Redesigning the designer drug ecstasy: non-psychoactive MDMA analogues exhibiting Burkitt's lymphoma cytotoxicity†
Michael N.
Gandy
a,
Matthew
McIldowie
a,
Katie
Lewis
a,
Agata M.
Wasik
b,
Danielle
Salomonczyk
c,
Keith
Wagg
d,
Zak A.
Millar
e,
David
Tindiglia
e,
Philippe
Huot
f,
Tom
Johnston
f,
Sherri
Thiele
f,
Blake
Nguyen
a,
Nicholas M.
Barnes
g,
Jonathan M.
Brotchie
f,
Mathew T.
Martin-Iverson
e,
Joanne
Nash
c,
John
Gordon
b and
Matthew J.
Piggott
*a
aSchool of Biomedical, Biomolecular and Chemical Sciences, The University of Western Australia, Crawley, WA 6009, Australia. E-mail: piggott@cyllene.uwa.edu.au; Fax: +61 6488 1005; Tel: +61 6488 3170
bMRC Centre for Immune Regulation, The Medical School, University of Birmingham, UK B15 2TT
cCentre for Neurobiology of Stress, Department of Biological Sciences, University of Toronto at Scarborough, 1265 Military Trail, Toronto, ON M1C 1A4, Canada
dDepartment of Chemistry, The Australian National University, Canberra, ACT 0200, Australia
eSchool of Medicine and Pharmacology, The University of Western Australia, Crawley, WA 6009, Australia
fToronto Western Research Institute, Toronto Western Hospital, 399 Bathurst Street, MC 11-419, Toronto, ON M5T 2S8, Canada
gClinical and Experimental Medicine, The Medical School, University of Birmingham, UK B15 2TT
First published on 7th September 2010
Abstract
Burkitt's lymphoma (BL) is a particularly aggressive cancer that primarily affects African children. Unfortunately, effective and affordable treatment is out of reach of most of the afflicted. The illicit psychoactive drug methylenedioxymethamphetamine (MDMA, ‘ecstasy’) is cytotoxic to BL cell lines, but its low potency, psychoactivity and neurotoxicity preclude consideration as a therapeutic drug candidate. This paper describes the discovery of novel α-aryl analogues of MDMA that lack psychoactivity and reduce BL cell line viability with significantly more potency than the lead compound. Preliminary in vitro studies also indicate that the compounds are non-toxic to a relevant neuronal cell line.
Introduction
Burkitt's lymphoma (BL) is an extremely aggressive cancer that primarily affects children and young adults. The sporadic form of the disease is rare, with an incidence of 0.002–0.003% of population per year, although its prevalence has increased rapidly in recent times due to the susceptibility of HIV-infected individuals, who have a 1000-fold greater incidence relative to the general population.1 In equatorial Africa, where it is associated with Epstein-Barr virus and malarial infection, BL is endemic and the most common childhood cancer, with a frequency of 0.005–0.020%.2,3 As the population of the ‘lymphoma belt’4 exceeds 500 million, this equates to 25–100,000 cases per year. Endemic BL commonly manifests as horrific facial tumours, which can double in size in one day (Fig. 1).1 | ||
| Fig. 1 A graphic example of a facial tumour characteristic of endemic Burkitt's lymphoma. (Image by Mike Blythe5). | ||
Current therapies for BL involve aggressive combination chemotherapy and frequent hospitalisation. While offering cure rates of 80%, these therapeutic regimes are not readily accessible to poor Africans, are not nearly as effective in AIDS sufferers and patients with disseminated tumours, and are associated with significant toxicity.1,6 Accordingly, there is an urgent demand for BL treatments that are cheaper, more efficacious and more amenable to patient compliance. Despite this need, BL is a very low priority disease for pharmaceutical companies due to its low profitability.
Thus, it seems that the burden of BL drug discovery falls on academia. Indeed, some reasonably simple compounds with very potent activity against BL cell lines, notably 2-benzoxazolyl hydrazones7and E-styrylbenzylsulfones,8 have recently been discovered by academic research groups. However, given the many barriers to successful drug development, a multipronged attack is probably required.
In 2005, the illicit drug MDMA (‘ecstasy’, 1) (Fig. 2) was shown to induce apoptosis in BL cell lines.9 Although MDMA is cheap to make and has excellent pharmacokinetic properties, it is not suitable for development as a treatment for BL. Firstly, its potency with respect to killing BL cells (IC50 0.2–1 mM)9 is inadequate. Secondly, MDMA is both psychoactive10 and neurotoxic.11
 | ||
| Fig. 2 Methylenedioxymethamphetamine (MDMA, ‘ecstasy’). The hydrochloride is the most commonly ‘marketed’ form. | ||
Accordingly, we set out to discover MDMA analogues with enhanced anti-BL potency and selectivity but without psychoactivity and neurotoxicity. Herein we detail our initial progress towards this goal. Although the structure-psychoactivity relationships of MDMA analogues have been explored, to the best of our knowledge, the use of MDMA as a lead compound in a medicinal chemistry program has not been reported previously.
Target choice
Our rationale for the choice of MDMA analogue targets has been guided by the brave and extensive work of Shulgin and co-experimenters.12–15 Two salient observations were apparent from their studies: extension of the α- or N-substituent of MDMA to anything larger than an ethyl group abolished psychoactivity (at least anecdotally). As part of our goal was to dissociate psychoactivity from anti-BL activity, modification of these positions provided a good starting point. Herein we focus on the α-substituent; our investigation of variously N-substituted MDMA analogues is ongoing.Synthesis
Because of the wide interest in amphetamine derivatives, abundant syntheses of the simpler members of the family exist, most of which proceed via reductive amination of benzyl ketones. Two of the most common routes to such ketones involve Knoevenagel-Walter condensation (an Henry reaction followed by dehydration) to give a β-nitrostyrene, followed by reductive hydrolysis;16 or epoxidation of a styrene followed by acid catalysed rearrangement.14 While the latter strategy lacks efficiency because of an early divergent step, and is probably not applicable to regioselective synthesis of benzyl aryl ketones, we have found that the Knoevenagel-Walter reaction lacks scope (see also ref. 16). For example, the reaction of α-nitrotoluene (2) with piperonal (4) failed to give the required β-nitrostyrene 5 under a variety of conditions (Fig. 3). This is presumably due to the combined effects of the stereoelectronically-stabilised intermediate nitronate 3 and relatively unreactive, electron-rich aldehyde 4. Accordingly, a more general and efficient synthetic strategy was investigated. | ||
| Fig. 3 Failed Knoevenagel-Walter condensation of α-nitrotoluene and piperonal. | ||
Our initial plan involved the reaction of the known piperonylmagnesium chloride (6)17 with various nitriles, which, after hydrolytic workup, would give a series of piperonyl ketones (7) (Fig. 4). In practice, the reaction of 6 with benzonitrile gave the desired acetophenone 7g in good yield, but with butyronitrile the yield of the ketone 7c was only 29%, presumably due to competing α-deprotonation, as supported by the isolation of the proto-demetallated product, 3,4-methylenedioxytoluene.
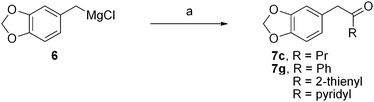 | ||
| Fig. 4 Synthesis of piperonyl ketones from nitriles. Reagents, conditions and yields: (a) (i) RCN, Et2O or THF, reflux, 7c (29%), 7g (80%), R = 2-thienyl (17%), R = 2-, 3- or 4-pyridyl (0%). | ||
An attempt to attenuate the basicity of the organometallic reagent by transmetallation with ceric chloride failed to improve the yield of 7c. Furthermore, heteroaromatic nitriles lacking an α-proton also gave very low yields of the corresponding ketones.
Knochel and co-workers have shown that benzylic organozinc reagents can be transmetallated with the THF-soluble complex CuCN.2LiCl, and that the resulting organocuprates react cleanly with acid chlorides to give benzyl ketones in excellent yields.18 However, they were unable to prepare piperonylzinc halides, as homocoupling predominates. This problem was solved by using a piperonylzinc phosphate.19
We have found that the organocuprate 8 derived from the Grignard reagent 6 also adds smoothly to acid chlorides (Fig. 5). Thus, various acid chlorides were added, in parallel, to solutions of the organocuprate 8, giving the piperonyl ketones 7 in fair to excellent yields (Tables 1 and 3). The reaction of 8 with phenylacetyl chloride gave an intractable mixture, thus benzyl piperonyl ketone (7p) was prepared using Baldwin's acyl anion-equivalent methodology,20 from the N-t-butylhydrazone 9. The symmetrical dipiperonyl ketone (7q) was more conveniently prepared by self-condensation21 of commercially available homopiperonylic acid (10).
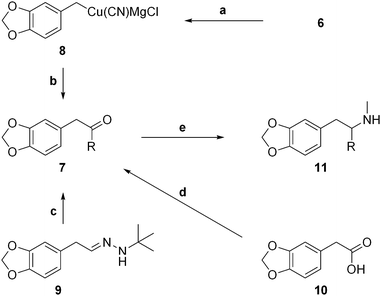 | ||
Fig. 5 Synthesis of MDMA analogues: Reagents and conditions: (a) CuCN.2LiCl, THF, −78 °C → −30 °C; (b) (i) RCOCl, THF, −78 °C → 0 °C; (ii) H3O+; (c) (i) BuLi, THF, −78 °C, then BnBr −78 °C → rt (ii) sat. NH4Cl; (ii) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 TFA/H2O (7p); (d) DCC, DMAP, DCM (7q) (e) MeNH2, AcOH, NaCNBH3, EtOH, THF, 3A sieves, 50 °C. (See Tables 1 and 3 for yields). 1 TFA/H2O (7p); (d) DCC, DMAP, DCM (7q) (e) MeNH2, AcOH, NaCNBH3, EtOH, THF, 3A sieves, 50 °C. (See Tables 1 and 3 for yields). | ||
| R | Ketone | % Yielda | Amine | % Yieldb | IC50/μMc |
|---|---|---|---|---|---|
| a Yields are based upon on Grignard concentration (determined by titration23). b The optimized conditions were not used for all reactions, hence the variable yields. c Based on reduced [3H]thymidine incorporation as per ref. 9; mean value ± SEM, n = 3 except for 1 and 7g, where n = 14 and 8, respectively. | |||||
| Me | 7a | 85 | 1 | 90 | 507 ± 80 |
| Et | 7b | 87 | 11b | 87 | 707 ± 23 |
| Pr | 7c | 85 | 11c | 70 | 379 ± 25 |
| c-Pr | 7d | 65 | 11d | 70 | 801 ± 31 |
| i-Pr | 7e | 84 | 11e | 84 | 651 ± 102 |
| t-Bu | 7f | 64 | 11f | 69 | 447 ± 61 |
| Ph | 7g | 97 | 11g | 91 | 76 ± 5 |
The ketones were reductively aminated to give MDMA (1) and the analogous N-methylamines (11b–t) in fair to good yields (Fig. 5, Tables 1 and 3). Crushed 4A molecular sieves were deleterious in these reactions, presumably due to sequestration of the methylamine (diameter 2.9 Å22); however, 3A sieves were beneficial. The solubility of the ketones in EtOH was poor and the use of the co-solvent THF improved yields by up to 20%. Also of note are the solubilities of the protonated forms of the amines in organic solvents; the usual acid–base-cycle purification step leads to substantial losses and should be avoided. The amines were converted to their crystalline hydrochlorides and were tested as such.
Results and discussion
Series 1 analogues: Anti-BL activity
MDMA (1) and the analogues 11b–t were assessed for toxicity to the L3055 Burkitt's lymphoma cell line.9 Initial studies began with 11b–g (Table 1), which were chosen to explore the effects of an incremental increase in the size of the α-substituent. The α-Ph analogue 11g was the standout performer, being almost seven times more potent than MDMA. Before making analogues of 11g, it was important to establish whether the negative attributes of MDMA had been retained in this compound, and thus it was assessed for potential to be psychoactive and neurotoxic.Psychoactivity
MDMA was originally described as inducing “an easily controlled altered state of consciousness with emotional and sensual overtones”.24 However, it is most likely the associated intense euphoria that has made MDMA such a popular recreational drug.15 Whilst mild euphoria might be a side-effect with some benefit in a cancer treatment, the powerful psychoactivity of MDMA is probably excessive for this purpose and has abuse liability. Thus, as mentioned above, one of our goals was to dissociate this psychoactivity from the BL cytotoxicity.We assessed potential psychoactivity with prepulse inhibition of the acoustic startle reflex (PPI), which is a reduction in the magnitude of the obligatory startle reflex induced by loud abrupt sounds produced by preceding the startling sound with a quiet, non-startling sound (the prepulse), by 100 ms in the present case. In this study, a wide range of intensities of startling stimuli were used, from below threshold to the asymptotic range, and we measured the prepulse-induced reduction in the asymptotic magnitude of response.25,26
A reduction in PPI is a widely replicated endophenotype of schizophrenia (for reviews see ref. 27–29), which may correlate with psychotic symptoms.30–32 In particular, we have observed prepulse-induced inhibition of the asymptotic startle magnitude in patients with schizophrenia,33 and decreased PPI of this measure in people is associated with poor attention and inhibitory control of irrelevant stimuli in the Stroop test.34 The most common animal models of psychosis produce a similar PPI deficit35 with psychotomimetic or hallucinogenic drugs,36,37 including dopaminergic drugs like amphetamines,38 MDMA39 or other psychotomimetics such as N-methyl-D-aspartate (NMDA) receptor antagonists like phencyclidine, ketamine and MK801.40,41 On the other hand, antipsychotic drugs have been shown to reverse the effects of some of these psychotomimetic compounds on PPI,42 and some antipsychotics increase PPI on their own.43 Thus, drug-induced reductions in PPI in rats correlate well with their psychotomimetic and hallucinogenic effects, and PPI provides an objectively quantifiable and reliable measure of psychoactivity of MDMA and related compounds.
para-Methoxyamphetamine (PMA) was included as a control as it is a related amphetamine analogue that has extremely potent hallucinogenic effects, and has similar subjective effects to MDMA (based on it being found in pills sold as “ecstasy”44), but is devoid of stimulant effects,45 indicating that reductions in PPI are likely more related to psychotomimetic or hallucinogenic effects than to motor stimulant effects.
As indicated in Fig. 6, the human-psychoactive drugs MDMA and PMA reduce prepulse inhibition in a dose-dependent manner. Conversely, compound 11g clearly shows the opposite trend, marginally increasing prepulse inhibition, much like some antipsychotic drugs (see above). These data strongly suggest that 11g does not exhibit psychoactivity. By extrapolation, other MDMA analogues with large α-substituents (see below) are also unlikely to be psychoactive (and therefore were not tested for this purpose).
 | ||
| Fig. 6 Effect of MDMA, p-methoxyamphetamine (PMA) and 11g on prepulse inhibition of RMAX (maximum response achievable from a subject under a given drug and prepulse condition). Dose-response curves were fitted using the equation y = yi + (RMAX - yi)/(1 + 10^(ES50 - x)), where yi was defined by the percent prepulse inhibition under the control condition, and RMAX by the percent prepulse inhibition at the maximum dose. Dose-response curves for MDMA and PMA showed good agreement with observed values (r2 = 0.862 and 0.966, respectively), while that for 11g did not (r2 = 0.261). *Significantly different from MDMA and PMA, p < 0.05; + significantly different from saline (y-intercept), p < 0.05. | ||
Receptor binding studies
In a preliminary investigation of the mode-of-action against the L3055 cell line, and to explain its lack of psychoactivity, the binding of 11g to a panel of receptors and transporters was assessed. MDMA is known to interact directly with several serotonin (5-hydroxytrypamine, 5HT) receptors,46–48 as well as the transporters for serotonin (SERT),46,49–54 noradrenaline (NET) and dopamine (DAT).46,50,52,54 Moreover, L3055 cells have been shown to express SERT9 and DAT,55 as well as 5HT2C, 5HT1B and 5HT1D,56 hence the choices in the panel. Inhibition constant (Ki) data for MDMA and 11g at 5HT2A, NET, SERT and DAT sites are presented in Table 2. Full dose-response relationships were not established for 5HT1A, 5HT2C, and 5HT1B/1D, because the affinities of both MDMA and 11g were too low based on a preliminary 10 μM screen.| Receptor/transporter | MDMA | 11g |
|---|---|---|
| a The affinity is provided as the half-maximal inhibitory constant (Ki, μM). The error is the SEM of the Ki provided by triplicate experiments. b P = 0.04 (Student t test). | ||
| 5HT1A | >10 | >10 |
| 5HT1B/D | >10 | >10 |
| 5HT2C | >10 | >10 |
| 5HT2A | >10 | 1.23 ± 0.71b |
| NET | >10 | 0.70 ± 0.2 |
| SERT | 0.21 ± 0.2 | 1.32 ± 0.4 |
| DAT | >10 | >10 |
Since the affinity of 11g for the SERT is comparable to that of MDMA, the lack of psychoactivity of the former could be explained if the two compounds elicit different responses at the transporter. The pharmacology of MDMA is complex and by no means fully understood, but a major effect is the amplification of extracellular levels of serotonin by blocking reuptake and reversing the actions of the SERT, i.e., releasing intracellular stores of serotonin.54 It is possible that 11g binds to the SERT but does not induce one or both of these effects. Alternatively, the lack of psychoactivity may reflect an increased affinity of 11g for the 5-HT2A receptor and/or the noradrenaline transporter, compared to MDMA.
Similarly, the enhanced BL-killing potency of 11g relative to MDMA is probably not due to its interaction with the SERT, but could be associated with an enhanced affinity for the NET and/or 5HT2A receptor. Of course at this early juncture, other modes of action cannot be ruled out.
Neurotoxicity
It has been well established that MDMA is neurotoxic, both in humans and experimental models such as mice, rats and primates.57–60 Neuronal death has been observed throughout the brain, (e.g hippocampus, striatum, cortex, amygdala and thalamus), and it appears that catecholaminergic and serotonergic neurons are most susceptible.60–64 Thus, as a step towards the discovery of realistic candidates for the treatment of BL, we assessed the neurotoxicity of MDMA and analogues in a neuroblastoma cell line, SH-SY5Y (Fig. 7). This is a catecholaminergic cell line, and so represents dopaminergic and adrenergic neurons, which have been shown to degenerate following exposure to MDMA.57,59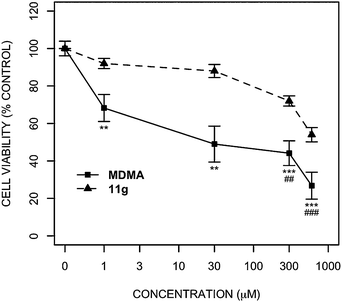 | ||
| Fig. 7 Effect of MDMA and 11g on SH-SY5Y cell viability. SH-SY5Y cells were exposed to various concentrations of MDMA or 11g (1, 30, 300, 600 μM) for 24 h. Cell viability was assessed using Alamar Blue. Data are presented as mean ± SEM (n = 6). Two way analysis of variance using concentration and compound as factors showed significant effects of concentration (p < 0.001, F4 = 8.10) and compound (p < 0.001, F3 = 92.37) and a significant interaction between the two (p < 0.001, F12 = 9.44). Bonferoni post hoc showed significant effects of MDMA at 1–600 μM, and 11g at 300 and 600 μM, on cell viability compared to vehicle (media). ##, ### indicates a p < 0.01 and p < 0.001, respectively for 11g compared to vehicle. **, *** indicates a p < 0.01 and p < 0.001, respectively for MDMA compared to vehicle. | ||
As illustrated in Fig. 7, although 11g is toxic to the SH-SY5Y cell line at high concentrations, it is significantly less toxic than MDMA at all concentrations tested. Although a cell-based assay is not always sufficient to predict in vivo toxicity, especially where metabolism and hyperthermia are implicated,65 the reduced toxicity of 11g relative to MDMA was encouraging, and led us to adopt it as the new lead compound.
Series 2 analogues
The enhanced potency and reduced toxicity of 11g to the neuronal cell line, coupled with the likelihood that it is not psychoactive, made it the basis for further structural modifications. Series 2 analogues that explored tolerance to steric (11h–j) and (stereo)electronic (11k–n) modifications to, and reduction (11o) or extension (11p) of, the phenyl substituent did not have a dramatic effect on activity (Table 3). However, a modest improvement in potency as like substituents were ‘shifted’ from the ortho-meta-para position (11h–m) was noted.| R | Ketone | % Yielda | Amine | % Yield | IC50 /μM |
|---|---|---|---|---|---|
| a Based upon 100% conversion of piperonyl chloride into organocuprate 8; as the actual conversion is somewhat lower, the yields shown here are lower estimates. b Prepared using Baldwin's acyl anion equivalent methodology from 9. c Prepared by self-condensation of homopiperonylic acid (10). d Mean value ± SEM, n = 3 except for 11q where n = 2. | |||||

|
7h | 57 | 11h | 76 | 65 ± 8 |

|
7i | 88 | 11i | 83 | 51 ± 6 |
|
7j | 67 | 11j | 83 | 42 ± 3 |

|
7k | 58 | 11k | 86 | 92 ± 8 |

|
7l | 68 | 11l | 74 | 80 ± 3 |

|
7m | 49 | 11m | 64 | 69 ± 3 |

|
7n | 71 | 11n | 70 | 63 ± 2 |

|
7o | 64 | 11o | 85 | 81 ± 8 |

|
7p | 36b | 11p | 88 | 69 ± 4 |

|
7q | 50c | 11q | 67 | 36.7 ± 0.1 |
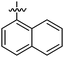
|
7r | 63 | 11r | 79 | 12.6 ± 0.5 |

|
7s | 75 | 11s | 84 | 6.6 ± 0.4 |

|
7t | 71 | 11t | 83 | 6 ± 1 |
Symmetrical analogue 11q was roughly twice as potent as the series 2 lead 11g, which could reflect an effective doubling of concentration due to the absence of chirality, or simply an enhancement due to a larger α-substituent. The latter reasoning is certainly supported by the results for 11r–s, which are approximately ten fold more potent than 11g, and nearly two orders of magnitude more potent than MDMA.
Neurotoxicity – series 2 analogues
Two of the most promising leads, 11r and 11s, were assessed for toxicity to the SH-SY5Y cell line alongside MDMA, as described above for 11g. As indicated in Fig. 8, there was no significant effect of 11r or 11s on cell viability compared to vehicle. This suggests that modifications that enhance toxicity to the BL cell line, namely incorporation of large aromatic α-substituents, also eliminate inherent neurotoxicity. Of course, further studies are required to see if the lack of toxicity in this cell-based assay is replicated in animal models. | ||
| Fig. 8 Effect of MDMA and analogues on SH-SY5Y cell viability. SH-SY5Y cells were exposed to various concentrations of MDMA (1), 11r or 11s (1, 30, 300, 600 μM) for 24 h. Cell viability was assessed using Alamar Blue. Data are presented as mean ± SEM (n = 6). Two way analysis of variance using concentration and compound as factors showed significant effects of concentration (p < 0.001, F4 = 8.10) and compound (p < 0.001, F3 = 92.37) and a significant interaction between the two (p < 0.001, F12 = 9.44). There was no significant effect of 11r or 11s compared to vehicle. **, *** indicates p < 0.01 and p < 0.001, respectively for MDMA compared to vehicle. | ||
Conclusion
Readily synthesised analogues of the illicit, psychoactive drug MDMA bearing alternative substituents (to methyl) at the α-position, reduce viability of L3055 Burkitt's lymphoma (BL) cells at micromolar concentrations. Pre-pulse inhibition studies indicate that the most potent series 1 analogue 11g, with an α-phenyl substituent, is very unlikely to be psychoactive. However, 11g is toxic to the dopaminergic neuroblastoma cell line SH-SY5Y, albeit less so than MDMA. Series 2 analogues, based on 11g (11h–11t), also killed BL cells, in some instances (11r–11t) with low micromolar potency. Furthermore, 11r and 11s exhibited no toxicity in the dopaminergic cell line and are unlikely to be psychoactive. Accordingly, 11r and 11s are promising new drug leads for Burkitt's and related lymphomas.Our efforts to further improve selective BL-killing potency and determine the mode of action of this class of compounds are ongoing. Future studies will include an examination of the importance of configuration (i.e. evaluation of individual enantiomers as opposed to the racemates used in this study), receptor/transporter functionality studies for the more potent analogues, further optimisation of the α-substituent, and modification of other parts of the amphetamine skeleton. The toxicity of these compounds to a broader range of cancerous cells lines is also of interest.
Moreover, in this paper we have shown, for the first time, that it is possible to dissociate the psychoactivity exhibited by MDMA from one of its useful biological properties. This opens the door to the use of MDMA as a lead compound in other indications for which it has shown promising activity; for example, in Parkinson's disease,66 and post-traumatic stress disorder.67,68
Acknowledgements
The authors thank Dr Lindsay Byrne for assistance with NMR spectroscopy and Dr Tony Reeder for mass spectra. BN was the recipient of a PhD scholarship from Atuka Ltd (http://www.atuka.com), MNG and KDL are recipients of UWA Postgraduate and Australian Postgraduate Awards, respectively. AMW, NMB and JG were supported by Leukaemia and Lymphoma Research (U.K.). PH held a Fellowship from the Edmund J Safra Philanthropic Foundation and the Parkinson Society Canada.References
- A. Serafeim, M. J. Holder, G. Grafton, A. Chamba, M. T. Drayson, Q. T. Luong, C. M. Bunce, C. D. Gregory, N. M. Barnes and J. Gordon, Blood, 2003, 101, 3212 CrossRef CAS.
- J. L. Hecht and J. C. Aster, J. Clin. Oncol., 2000, 18, 3707 CAS.
- R. Rochford, M. J. Cannon and A. M. Moormann, Nat. Rev. Microbiol., 2005, 3, 182 Search PubMed.
- C. A. van den Bosch, Lancet Oncol., 2004, 5, 738 CrossRef CAS.
- http://en.wikipedia.org/wiki/File%3ALarge_facial_Burkitt%27s_Lymphoma.JPG, Accessed 20th May, 2009.
- J. T. Yustein and C. V. Dang, Curr. Opin. Hematol., 2007, 14, 375 CrossRef.
- J. Easmon, G. Puerstinger, K.-S. Thies, G. Heinisch and J. Hofmann, J. Med. Chem., 2006, 49, 6343 CrossRef CAS.
- M. V. R. Reddy, M. R. Mallireddigari, S. C. Cosenza, V. R. Pallela, N. M. Iqbal, K. A. Robell, A. D. Kang and E. P. Reddy, J. Med. Chem., 2008, 51, 86 CrossRef CAS.
- E. J. Meredith, M. J. Holder, A. Chamba, A. Challa, A. D. Lee, C. M. Bunce, M. T. Drayson, G. Pilkington, R. D. Blakely, M. J. S. Dyer, N. M. Barnes and J. Gordon, FASEB J., 2005, 19, 1187 CAS.
- S. R. White, T. Obradovic, K. M. Imel and M. J. Wheaton, Prog. Neurobiol., 1996, 49, 455 CrossRef CAS.
- G. A. Ricaurte, L. S. Forno, M. A. Wilson, L. E. DeLanney, I. Irwin, M. E. Molliver and J. W. Langston, JAMA, J. Am. Med. Assoc., 1988, 260, 51 Search PubMed.
- U. Braun, A. T. Shulgin and G. Braun, J. Pharm. Sci., 1980, 69, 192 CrossRef CAS.
- U. Braun, A. T. Shulgin and G. Braun, Arzneimittelforschung., 1980, 30, 825 CAS.
- D. E. Nichols, A. J. Hoffman, R. A. Oberlender, P. Jacob, III and A. T. Shulgin, J. Med. Chem., 1986, 29, 2009 CrossRef CAS.
- A. T. Shulgin, A. Shulgin and D. E. Nichols, Phenethylamines I have known and loved: a chemical love story., Transform Pr.USA, 1991 Search PubMed.
- M. P. Johnson, S. P. Frescas, R. Oberlender and D. E. Nichols, J. Med. Chem., 1991, 34, 1662 CrossRef CAS.
- K. V. Baker, J. M. Brown, N. Hughes, A. J. Skarnulis and A. Sexton, J. Org. Chem., 1991, 56, 698 CrossRef CAS.
- S. C. Berk, P. Knochel and M. C. P. Yeh, J. Org. Chem., 1988, 53, 5789 CrossRef.
- C. Jubert and P. Knochel, J. Org. Chem., 1992, 57, 5425 CrossRef CAS.
- R. M. Adlington, J. E. Baldwin, J. C. Bottaro and M. W. D. Perry, J. Chem. Soc., Chem. Commun., 1983, 1040 RSC.
- S. Bhandari and S. Ray, Synth. Commun., 1998, 28, 765 CrossRef CAS.
- Calculated using Spartan.
- H.-S. Lin and L. A. Paquette, Synth. Commun., 1994, 24, 2503.
- A. T. Shulgin and D. E. Nichols, Psychopharmacol. Hallucinogens, [Workshop], 1978, 74 Search PubMed.
- A. Hince Dana and T. Martin-Iverson Mathew, Behav. Neurosci., 2005, 119, 66 CrossRef CAS.
- C. W. Stoddart, J. Noonan and M. T. Martin-Iverson, Behav. Neurosci., 2008, 122, 516 CrossRef CAS.
- K. S. Cadenhead, Psychiatric Clinics of North America, 2002, 25, 837 Search PubMed.
- I. C. Weiss and J. Feldon, Psychopharmacology, 2001, 156, 305 CrossRef CAS.
- N. R. Swerdlow, M. Weber, Y. Qu, G. A. Light and D. L. Braff, Psychopharmacology, 2008, 199, 331 CrossRef CAS.
- K. M. Abel, S. Jolley, D. R. Hemsley and M. A. Geyer, J. Psychopharmacol., 2004, 18, 181 Search PubMed.
- E. A. Hazlett, M. J. Romero, M. M. Haznedar, A. S. New, K. E. Goldstein, R. E. Newmark, L. J. Siever and M. S. Buchsbaum, Schizophr. Res., 2007, 93, 288 CrossRef.
- U. Meincke, D. Morth, T. Voss, B. Thelen, M. A. Geyer and E. Gouzoulis-Mayfrank, Eur. Arch. Psychiatry Clin. Neurosci., 2004, 254, 415 CrossRef.
- K. E. Scholes and M. T. Martin-Iverson, Psychophysiology, 2010, 47, 223 CrossRef.
- K. E. Scholes and M. T. Martin-Iverson, Behav. Brain Res., 2009, 205, 456 CrossRef.
- M. A. Geyer and B. Ellenbroek, Progress Neuro-Psychopharmacol. Biol. Psychiatry, 2003, 27, 1071 Search PubMed.
- M. A. Geyer, Pharmacopsychiatry, 1998, 31, 73 CrossRef CAS.
- M. A. Geyer, K. Krebs-Thomson, D. L. Braff and N. R. Swerdlow, Psychopharmacology, 2001, 156, 117 CrossRef CAS.
- N. R. Swerdlow, R. S. Mansbach, M. A. Geyer, L. Pulvirenti, G. F. Koob and D. L. Braff, Psychopharmacology, 1990, 100, 413 CrossRef CAS.
- V. Bubenikova, M. Votava, J. Horacek and T. Palenicek, Behavioural Pharmacology, 2005, 16, 127 CrossRef CAS.
- V. A. Keith, R. S. Mansbach and M. A. Geyer, Biol. Psychiatry, 1991, 30, 557 CrossRef CAS.
- N. M. W. J. De Bruin, B. A. Ellenbroek, A. R. Cools, A. M. L. Coenen and E. L. J. M. Van Luijtelaar, Psychopharmacology, 1999, 142, 9 CrossRef CAS.
- N. R. Swerdlow and M. A. Geyer, Pharmacol., Biochem. Behav., 1993, 44, 741 CrossRef CAS.
- C. Johansson, D. M. Jackson, J. Zhang and L. Svensson, Pharmacol., Biochem. Behav., 1995, 52, 649 CrossRef CAS.
- L. H. Ling, C. Marchant, N. A. Buckley, M. Prior and R. J. Irvine, Med. J. Aust., 2001, 174, 453 Search PubMed.
- M. T. Martin-Iverson, N. Yamada, A. W. By and B. A. Lodge, J. Psychiatry Neurosci., 1991, 16, 253 CAS.
- G. Battaglia, B. P. Brooks, C. Kulsakdinun and E. B. De Souza, Eur. J. Pharmacol., 1988, 149, 159 CrossRef CAS.
- R. A. Lyon, R. A. Glennon and M. Titeler, Psychopharmacology, 1986, 88 Search PubMed.
- T. D. Steele, D. E. Nichols and G. K. W. Yim, Biochem. Pharmacol., 1987, 36, 2297 CrossRef CAS.
- U. V. Berger, X. F. Gu and E. C. Azmitia, Eur. J. Pharmacol., 1992, 215, 153 CrossRef CAS.
- M. P. Johnson, P. F. Conarty and D. E. Nichols, Eur. J. Pharmacol., 1991, 200, 9 CrossRef CAS.
- D. C. Jones, S. S. Lau and T. J. Monks, J. Pharmacol. Exp. Ther., 2004, 311, 298 CrossRef CAS.
- R. B. Rothman, M. H. Baumann, C. M. Dersch, D. V. Romero, K. C. Rice, F. I. Carroll and J. S. Partilla, Synapse, 2001, 39, 32 CrossRef CAS.
- G. Rudnick and S. C. Wall, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 1817 CrossRef CAS.
- C. D. Verrico, G. M. Miller and B. K. Madras, Psychopharmacology, 2006, 189, 489 CrossRef.
- E. J. Meredith, M. J. Holder, A. Rosen, A. D. Lee, M. J. S. Dyer, N. M. Barnes and J. Gordon, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 13485 CrossRef CAS.
- J. G., unpublished data.
- J. P. Capela, H. Carmo, F. Remiao, M. L. Bastos, A. Meisel and F. Carvalho, Mol. Neurobiol., 2009, 39, 210 Search PubMed.
- M. de Win, M. L. G. Jager, J. Booij, L. Reneman, T. Schilt, C. Lavini, S. D. Olabarriaga, G. J. den Heeten and W. van den Brink, Brain, 2008, 131, 2936 CrossRef.
- A. R. Green, A. O. Mechan, J. M. Elliott, E. O'Shea and M. I. Colado, Pharmacol. Rev., 2003, 55, 463 CrossRef CAS.
- T. Xie, L. Tong, U. D. McCann, J. Yuan, K. G. Becker, A. O. Mechan, C. Cheadle, D. M. Donovan and G. A. Ricaurte, J. Neurosci., 2004, 24, 7043 CrossRef CAS.
- D. L. Commins, G. Vosmer, R. M. Virus, W. L. Woolverton, C. R. Schuster and L. S. Seiden, J. Pharmacol. Exp. Ther., 1987, 241, 338 CAS.
- E. O'Hearn, G. Battaglia, E. B. De Souza, M. J. Kuhar and M. E. Molliver, J. Neurosci., 1988, 8, 2788 CAS.
- C. J. Schmidt, J. A. Levin and W. Lovenberg, Biochem. Pharmacol., 1987, 36, 747 CrossRef CAS.
- T. Xie, L. Tong, M. W. McLane, G. Hatzidimitriou, J. Yuan, U. McCann and G. Ricaurte, Neuropsychopharmacology, 2006, 31, 2639 CrossRef CAS.
- E. Puerta, I. Hervias and N. Aguirre, Neuropsychobiology, 2009, 60, 119 CrossRef CAS.
- M. M. Iravani, M. J. Jackson, M. Kuoppamaeki, L. A. Smith and P. Jenner, J. Neurosci., 2003, 23, 9107 CAS.
- J. C. Bouso, R. Doblin, M. Farre, M. A. Alcazar and G. Gomez-Jarabo, J. Psychoactive Drugs, 2008, 40, 225 Search PubMed.
- P. O. Johansen and T. S. Krebs, J. Psychopharmacol., 2009, 23, 389 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Full synthetic details and complete characterisation of all ketone precursors and target amines; protocols for the biological assays. See DOI: 10.1039/c0md00108b |
| This journal is © The Royal Society of Chemistry 2010 |
