ion (vanadium, zinc or molybdenum) complexes with (O,O) chelators, namely hydroxy-pyrone and –pyridinones,1,16,17 a few recent studies are known with the corresponding thio derivatives containing (O,S) donor atoms,10 some of them appearing while this manuscript was in progress.18 Herein we present preliminary results of in vitro studies on anti-diabetic activity as well as solution equilibrium studies for the characterization (stoichiometry, thermodynamic stability, speciation) of Zn(II) and Mo(VI) complexes of (O,S) donor ligands, 3-hydroxy-4-thiopyrone (thiomaltol) and 3-hydroxy-2-methyl-4-thiopyridinone (DMHTP). These studies are also compared with the corresponding results obtained for the oxo-analogues (maltol and DMHP, see Scheme 1), aimed at shedding some light on structure activity relationships.
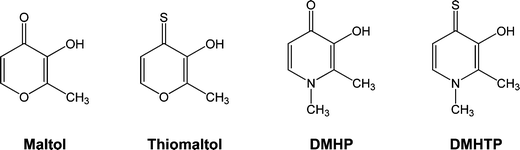 | ||
| Scheme 1 Structural formulae of the ligands. | ||
2. Experimental
2.1 Reagents and materials
All the chemicals were of analytical reagent grade and used as supplied without further purification. Whenever necessary, the organic solvents were dried according to standard methods.19 Chemical reactions were monitored by TLC, using alumina plates coated with silica gel 60 F254 (Merck). DMHP (1,2-dimethyl-3-hydroxy-4-pyridinone) and maltol (3-hydroxy-2-methyl-4-pyranone) were purchased from Aldrich; thiomaltol and DMHTP were synthesized in our laboratory.20 In the complexation studies, the ZnCl2 (0.0156 M) solution from Merck was standardized by titration with K2H2EDTA (EDTA ≡ ethylenediaminetetraacetic acid).2.2 Apparatus
Melting points were measured with a Leica Galen III hot stage apparatus and are uncorrected. IR spectra were recorded on a Bio-Rad Merlin, FTS 3000 MX. The 1H NMR spectra were recorded on a Bruker Advance II 400 spectrometer at 25 °C. Chemical shifts (δ) are reported in ppm, using tetramethylsilane (TMS) as reference. The following abbreviations are used: s = singlet, d = doublet. Elemental analyses were performed on a Fisons EA1108 CHNF/O instrument. The potentiometric equipment used was according to that previously described.212.3 Procedures
Zn–maltol complex. Maltol (0.15 g, 1.2 mmol) was suspended in water (7 mL) and dissolved by the addition of 1 M KOH solution (0.6 mL; 1.2 mmol). Zn(NO3)2·4H2O (0.155 g, 0.6 mmol) was added to the reaction mixture, which was left stirring for 2 h at room temperature. There was formation of a white precipitate, which was filtered, washed with cold water and recrystallized from ethanol to yield a white powder (66%); mp 238–239 °C. IR (KBr) 1616 cm−1 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H-NMR (MeOD/TMS): 7.95 (d, 1 H, H-Py), 6.54 (d, 1 H, H-Py), 2.36 (s, 3 H, CH3). Elemental analysis calc (%) C12H10O6Zn: C, 45.66; H, 3.17. Found: C, 45.21; H, 3.30.
O). 1H-NMR (MeOD/TMS): 7.95 (d, 1 H, H-Py), 6.54 (d, 1 H, H-Py), 2.36 (s, 3 H, CH3). Elemental analysis calc (%) C12H10O6Zn: C, 45.66; H, 3.17. Found: C, 45.21; H, 3.30.
Zn–DMHP complex. DMHP (0.15 g, 1 mmol) was suspended in water (7 mL) and dissolved by the addition of a 0.1 M HCl solution. Zn(NO3)2·4H2O (0.131 g, 0.5 mmol) was added to the reaction mixture. A 0.1 M KOH solution was added until pH 7 and the mixture was left stirring for 2 h. There was formation of a white precipitate, which was filtered and washed with cold water and subsequently recrystallized from methanol to obtain the pure product as white powder (60%); mp: > 350 °C. IR (KBr) 1605 cm−1 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H-NMR (MeOD/TMS): 7.36 (d, 1 H, H-Py), 6.41 (d, 1 H, H-Py), 3.72 (s, 3 H, CH3), 2.40 (s, 3 H, CH3). Elemental analysis calc (%) C14H16N2O4Zn·0.7H2O: N, 8.16; C, 48.95; H, 5.07. Found: N, 8.22;C, 48.57; H, 4.90.
O). 1H-NMR (MeOD/TMS): 7.36 (d, 1 H, H-Py), 6.41 (d, 1 H, H-Py), 3.72 (s, 3 H, CH3), 2.40 (s, 3 H, CH3). Elemental analysis calc (%) C14H16N2O4Zn·0.7H2O: N, 8.16; C, 48.95; H, 5.07. Found: N, 8.22;C, 48.57; H, 4.90.
Zn–thiomaltol complex. As described for Zn–maltol complex. Yield: 87% (0.106 g); mp > 350 °C. IR (KBr) 1576 cm−1 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S). 1H-NMR (DMSO/TMS): 8.22 (d, 1 H, H-Py), 7.66 (d, 1 H, H-Py), 2.48 (s, 3 H, CH3). Elemental analysis calc (%) C12H10O4S2Zn: C, 41.45; H, 2.90; S, 18.44. Found: C, 41.10; H, 2.82; S, 18.26.
S). 1H-NMR (DMSO/TMS): 8.22 (d, 1 H, H-Py), 7.66 (d, 1 H, H-Py), 2.48 (s, 3 H, CH3). Elemental analysis calc (%) C12H10O4S2Zn: C, 41.45; H, 2.90; S, 18.44. Found: C, 41.10; H, 2.82; S, 18.26.
Zn–DMHTP complex. As described for Zn–DMHP complex. Yield: 84% (0.071 g); mp: 327–330 °C. IR (KBr) 1587 cm−1 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S). 1H-NMR (DMSO/TMS): 7.50 (d, 1 H, H-Py), 7.39 (d, 1 H, H-Py), 3.89 (s, 3 H, CH3), 2.47 (s, 3 H, CH3). Elemental analysis calc (%) C14H16N2O2S2Zn: N, 7.63; C, 44.03; H, 4.22; S, 16.79. Found: N, 7.23; C, 43.92; H, 4.24; S, 17.24.
S). 1H-NMR (DMSO/TMS): 7.50 (d, 1 H, H-Py), 7.39 (d, 1 H, H-Py), 3.89 (s, 3 H, CH3), 2.47 (s, 3 H, CH3). Elemental analysis calc (%) C14H16N2O2S2Zn: N, 7.63; C, 44.03; H, 4.22; S, 16.79. Found: N, 7.23; C, 43.92; H, 4.24; S, 17.24.
Mo–maltol complex. To a solution of maltol (0.1 g, 0.79 mmol) in ethanol (7 mL), an aqueous solution (11 mL) of ammonium molybdate (0.245 g, 0.2 mmol) was added. A 0.1 M HCl solution was added until pH 3.5 and left stirring for 6 h. The orange precipitate was filtered and washed three times with water, ethanol and ether. Recrystallization from ethanol gave the pure complex as orange solid (89%); mp: 347–349 °C. IR (KBr) 1617 cm–1 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H-NMR (MeOD/TMS): 8.24 (d, 1 H, H-Py), 6.79 (d, 1 H, H-Py), 2.47 (s, 3 H, CH3). Elemental analysis calc (%) C12H10MoO8.5HCl: C, 25.72; H, 1.80. Found: C, 25.72; H, 1.66.
O). 1H-NMR (MeOD/TMS): 8.24 (d, 1 H, H-Py), 6.79 (d, 1 H, H-Py), 2.47 (s, 3 H, CH3). Elemental analysis calc (%) C12H10MoO8.5HCl: C, 25.72; H, 1.80. Found: C, 25.72; H, 1.66.
Mo–DMHP complex. To a solution of DMHP (0.1 g, 0.7 mmol) in hot water (7 mL), an aqueous solution (11 mL) of ammonium molybdate (0.44 g, 0.35 mmol) was added. A 0.1 M HCl solution was added until pH 4 and left stirring for 3 h. The yellow precipitate obtained was filtered off and washed three times with water, ethanol and ether. The pure complex was obtained as a yellow solid. Recrystallization from ethanol yielded the final product as yellow crystals (87%); mp > 350 °C. IR (KBr) 1616 cm−1 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H-NMR (DMSO/TMS): 7.82 (d, 1 H, H-Py), 7.41 (d, 1 H, H-Py), 3.81 (s, 3 H, CH3), 2.37 (s, 3 H, CH3). Elemental analysis calc (%) C14H16MoN2O6·1.5H2O: N, 6.50; C, 38.99; H, 4.44. Found: N, 6.39; C, 39.00; H, 4.01.
O). 1H-NMR (DMSO/TMS): 7.82 (d, 1 H, H-Py), 7.41 (d, 1 H, H-Py), 3.81 (s, 3 H, CH3), 2.37 (s, 3 H, CH3). Elemental analysis calc (%) C14H16MoN2O6·1.5H2O: N, 6.50; C, 38.99; H, 4.44. Found: N, 6.39; C, 39.00; H, 4.01.
Mo–thiomaltol complex. As previously described.20 Yield: 30% (0.013 g); mp: decomposed before melting. IR (KBr) 1572 cm−1 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S). 1H-NMR (MeOD/TMS): 8.46 (d, 1 H, H-Py), 7.68 (d, 1 H, H-Py), 2.44 (s, 3 H, CH3). Elemental analysis calc (%) C12H10MoO6S2·0.2H2O: C, 35.13; H, 2.46; S, 15.63. Found: C, 34.82; H, 2.53; S, 15.50.
S). 1H-NMR (MeOD/TMS): 8.46 (d, 1 H, H-Py), 7.68 (d, 1 H, H-Py), 2.44 (s, 3 H, CH3). Elemental analysis calc (%) C12H10MoO6S2·0.2H2O: C, 35.13; H, 2.46; S, 15.63. Found: C, 34.82; H, 2.53; S, 15.50.
Mo–DMHTP complex. To a solution of DMTHP (0.03 g, 0.20 mmol) in ethanol (2 mL), an aqueous solution (3 mL) of ammonium molybdate (0.124 g, 0.1 mmol) was added. After 1 h stirring the yellow precipitate was filtered off and washed three times with water, ethanol and ether. Recrystallization from ethanol gave the pure complex as a dark yellow solid (65%); mp: > 230 °C. IR (KBr) 1600 cm−1 (νC
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S). 1H-NMR (MeOD/TMS): 7.79 (d, 1 H, H-Py), 7.18 (d, 1 H, H-Py), 3.98 (s, 3 H, CH3), 2.79 (s, 3 H, CH3). Elemental analysis calc (%) C14H16MoN2O4S2: N, 6.42; C, 38.53; H, 3.70; S, 14.70. Found: N, 6.05; C, 38.10; H, 3.28; S, 14.35.
S). 1H-NMR (MeOD/TMS): 7.79 (d, 1 H, H-Py), 7.18 (d, 1 H, H-Py), 3.98 (s, 3 H, CH3), 2.79 (s, 3 H, CH3). Elemental analysis calc (%) C14H16MoN2O4S2: N, 6.42; C, 38.53; H, 3.70; S, 14.70. Found: N, 6.05; C, 38.10; H, 3.28; S, 14.35.
Titrant solution. The titrant (0.1 M KOH) was prepared from a carbonate-free commercial concentrate (Titrisol), standardized by potentiometric titration with potassium hydrogen phthalate and discarded whenever the percentage of carbonate (Gran’s method)22 was about 0.5% of the total amount of base.
Measurements. Potentiometric titrations of the ligands and their zinc complexes in 15% (v/v) CH3OH/H2O solution were performed at ionic strength (I) 0.1 M KCl and 25.0 ± 0.1 °C. For all the samples prepared, the total volume was 20 mL, the ligand concentration was 2.0–4.0 × 10−3 M and the metal ion-to-ligand molar ratio was 0
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 or 1
2 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. Under the experimental conditions used, the value determined for the water ionisation constant (pKw) was 13.84.
4. Under the experimental conditions used, the value determined for the water ionisation constant (pKw) was 13.84.
Calculation of equilibrium constants. The stepwise protonation constants, Ki = [HiL]/[Hi−1L][H], were previously determined by us.20 The overall metal-complex stability constants, βMmHhLl = [MmHhLl]/[M]m[H]h[L]l, for the Zn(II) systems with thiomaltol, DMHP and DMHTP were calculated by fitting the respective potentiometric data with the HYPERQUAD 2003 program.22 The Zn(II) hydrolytic species23 were included in the equilibrium model and the species distribution curves were plotted with the HYSS program.22
All animal experiments in the present study were approved by the Experimental Animal Research of Kyoto Pharmaceutical University (KPU) and were performed according to the Guideline for Animal Experimentation of KPU.
3. Results and discussion
3.1 Physical-chemical characterization of the compounds
The protonation constants of maltol, thiomaltol, DMHP and DMHTP were previously determined by us from spectrophotometric titrations in 15% (v/v) MeOH aqueous solutions, at 25 °C and 0.1 M KCl, except for DMHTP, which log K2 (<2) was determined by 1H NMRtitration.20 Spectrophotometric methods were also adopted in previous studies on Mo(VI) complexation because, under the usual potentiometric concentration conditions (mM), precipitation occurred.20 The fully protonated forms of the studied compounds have one (maltol and thiomaltol) or two (DMHP and DMHTP) dissociable protons and the corresponding protonation constants (see Table 1) are according with the values typically attributed to the corresponding hydroxyl and N-pyridinyl groups.12Table 1 shows also that the stepwise protonation constants determined in our laboratory are in good agreement with some literature data,20,26–29 obtained under different experimental conditions. Especially relevant is the fact that the hydroxyl protons of thiomaltol and DMHTP (8.27, 9.70) are considerably more acidic (lower log K1) than the respective oxo-derivatives (8.67, 9.82), which may be due to the lower electronegativity and higher polarizability of the thio-compounds, allowing a higher delocalization of the phenolate negative charge. Also the electronegativity of the ring O-atom is higher than that of the N–CH3 group, thus allowing a higher stabilization of the corresponding phenolate negative charges.| Compound | Maltol | Thiomaltol | DMHP | DMHTP |
|---|---|---|---|---|
| a Ref. 20 (I = 0.10 M KCl, T = 25 °C, in 15% MeOH/H2O); b ref. 26 (I = 0.16 M NaCl, T = 37 °C, in water); c ref. 27 (I = 0.20 M KCl, T = 25.0 °C, in water); d ref. 28 (I = 0.10 M KCl, T = 25.0 °C, in water); e ref. 29 (I = 0.16 M NaCl, T = 25.0 °C, in water); f ref. 20 determined by 1H NMRtitration; g in 1-octanol/water; h ref. 30; i ref. 31, in chloroform/saline system; j pM values (CM = 10−6 M, CL = 10−5 M, pH = 7.4); l ref. 32 (I = 0.2 M KCl, T = 25.0 °C, in water). | ||||
| log K1 | 8.67(3)a | 8.27(1)a | 9.82(1)a | 9.70(1)a |
| 8.67(3)b | 8.16(2)d | 9.77(2)c | 9.47d | |
| log K2 | 8.44(2)c | 8.12(2)e | 3.74(2)a | 9.44(1)e |
| — | — | 3.67(2)c | 0.95(3)f | |
| log Pg | −0.04 | 0.54 | −0.85 | −0.59 |
| 0.60i | −1.03h | |||
| log βZnL | 5.57(2)c | 7.05(3) | 7.35(1) | 9.00(1) |
| 7.24(2)c | ||||
| log βZnL2 | 10.29(2)c | 13.92(5) | 13.69(1) | 17.09(7) |
| 13.55(2)c | ||||
| log βZnL3 | 12.71(8)c | 16.7(1) | — | 20.53(5) |
| 15.2(2)c | ||||
| log βZnL2H−1 | −0.1(1)c | — | 2.62(3) | 6.82(6) |
| 2.3(3)c | ||||
| pZnj | 6.1 | 7.9 | 6.3 | 8.4 |
| log βMoO2L2a | 38.51(5) | 38.50(3) | 39.31(4) | 41.48(6) |
| 40.22(4)l | ||||
| log βMoO3La | 20.71(6) | 21.19(3) | 20.10(4) | 21.82(5) |
| 20.0(1)l | ||||
| pMoj | 6.15 | 6.57 | 6.00 | 6.18 |
From the species distribution curves of the compounds at different pH conditions (see Fig. S1 of Supplementary Material† ), it can be seen that for all the compounds the neutral HL species are predominant at the physiological pH: ca 95% for maltol, 88% for thiomaltol and 100% for both DMHP and DMHTP. This is a relevant feature in terms of membrane-crossing capacity.
The lipo/hydrophilic character of the ligands was also assessed through calculation of the corresponding partition coefficients (log P) between 1-octanol and a tris-buffered (pH = 7.4) aqueous solution. Analysis of log P values (see Table 1) clearly shows that the O-heterocyclic compounds, maltol (log P = −0.04) and thiomaltol (log P = 0.54), have higher lipophilic character then the corresponding N-heterocycles, DMHTP (log P = −0.59) and DMHP (log P = −0.85). DMHP presents a partition coefficient slightly below the limit usually accepted for hydrophilic compounds (log P < −1). The thiocarbonylic compounds are more lipophilic than the corresponding carbonylic analogues. This can mainly be due to the fact that the sulfur atom, being less electronegative and more polarizable than the oxygen atom, can establish weaker H-bond interactions with water molecules.
3.2 Complexation studies
The global formation constants calculated for the studied Zn(II) complexes are indicated in Table 1, which also includes some reported values for maltol and DMHP. For both the oxo and thio compounds, the mono- and bis-chelate complexes were found in our equilibrium model, corresponding to a (O,O)/(O,S) or a [2 × (O,O])/[2 × (O,S)] coordination mode (see Fig. 1). Fig. 1 shows that, under the experimental methanol–aqueous conditions, the bis-chelate species predominates above pH ca 5–7. Moreover, for all the compounds at pH > 7.0, the ZnL3 species, corresponding to a 6 coordination number for Zn(II), was also detected and the corresponding values are indicated in Table 1 for maltol, thiomaltol and DMHTP. In the case of the Zn(II)/DMHP system, the formation constant for the tris-chelate complex could be estimated (log βca 15.5) but it appears only at very low percentage (<5%) under 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 metal-to-ligand stoichiometric conditions, and so it is not depicted in Table 1. Besides the tris-chelate complex species found for all cases, mixed hydroxo species ZnL2OH were also admitted in the complexation model except for thiomaltol (due to earlier precipitation problems).
4 metal-to-ligand stoichiometric conditions, and so it is not depicted in Table 1. Besides the tris-chelate complex species found for all cases, mixed hydroxo species ZnL2OH were also admitted in the complexation model except for thiomaltol (due to earlier precipitation problems).
 | ||
Fig. 1 Species distribution diagrams for the Zn(II)–L 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 systems: L = maltol (a), L = thiomaltol (b), L = DMHP (c) and L = DMHTP (d). CL = 2 × 10−3 M. 4 systems: L = maltol (a), L = thiomaltol (b), L = DMHP (c) and L = DMHTP (d). CL = 2 × 10−3 M. | ||
A comparative analysis of the zinc(II)-binding affinity of the ligands, based on the overall stability constants, or more accurately on the pZn values (at physiological pH 7.4, CL/CZn = 10, CZn = 10−6 M, see Table 1), indicates that Zn(II) is more strongly bound by the softer O,S-donor ligands (thiomaltol and DMHTP) than the harder O,O-analogues (maltol and DMHP). The pZn values follow the order (indicated by the ligands) DMHTP > thiomaltol > DMHP > maltol, which reasonably agrees with the stability both of the mono and bis complexes, but not with the basicity of the coordinating donors. So the electronic stabilization through the N-ring that explains the higher basicity of the pyridinone relative to the pyrone derivatives is less relevant in the Zn-complex stability, which seems to be over-passed by the type of coordinating atoms, namely the π character of the thione.
The species distribution curves for the Zn(II)–ligand systems shown in Fig. 1 also indicate that, at the physiological pH, the percentage of neutral species (ZnL2) decreases according to the following trend order: DMHTP (98%) > thiomaltol (93%) > DMHP (90%) > maltol (73%). It can be seen that the percent formation of the neutral ZnL2 species is extremely high in all systems except for Zn(II)/maltol. In this last case, the simultaneous presence of the charged ZnL complex can be associated with a reduction of permeability and/or an increase of metal ion release, features of potential biological relevance. The above-mentioned trend follows the pZn value, but it does not correlate with the percentage of HL formation at physiological pH for the ligands (DMHTP ≈ DMHP > maltol > thiomaltol), which suggests that the lipophilicity order of the zinc complexes eventually does not parallel that of the ligands.
Therefore, the thio-derivatives seem to present two major advantages, as compared with the oxo-derivatives, namely the higher stability of the zinc complexes and also the higher lipophilicity of the ligands and complexes, two important requirements to facilitate the zinc-complex transport across apolar membranes
The solution Mo(VI) complexation with the same set of ligands was recently reported20 and the respective stability constants are also included in Table 1. The concentration distribution curves of the Mo complex species at a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand-to-metal ion molar concentration ratio (see Fig. S2 of Supplementary Material† ) showed that, under the experimental methanol–aqueous conditions, the bis-chelated species (MoO2L2) predominate below pH 4–5 and above these pH values they become decomposed through the formation of trioxo-molybdenum mono-chelate complexes (MoO3L).20 Moreover, both thiomaltol and DMHTP retard the formation of MoO42− to higher pH values (≥7) than those of the analogous oxo complexes. Thus, at the physiological pH there is a higher percent formation of MoO3L for the thio-compounds: ca 90% for thiomaltol and 60% for DMHTP; ca 52% for maltol and 5% for DMHP.
1 ligand-to-metal ion molar concentration ratio (see Fig. S2 of Supplementary Material† ) showed that, under the experimental methanol–aqueous conditions, the bis-chelated species (MoO2L2) predominate below pH 4–5 and above these pH values they become decomposed through the formation of trioxo-molybdenum mono-chelate complexes (MoO3L).20 Moreover, both thiomaltol and DMHTP retard the formation of MoO42− to higher pH values (≥7) than those of the analogous oxo complexes. Thus, at the physiological pH there is a higher percent formation of MoO3L for the thio-compounds: ca 90% for thiomaltol and 60% for DMHTP; ca 52% for maltol and 5% for DMHP.
Analysis of the pMo values in Table 1 shows that the softer O,S-donor ligands (thiomaltol and DMHTP) have a slightly higher affinity for the Mo(VI) than the harder O,O-analogues. However this metal–ligand affinity difference between the thio-and the oxo-derivatives is much more feeble for Mo(VI)– than the corresponding Zn(II)–ligand systems.
3.3 In vitro insulin mimetic activities of Zn and Mo complexes
Insulin-mimetic activities of eight Zn and Mo complexes and their ligands were estimated by in vitro experiments, in which the inhibition of free fatty acid (FFA) release from isolated rat adipocytes treated with epinephrine was compared with the activity of ZnSO4 (positive control).Fig. 2A shows the effects of the Zn complexes on the FFA release, in comparison with that of ZnSO4, and the apparent IC50 values are included in Table 2. Three Zn complexes exhibited concentration-dependency release in the range of 0.1–0.5 mM. On the basis of IC50 values, Zn(DMHP)2 was found to have low activity as insulin-mimics compared with ZnSO4. On the other hand, Zn(thiomaltol)2 shows very high insulin-mimetic activity compared with ZnSO4. We could not evaluate the insulin-mimetic activity of Zn(DMHTP)2 because it had very high lipophilicity. Thus, we evaluated the insulin mimetic activity by using Zn(DMHTP)2 in a DMSO suspension solution.
 | ||
| Fig. 2 Inhibitory effects of (A) ZnSO4 and new Zn complexes and (B) MoO2 and new Mo complexes on FFA release from rat adipocytes treated with epinephrine; b (blank) and c (control) are cells only and cells plus epinephrine, respectively. Data are expressed as the means ± SDs for three experiments. | ||
| Complex | IC50 value/mM |
|---|---|
| a Ref. 31; b ref. 35; c ref. 37; *nd (incapable evaluation). | |
| ZnSO4 | 0.518 ± 0.045 |
| 1.00 ± 0.07a | |
| Zn(maltol)2 | 0.380 ± 0.062 |
| 0.54 ± 0.07a | |
| Zn(DMHP)2 | 1.900 ± 0.041 |
| Zn(thiomaltol)2 | 0.0033 ± 0.0042 |
| Zn(DMHTP)2 | nd* |
| Zn(ethylmaltol)2 | 0.44 ± 0.05a |
| MoO2 | None |
| MoO2(maltol)2 | None |
| MoO2(DMHP)2 | None |
| MoO2(thiomaltol)2 | 43.6 ± 21.1 |
| MoO2(DMHTP)2 | 20.4 ± 6.3 |
| VOSO4 | 1.00b |
| VO(ethylmaltol)2 | 3.46c |
| VO(DMHP)2 | 1.36c |
In order to aid the further rationalization of the activity of the zinc complexes herein studied, we tried to identify eventual relationships between the in vitroinsulin-mimetic activity and the lipophilicity of the ligands in the Zn(II) complexes as well as the chelating affinity of the ligands. Therefore, the pIC50 values (−log IC50) on FFA release were plotted against the partition coefficients (log P) of the ligands and the pZn of the complexes (see Fig. 3).
 | ||
| Fig. 3 Trend relationship between the insulin-mimetic activity (pIC50) values of the Zn(II) complexes, chelating affinity (pZn, ■) and the lipophilicity of the corresponding ligands (log P, ▲). | ||
Analysis of Fig. 3 shows the existence of some correlation trend (although this conclusion is hampered by reduced number of points) between the insulin-mimetic activity of the Zn(II) complexes expressed as pIC50 (−log IC50), the partition coefficient of the correspondent ligands (log P) and also the zinc-chelating affinity of the ligands (pZn). Among the three complexes bioassayed, Zn(thiomaltol)2 exhibited the highest values for the insulin-mimetic activity as well as for the partition coefficient (log P = 0.54) and the chelating affinity (pZn = 7.9). These results suggest that the insulin-mimetic active sites of Zn(II) are mainly in the cells. The results are also supported by Zn uptake and the following translocation of glucose transporter (GLUT4) in the 3T3-L1 adipocytes, which are treated with other types of Zn complexes such as Zn-allixin (with Zn(O4) coordination mode)33 and Zn-thioallixin-N-methyl and Zn-1-oxy-2-puridine-thiolate (with Zn(S2O2) coordination mode).34
The Mo(VI)-complexes with the herein reported ligands were also in vitro assayed for their insulin-mimetic activity based on their effect over the FFA release from rat adipocytes treated with epinephrine, in comparison with that of MoO2 (see Fig. 2B). Analysis of IC50 values indicated for the Mo complexes unexpectedly lower activities as insulin-mimics (see Table 2) than expected values based on previous results.1 The similarly low insulin mimetic activity obtained for the Mo(VI) complexes may be related to the high percentage of hydrolytic processes occurring at the physiological pH, otherwise also in agreement with the closely related pMo values. Apparently, the same reason stands for the lower dependency of the activity on the complex strength and on the lipophilicity of the ligand. Nevertheless, some insulin-mimetic activity could be measured for the thio-compounds (thiomaltol and DMHTP), in opposition to the oxo-analogues.
The set of in vitro results obtained for the zinc complexes seem to evidence that pyrone complexes present an enhanced mimetic activity as compared with the corresponding pyridinone complexes and that complexes with S2O2 coordination mode show higher activity than those with O4 coordination mode. Table 2 also contains literature data for two ionic complexes (ZnSO4,31 and VOSO435), which are only sparingly absorbed in intestines, as well as for zinc and vanadium complexes of FDA-approved food additives, such as maltol (BMOV is already introduced in clinical trials with humans36) and ethylmaltol,31,37 besides the IC50 value for VO(DMHP)2.37 It can be concluded that the IC50 value herein obtained for Zn(thiomaltol)2 is quite lower than those from the literature in Table 2, as well as lower than the quite recently reported highest insulin mimetic activity for N-hydroxy-thiazole-thione zinc and vanadium complexes (IC50ca. 0.014–0.08 mM).18 This result is undoubtedly encouraging, even if the biological studies are only still of preliminary nature.
Since in animal models it has been reported that Zn chelation can increase the risk of developing type 1 diabetes,38 we have decided to analyze eventual effects of the ligands on the insulin-mimetic activity of the tested complexes. Therefore, the activity of the ligands alone was also bio-assayed, under the same experimental conditions of the metal complexes. It was observed that all the four ligands had little or no effect on the insulin-minetic activity (Fig. 4).
 | ||
| Fig. 4 Inhibitory effects of the ligands on FFA release from rat adipocytes treated with epinephrine. b (blank) and c (control) are cells only and cells plus epinephrine, respectively. Data are expressed as the means ± SDs for three experiments. | ||
Therefore, based on the herein performed in vitro studies, Zn(thiomaltol)2 can be considered promising as insulin-mimetics, although implications for therapy applications are obviously only speculative at this time. When considering the development of metal complexes for the treatment of DM, one has to bear in mind the choice of the metal ion and of the coordination compounds (low-toxic and low molecular weight ligands) since chelation reduces the polarity of the metal so that the complexes are able to better permeate through lipid bilayer cell membranes than the ionic compounds. Moreover, it is necessary to have an adequate hydro/lipophilicity for transport in the serum and trans-membrane transport with minimized toxicity and to make an analysis of the complex stability both in vitro and in vivo to avoid metal ion release by ligand exchange, redox processes or metabolic changes of the complex. Questions such as the role, the toxicity and the biological activity of the ligand as well as the quantity of metal ion internalized must be answered, but these issues have already been fully discussed in previous papers.1,2,10
The main reasons for pharma companies showing such adverse interest on vanadium compounds with high potential in oral treatment of DM seems to be the vanadate toxicity and the possible aerial oxidation of many vanadium(IV) compounds with concomitant changes in their outer appearance. Therefore, it seems to us that the results herein presented may provide new impetus for the future implementation of drugs based on non-toxic zinc complexes of heterocyclic low molecular weight ligands.
4. Conclusion
A set of Zn(II) and Mo(VI) complexes with 3-hydroxy-4-pyrone and –pyridinone, as well as the corresponding thione derivatives, have been assessed for their insulin-mimetic activity and solution physical-chemical properties. The thio-derivatives evidenced higher activity than the oxo-analogues and the Zn(II)–thiomaltol complex appears as the most promising compound. The in vitroinsulin-mimetic results have been somehow supported not only by the complexation properties but also by the lipo/hydrophilic character of the ligands. In fact, the higher activity presented by the O,S-compounds seems to be correlated with the higher Zn(II) affinity of the corresponding ligands, as compared with the corresponding O,O-derivatives; on the other hand, some trend was found between the insulin-mimetic activity of the zinc complexes and the lipophilicity of the ligands. So, the insulin-mimetic activity of the reported Zn(II) complexes seems to depend both on the stability of the complex and on its permeability through the cell membrane. Concerning the Mo(VI) complexes, a much lower insulin mimetic activity was revealed for these compounds than for the zinc analogues, which may be ascribed to the somewhat lower complex forming ability of the ligands towards this metal ion, with concomitantly higher competition by hydrolytic processes.Acknowledgements
The authors thank the Portuguese Fundação para a Ciência e Tecnologia (FCT) (project PCDT/QUI/56985/04) for financial support.References
- K. H. Thompson, J. Chiles, V. G. Yuen, J. Tse, J. H. McNeill and C. Orvig, J. Biol. Inorg. Chem., 2004, 9, 683–690.
- H. Sakurai, Expert Opin. Drug Discovery, 2007, 2, 873–887 Search PubMed.
- K. H. Thompson, J. H. McNeill and C. Orvig, Chem. Rev., 1999, 99, 2561–2671 CrossRef CAS.
- M. Anke, Anal. Real Acad. Nac. Farm., 2004, 70, 961–999 Search PubMed.
- P. Trumbo, A. A. Yates, S. Schlicker and M. Poos, J. Am. Diet. Assoc., 2001, 101, 294–301 CrossRef CAS.
- J. Li, G. Elberg, D. Gefel and Y. Shechter, Biochemistry, 1995, 34, 6218–6225 CrossRef CAS.
- Y. Goto, K. Kida, M. Ikeuchi, Y. Kaino and H. Matsuda, Biochem. Pharmacol., 1992, 44, 174–177 CrossRef CAS.
- A. Sigel and H. Sigel, Molybdenum and Tungsten: Their Roles in Biological Processes, in Metal Ions in Biological Systems, Marcel Dekker, New York, 2002, vol. 39, p. 810 Search PubMed.
- R. Hille, Chem. Rev., 1996, 96, 2757–281 CrossRef CAS.
- Y. Adachi, J. Yoshida, Y. Kodera, T. Kiss, T. Jakusch, E. A. Enyedy, Y. Yoshikawa and H. Sakurai, Biochem. Biophys. Res. Commun., 2006, 351, 165–70 CrossRef CAS.
- Z. D. Liu and R. C. Hider, Coord. Chem. Rev., 2002, 232, 151–171 CrossRef CAS.
- M. A. Santos, M. Gil, L. Gano and S. Chaves, J. Biol. Inorg. Chem., 2005, 10, 564–580 CrossRef CAS.
- M. A. Santos, Coord. Chem. Rev., 2008, 252, 1213–1224 CrossRef CAS.
- R. C. Hider, L. Ejim, P. D. Taylor, R. Gale, E. Huelns and J. B. Porter, Biochem. Pharmacol., 1990, 39, 1005–1012 CrossRef CAS.
- K. H. Thompson and C. Orvig, J. Chem. Soc., Dalton Trans., 2000, 2885–2892 RSC.
- Y. Yoshikawa, E. Uedo, H. Miyaki, H. Sakurai and Y. Koshima, Biochem. Biophys. Res. Commun., 2001, 281, 1190–1193 CrossRef CAS.
- S. L. Lord, N. A. Epstein, R. L. Paddock, C. M. Vogels, T. L. Hennigar, M. J. Zawaoroto, N. J. Taylor, W. R. Driedzic, T. L. Broderick and S. A. Wescott, Can. J. Chem., 1999, 77, 1249–1261 CrossRef CAS.
- A. Katoh, Y. Matsumura, Y. Yoshikawa, H. Yasui and H. Sakurai, J. Inorg. Biochem., 2009, 103, 567–574 CrossRef CAS.
- Purification of Laboratory Chemicals, ed. W. L. F. Armarego and D. D. Perrin, Butterworth-Heinemann Press, Oxford, 4th edn, 1999 Search PubMed.
- S. Chaves, M. Gil, S. Canário, R. Jelic, M. J. Romão, J. Trincão, E. Herdtweck, J. Sousa, C. Diniz, P. Fresco and M. A. Santos, Dalton Trans., 2008, 1773–1782 RSC.
- S. Chaves, S. M. Marques, A. Cachudo, M. A. Esteves and M. A. Santos, Eur. J. Inorg. Chem., 2006, 3853–3860 CrossRef CAS.
- P. Gans, A. Sabatini and A. Vacca, Talanta, 1996, 43, 1739–1753 CrossRef CAS.
- R. M. Smith and A. E. Martell, Critical Stability Constants, Plenum, New York, 1976, vol. 4, p. 9 Search PubMed.
- A. Leo, C. Hansch and D. Elkins, Chem. Rev., 1971, 71, 525–616 CrossRef CAS.
- M. Nakai, H. Watanabe, C. Fujisawa, H. Kakegawa, T. Satoh, J. Takada, R. Matsushita and H. Sakurai, Biol. Pharm. Bull., 1995, 18, 719–725 CAS.
- B. Song, N. Aebischer and C. Orvig, Inorg. Chem., 2002, 41, 1357–1364 CrossRef CAS.
- T. Jakusch, K. Gajda-Schrantz, Y. Adachi, H. Sakurai, T. Kiss and L. Horváth, J. Inorg. Biochem., 2006, 100, 1521–1526 CrossRef CAS.
- J. A. Lewis, B. L. Tran, D. T. Puerta, E. M. Rumberger, D. N. Hendrickson and S. M. Cohen, Dalton Trans., 2005, 2588–2596 RSC.
- V. Monga, B. O. Patrick and C. Orvig, Inorg. Chem., 2005, 44, 2666–2677 CrossRef CAS.
- R. Yokel, A. K. Datta and E. G. Jackson, J. Pharmacol. Exp. Ther., 1991, 257, 100–106 CAS.
- Y. Adachi, J. Yoshida, Y. Kodera, A. Kato, Y. Yoshikawa, Y. Kojima and H. Sakurai, J. Biol. Inorg. Chem., 2004, 9, 885–893 CrossRef CAS.
- M. A. Santos, S. Gama, J. C. Pessoa, M. C. Oliveira, I. Tóth I and E. Farkas, Eur. J. Inorg. Chem., 2007, 1728–1737 CrossRef CAS.
- A. Nakayama, M. Hiromura, Y. Adachi and H. Sakurai, J. Biol. Inorg. Chem., 2008, 13, 675–684 CrossRef CAS.
- W. Basuki, M. Hiromura and H. Sakurai, J. Inorg. Biochem., 2007, 101, 692–699 CrossRef CAS.
- H. Sakurai, Y. Kojima, Y. Yoshikawa, K. Kawabe and H. Yasui, Coord. Chem. Rev., 2002, 226, 187–198 CrossRef CAS.
- D. Rehder, Inorg. Chem. Commun., 2003, 6, 604–617 CrossRef CAS.
- M. Rangel, A. Tamura, C. Fukushima and H. Sakurai, J. Biol. Inorg. Chem., 2001, 6, 128–132 CrossRef CAS.
- Y. Zheng, X.-K. Li, Y. Wang and L. Cai, Hemoglobin, 2008, 32, 135–145 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1, Fig. S2. See DOI: 10.1039/b914169c |
| This journal is © The Royal Society of Chemistry 2010 |
