Poly(methyl methacrylate)-graft-oligoamines as low cytotoxic and efficient nonviral gene vectors
Yong-Qiang
Wang
a,
Yun-Xia
Sun
ab,
Xin-Lin
Hong
*a,
Xian-Zheng
Zhang
*ab and
Gao-Yong
Zhang
a
aDepartment of Chemistry, Wuhan University, Wuhan 430072, China. E-mail: hongxl@whu.edu.cn
bKey Laboratory of Biomedical Polymers, Ministry of Education, Wuhan University, Wuhan 430072, China. E-mail: xz-zhang@whu.edu.cn; Fax: +86-27-68756619; Tel: +86-27-68756619
First published on 6th October 2009
Abstract
A series of poly(methyl methacrylate)-graft-oligoamines (PMMA-g-oligoamines), including PMMA-g-DETA, PMMA-g-TETA and PMMA-g-TEPA, were synthesized through aminolysis of the PMMA with diethylenetriamine, triethylenetetramine and tetraethylenepentamine. Agarose gel retardation assay indicated that PMMA-g-oligoamines had good binding capability with plasmidDNA, and the binding capability increased with increasing length of oligoamines and content of nitrogen (N%). The results of particle size, zeta potential and morphology observation further showed that the PMMA-g-oligoamines could condense DNA efficiently and the PMMA-g-oligoamine/DNA complexes were uniform nanospheres. The in vitro cell viability indicated that PMMA-g-oligoamines were less toxic than 25 kDa PEI, though the cytotoxicity of PMMA-g-oligoamines increased slightly with increasing length of oligoamines as well as the N% of PMMA-g-oligoamines. The transfection efficiency of PMMA-g-oligoamines/DNA complexes in 293 T and HeLa cells demonstrated that PMMA-g-oligoamines could transfect cells efficiently with increasing the length of oligoamines, especially PMMA-g-TEPA with highest N%, and showed similar transfection capability as 25 kDa PEI. The cellular uptake study showed that the distribution of YOYO-1 labeled DNA in the cytoplasm and nuclei increased gradually with increasing length of oligoamines.
Introduction
Gene therapy has gained significant research attention over the past two decades due to its great potential in treating inherited or acquired diseases related to genetic disorders.1 In gene therapy, gene vectors are necessary to protect the gene from extracellularnuclease degradation and to condense the gene for cellular uptake. Initially, viruses were utilized as gene vectors owing to their high efficiency in delivering both DNA and RNA to numerous cell lines .2 However, several defects, including toxicity, immunogenicity and limitations with respect to scale-up, restrict the wide application of viral vectors. Recently, polycations, as nonviral vectors, have attracted increasing attention due to their low toxic, potential for large-scale production and adjustable structures.3–5A variety of natural and synthesized polycations, such as poly(L-lysine) (PLL),6poly(ethylenimine) (PEI),7 polyamidoamine dendrimer,8,9chitosan,10 and so forth, have been widely used as gene carriers. Among these polymeric gene vectors, 25 kDa PEI has become the standard for designing new polymeric gene vectors due to its high transfection efficiency in vitro and in vivo.11–13 However, the cytotoxicity of 25 kDa PEI is also high in addition to its high transfection efficiency. Many efforts have focused on reducing the cytotoxicity of PEI while preserving the high transfection efficiency by introducing biocompatible materials, such as dextran, chitosan and polyaspartamide.14–16Poly(dimethylamino)ethyl methacrylate (PDMAEMA) has also been utilized in gene delivery.17–19 The homopolymers and copolymers of DMAEMA show low or acceptable cytotoxicity and possess promising transfection activity. The PDMAEMA vectors have good binding capability and endosome escape. Moreover, various modifications to the PDMAEMA structure have also been investigated in attempts to further improve transfection efficiency.20–26 A series of oligoamine polymers based on grafted oligoamine residues on natural polysaccharides27,28 and poly(amino amine)s containing oligoamines in the main chain or with functional groups in the side chain29–32 were also developed as nonviral gene vectors, which show efficient transfection and low cytotoxicity.
In this study, in order to combine the high transfection efficiency of PEI and the advantages of PDMAEMA, oligoamines (the basic unit for PEI) were grafted onto poly(methyl methacrylate) (PMMA) through the aminolysis method. The acquired PMMA-g-olgoamines have a structure resembling both PEI and PDMAEMA. Besides, the length of oligoamine side chains can be altered, and the effect of the pendant oligoamine on the transfection efficiency and cytotoxicity of PMMA-g-olgoamines as gene vectors was evaluated systematically.
Results and discussion
Synthesis of PMMA-graft-oligoamines
The PMMA-g-oligoamines were prepared by aminolysis of the PMMA with oligoamines, and the schematic synthesis was illustrated in Scheme 1. Due to the fact that catalytic chain transfer polymerization (CCTP) could efficiently control the molecular weight of the polymer chain, this method was utilized to prepare PMMA with appropriate molecular weight. The structure of PMMA was confirmed by 1H NMR spectroscopy which shows 1H NMR peaks at 5.4 and 6.2 ppm (Fig. 1), which belong to terminal vinyl groups of PMMA, and indicate the CCTP of MMA is successful.33 | ||
| Scheme 1 Schematic illustration of the synthesis of PMMA-g-oligoamines and the structure of COPhBF. | ||
 | ||
| Fig. 1 1H NMR spectra of PMMA, PMMA-g-DETA, PMMA-g-TETA and PMMA-g-TEPA. | ||
The oligoamines were grafted onto PMMAviaaminolysis to synthesize the polycations. We found that the aminolysis reaction between PMMA and oligoamines was unsuccessful at room temperature even for several days; hence, the reaction temperature was raised to 130 °C. Besides, it should be noted that crosslinking may occur since both PMMA and the oligoamines have multifunctional groups. In order to avoid crosslinking, a large excess of oligoamine was added. After reaction, the non-reacted oligoamines as well as residual solvent, were removed by dialysis. 1H NMR spectra of the PMMA-g-oligoamines are shown in Fig. 1. For PMMA-g-oligoamines, the reduced intensity of peak at 3.5 ppm, ascribed to –OCH3 groups, relative to that of PMMA was evident, and signals of oligoamine units (–NHCH2CH2–) at 2.4–3.4 ppm were evident. In addition, it should be pointed out that after aminolysis reaction the peaks ascribed to the vinyl group of PMMA (5.4 and 6.2 ppm) disappeared, indicating that Michael addition occurred between the oligoamines and the terminal active vinyl groups. These results indicate the success of the aminolysis reaction. The degree of aminolysis of oligoamines was calculated from 1H NMR spectra and results are listed in the Experimental section.
Buffer capability
The buffer capability of gene vectors is very important for the release of complexes from endosomes. In this study, the buffer capabilities of 25 kDa PEI and PMMA-g-oligoamines were evaluated in terms of acid–base titration. As illustrated in Fig. 2, the three PMMA-g-oligoamines have lower buffer capabilities than that of 25 kDa PEI. This is ascribed to the fact that the number of primary, secondary and tertiary amines groups, as well as the N% which could be protonated in PMMA-g-oligoamines, was less than that in 25 kDa PEI.For the oligoamine modified polymers, it was reported that the buffer capability depends on the length of oligoamines. However, PMMA-g-DETA shows higher buffer capability than PMMA-g-TETA and PMMA-g-TEPA. The higher buffer capability of PMMA-g-DETA was attributed to the higher degree of aminolysis (DA) of PMMA-g-DETA (93%), than that of PMMA-g-TETA and PMMA-g-TEPA (74 and 73% respectively). On the other hand, PMMA-g-TETA and PMMA-g-TEPA exhibit higher hydrophobicity than that of PMMA-g-DETA due to the lower DA of oligoamines, thus, PMMA-g-TETA and PMMA-g-TEPA could coil or partially self-assemble in solution, resulting in shielding of protonation of the amines. For PMMA-g-TETA and PMMA-g-TEPA, having a similar DA of oligoamines, the buffer capability increases with increasing length of the pendant oligoamines, which is in accordance with literature findings.
Gel retardation assay
The DNA binding capability of polycations is a prerequisite for nonviral gene carriers. As shown in Fig. 3, all polymers show good DNA binding capability. Based on the increasing N%, the binding capability of PMMA-g-oligoamines lies in the sequence of PMMA-g-TEPA ≥ PMMA-g-TETA > PMMA-g-DETA. In the case of PMMA-g-TEPA and PMMA-g-TETA, no migration bands of plasmidDNA were observed at an N/P ratio of 4 or above. However, PMMA-g-DETA completely condenses plasmidDNA at N/P ratio of 10 or above. The results indicate that the binding capability of PMMA-g-oligoamines increases with increasing length of pendant oligoamines as well as the N% of PMMA-g-oligoamines. | ||
| Fig. 3 Agarose gel electrophoresis retardation assay of PMMA-g-oligoamine/DNA complexes: (A) PMMA-g-DETA, (B) PMMA-g-TETA, (C) PMMA-g-TEPA. | ||
Particle size and ζ-potential
Appropriate particle size of polycation/DNA complexes is also important for cellular uptake and efficient transfection. Liu et al. reported that cells typically uptake particles ranging from 54 to 625 nm, which would be endocytosed profitably by cells.34 As shown in Fig. 4(A), it was found that the particle size of the complexes decreases with increasing N/P ratio, and the three PMMA-g-oligoamines could efficiently condense the plasmidDNA into particles ranging from 170 to 220 nm at N/P ratios from 30 to 70. Besides, it was observed that the effect of length of pendant oligoamines on the size of polycation/DNA complexes is nearly negligible. | ||
| Fig. 4 Particle size (A) and zeta potential (B) of PMMA-g-oligoamine/DNA complexes at N/P ratios ranging from 10 to 70. Data are shown as mean ± SD (n = 3). TEM micrographs of PMMA-g-DETA/DNA complexes at N/P ratio of 40 (C) and 60 (D). | ||
The positively charged polycation/DNA complexes can bind to negatively charged cell membranes, which facilitates the entering of complexes into cells by cellular uptake. The ζ-potential values of PMMA-g-oligoamine/DNA complexes were also measured at N/P ratios ranging from 10 to 70. As presented in Fig. 4(B), the ζ-potential of PMMA-g-oligoamine/DNA complexes increases with increasing of N/P ratios. Moreover, the ζ-potential increases slightly with increasing length of oligoamines and N% of PMMA-g-oligoamines. The results demonstrate that the ζ-potential of PMMA-g-oligoamine/DNA complexes strongly depend on not only the length of the pendant oligoamine but also the N% of PMMA-g-oligoamines.
Morphology observation
The morphology of PMMA-g-oligoamine/DNA complexes was observed by TEM. As presented in Fig. 4(C) and (D), the micrographs of PMMA-g-DETA/DNA complexes at N/P ratios of 40 and 60 shows that most of the complexes are uniform nanospheres with size of about 200 nm which is consistent with the particle size measured by the Nano-ZS ZEN3600 apparatus. The morphology of PMMA-g-DETA/DNA complexes show that the PMMA-g-oligoamines could condense DNA compactly and form regular spheres.In vitro cytotoxicity
The cytotoxicity of PMMA-g-oligoamines in 293 T and HeLa cells was evaluated by MTT assay and 25 kDa PEI was used as the positive control. As shown in Fig. 5, the PMMA-g-oligoamines have much lower cytotoxicity to 293 T and HeLa cells as compared with 25 kDa PEI, and the cell viability of PMMA-g-oligoamines in both cell lines decreases slightly with increasing concentration of PMMA-g-oligoamines. Additionally, the cytotoxicity of PMMA-g-oligoamines increases slightly with the increasing length of oligoamines. It is known that the cytotoxicity depends on the molecular weight of the polycations and increases with an increase of molecular weight. Moreover, the IC50 (the half maximal inhibitory concentration) decreases with increase of the molecular weight of PMMA-g-oligoamines. PMMA-g-TEPA has the lowest IC50, which is >1300 μg μL−1 in both cell lines and markedly is 100 times higher than that of 25 kDa PEI. These results indicate that PMMA-g-oligoamines have no apparent cytotoxicity. | ||
| Fig. 5 The cytotoxicity of 25 kDa PEI and PMMA-g-oligoamines in (A) 293 T as well as in (B) HeLa cells. | ||
In vitro transfection efficiency
To evaluate the potential of PMMA-g-oligoamines as gene vectors, the in vitro transfection studies of PMMA-g-oligoamines/DNA complexes were conducted with 293 T and HeLa cells at N/P ratios ranging from 20 to 70. The naked plasmidDNA was used as a negative control and 25 kDa PEI/DNA complexes at N/P ratio of 10 was used as a positive control.As shown in Fig. 6(A) and (B), the transfection efficiency of PMMA-g-oligoamines/DNA complexes in both cell lines increases with increasing length of the pendant oligoamines and the N% of PMMA-g-oligoamines, i.e., the transfection efficiency of PMMA-g-oligoamine/DNA complexes changes in the order of PMMA-g-TEPA > PMMA-g-TETA > PMMA-g-DETA. When transfecting the 293 T cells (Fig. 6(A)), the transfection efficiency of PMMA-g-oligoamine/DNA complexes, such as PMMA-g-TEPA/DNA complexes, increases with increasing N/P ratio from 20 to 50 (from 1.32 × 109 to 2.96 × 109 RLU/mg protein), then the transfection efficiency decreases slightly with a further increase of N/P ratio from 50 to 70 (from 2.96 × 109 to 1.39 × 109 RLU/mg protein). In addition, the transfection efficiency of PMMA-g-TEPA/DNA complexes at an N/P ratio of 50 (average 2.96 × 109 RLU/mg protein) is comparable to that of 25 kDa PEI/DNA at an N/P ratio of 10 (average 3.58 × 109 RLU/mg protein). However, when transfecting the HeLa cells, the transfection efficiency of PMMA-g-oligoamines/DNA complexes increases gradually with increasing N/P ratios from 20 to 70, and the transfection efficiency of PMMA-g-TEPA/DNA complexes at N/P ratio of 70 (average 0.95 × 109 RLU/mg protein) is a little lower than that of 25 kDa PEI/DNA at N/P ratio of 10 (average 2.20 × 109 RLU/mg protein). The different transfection trends of PMMA-g-oligoamine/DNA complexes in 293 T and HeLa cells are ascribed to the fact that the transfection capability of gene vectors is cell line dependent.
 | ||
| Fig. 6 The transfection efficiency of PMMA-g-oligoamine/DNA complexes at N/P ratios ranging from 20 to 70 in (A) 293 T and (B) HeLa cells. (C) Fluorescence microscopy images (a–f) and corresponding light merged micrographs (a′–f′) of PMMA-g-oligoamine/DNA complexes at N/P ratios of 40 and 60 in 293 T cells. The micrographs are obtained at a magnification of 100×. | ||
It was found that the transfection efficiency of PMMA-g-oligoamine/DNA complexes greatly depends on the structure of the pendant chains, and the transfection efficiency increases with increasing number of secondary aminoethylene groups in the pendant chain. This would correlate with the optimum particle size and higher surface charge of the PMMA-g-oligoamine/DNA complexes.32 Smaller particle size and higher surface charge of complexes are favorable for endocytosis and transgene expression. With increasing number of secondary aminoethylene groups in the pendant chain of PMMA-g-oligoamines, the ζ-potential of complexes is higher and the complex is more compact, which induces relatively high transfections.
The green fluorescent protein expressions of transfected 293 T cells by PMMA-g-oligoamine/DNA complexes at N/P ratios of 40 and 60 are shown in Fig. 6(C). The fluorescence micrographs of 293 T cells are shown as a–f, while light merged micrographs are shown as a′–f′. It can be seen from the fluorescence micrographs that the number of transfected 293 T cells by PMMA-g-TEPA/DNA complexes at N/P ratios of 40 and 60 are much more than for PMMA-g-TETA/DNA and PMMA-g-DETA/DNA complexes.
Intracellular distribution of complexes
The intracellular distribution of PMMA-g-oligoamine/DNA complexes at N/P ratio of 60 in 293 T and HeLa cells was visualized using a confocal laser scanning microscope. As shown in Fig. 7, after transfection for 4 h, the green dots of YOYO-1 labeled pGL-3 DNA are located mainly in the cytoplasm for most cells and a few green dots are visualized in the nucleus (Fig. 7 (iii)) in both cell lines . Moreover, it is found that the YOYO-1 labeled pGL-3 in the cytoplasm and nucleus increases gradually with increasing length of pendant oligoamines as well as the N% of the PMMA-g-oligoamines (Fig. 7, Aiii–Ciii and Diii–Fiii). The results indicate that the cellular uptake of PMMA-g-oligoamine/DNA complexes in both cell lines increases with the increasing length of oligoamines. Additionally, these results also support that the transfection efficiency of PMMA-g-oligoamine/DNA complexes depends on the length of the oligoamines.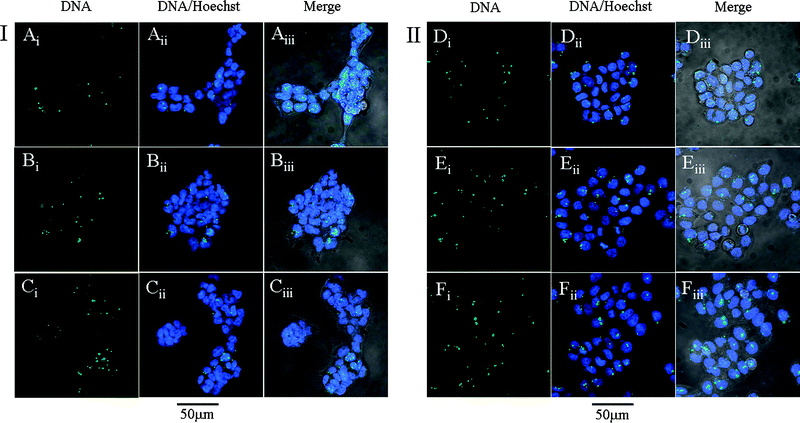 | ||
| Fig. 7 Intracellular distribution of PMMA-g-oligoamine/DNA complexes at N/P ratio of 60 in (I) 293 T and (II) HeLa cells. (A, D) refer to PMMA-g-DETA/DNA complexes, (B, E) refer to PMMA-g-TETA/DNA, and (C, F) refer to PMMA-g-TEPA/DNA complexes. YOYO-1 labeled DNA (i), DNA and Hoechst 33258 (ii) and merged images (iii). | ||
Experimental
Branched poly(ethylenimine) (bPEI) with molecular weight of 25 kDa was purchased from Sigma-Aldrich. Diethylenetriamine (DETA), triethylenetetramine (TETA) and tetraethylenepentamine (TEPA) were obtained from Shanghai Chemical Reagent Co. China (SCRC) and used as received. Methyl methacrylate (MMA) was obtained from SCRC and purified by distillation. 2,2′-Azobisisobutyronitrile (AIBN) was purchased from SCRC and recrystallized from ethanol. Tetraphenyl cobaloxime boron fluoride (COPhBF, as shown in Scheme 1), as the chain transfer reagent for the catalytic chain transfer polymerization (CCTP), was prepared according to the literature.35,36 QIAfilter™ plasmidpurification Giga Kit (5) was purchased from Qiagen (Hilden, Germany). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin, trypsin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and Dubelcco’s phosphate buffered saline (PBS) were purchased from Greiner bio-one. The Micro BCA proteinassay kit was purchased from Pierce. All other reagents were analytical grade and used as received.Synthesis of poly(methyl methacrylate) (PMMA)
The poly(methyl methacrylate) was acquired by polymerization of MMAvia catalytic chain transfer polymerization (CCTP). In brief, 80 g of MMA, 60 g of toluene and 60 g of butyl acetate were charged into a three-necked flask. The mixture was bubbled with highly pure nitrogen (99.999%) to exclude oxygen. After 1.5 h, 20 g of the mixtures were injected to a sealed flask with 2.4 mg of COPhBF and 0.4 g of AIBN, and the COPhBF and AIBN were dissolved into the above mixture through ultrasonication, then the solution was injected into a three-necked flask. It should be noted that the chain transfer reagent COPhBF is very sensitive to oxygen, so it must be guaranteed that the COPhBF and reactant are protected from oxygen in all operations. Charging with high pure nitrogen, the mixture was refluxed at 75 °C for 6 h for the polymerization. After the polymerization, the solvent was removed by rotary evaporation. Then the product was dissolved in THF and precipitated in methanol three times, and then dried under vacuum evaporation to obtain the PMMA. 1H NMR (CDCl3, ppm) δ 0.52–1.00 (m, –CH3), 1.59–2.00 (m, –CH2). 3.52 (–OCH3), 5.40 and 6.20 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2); Mn = 1.50 × 104, Mw/Mn = 1.54.
CH2); Mn = 1.50 × 104, Mw/Mn = 1.54.
Synthesis of the PMMA-graft-oligoamines
The PMMA-g-oligoamines were obtained by aminolysis of PMMA with DETA, TETA and TEPA. In brief, 1 g of PMMA (having 0.01 mol MMA units) was dissolved in 25 g of dimethyl sulfoxide (DMSO), and then 0.3 mol of oligoamine (30 eq. relative to the MMA units) was added into the above solution. The mixture was refluxed at 130 °C for 17 h. Then the product was dialyzed (MW cut-off = 3500) against distilled water for 4 days and then lyophilized for 3 days. The three polymers were denoted as PMMA-g-DETA, PMMA-g-TETA and PMMA-g-TEPA, respectively.Characterizations
1H nuclear magnetic resonance (NMR) spectra were recorded on a Varian Mercury VX-300 MHz instrument. The molecular weight and polydispersity (Mw/Mn) of the PMMA was measured on a gel permeation chromatography (GPC ) relative to a polystyrene standard using a Waters 515 HPLC equipped with MZ gel SDplus linear 5 μm and 500 Å column. Sample was detected with a Wyatt DAWN® EOS™ multiangle light scatteringdetector and a Wyatt Optilab DAP differential refractive index detector. THF was used as eluent at a flow rate of 1.0 ml min−1. The column temperature was 30 °C. The molecular weights and polydispersity of the PMMA-g-oligoamines were measured on a GPC relative to PEG standard using a Waters 2690D HPLC equipped with an Ultrahydrogel 120, 250 and 2000 column. Samples were detected with a Wyatt multiangle light scatteringdetector and a Waters 2100 differential refractive index detector. HOAc–NaOAcbuffer solution (0.03 M, pH 4.0) was used as eluent at a flow rate of 0.6 ml min−1. The column temperature was 25 °C.Cell culture
Human embryonic kidney transformed 293 (293 T) cells and human cervix carcinoma (HeLa) cells were incubated in DMEM containing 10% FBS and 1% antibiotics (penicillin-streptomycin, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U ml−1) at 37 °C in a humidified atmosphere containing 5% CO2.
000 U ml−1) at 37 °C in a humidified atmosphere containing 5% CO2.
Amplification and purification of plasmidDNA
The pGL-3 and pEGFP-C1 plasmids were used in this study. The former one as a luciferase reporter gene was transformed in E. coli JM109 and the latter one as a green fluorescent proteingene was transformed in E. coli DH5α. Both plasmids were amplified in terrific broth media at 37 °C overnight at 250 rpm. The plasmids were purified by an EndoFree QiAfilter™ Plasmid Giga Kit (5). Then the purified plasmids were dissolved in TE buffer solution and stored at −20 °C. The integrity of plasmid was confirmed by agarose gel electrophoresis. The purity and concentration of plasmid were determined by ultraviolet (UV) absorbance at 260 and 280 nm.Acid–base titration
The buffer capability of 25 kDa PEI, PMMA-g-DETA, PMMA-g-TETA and PMMA-g-TEPA were determined by acid–base titrationassay between pH 10.30 to 2.50 as described by Benns et al.37,38 Briefly, the sample was dissolved in 30 mL of 150 mM NaCl solution (pH 7.4). The sample solution was first titrated by 0.1 M NaOH to a pH of 10.30; increments of 0.1 M HCl were then added to the solution and the pH was measured using a microprocessor pH meter.Agarose gel retardation assay
The PMMA-g-oligoamine/DNA complexes at different N/P ratios ranging from 1 to 15 (ratio of amino groups of the oligoamine in the PMMA-g-oligoamine to phosphate groups of DNA) were prepared by adding an appropriate volume of PMMA-g-oligoamine solution (in 150 mM NaCl solution, pH 7.4) to 1 μL of pGL-3 DNA (in 40 mM Tris-HCl buffer solution). The complexes were diluted by 150 mM NaCl solution to a total volume of 6 μL, and then the complexes were incubated at 37 °C for 30 min. After that the complexes were electrophorised on 0.7% (w/v) agarose gel containing GelRed™ and with Tris-acetate (TAE) running buffer at 80 V for 80 min. DNA was visualized with a UV lamp using a Vilber Lourmat imaging system (France).Particle size and ζ-potential measurement
Particle size and ζ-potential were measured by Nano-ZS ZEN3600 (MALVERN Instr.) at 25 °C. The complexes at various N/P ratios ranging from 10 to 70 were prepared by adding an appropriate volume of PMMA-g-oligoamine solution to an appropriate volume of pGL-3 DNA solution. Then the complexes were incubated at 37 °C for 30 min. Then the complexes were diluted by 150 mM NaCl solution (pH 7.4) to 1 mL volume prior to measurement.Transmission electron microscopy (TEM)
The morphologies of PMMA-g-DETA/DNA complexes at N/P ratios of 40 and 60 as representative examples were observed by TEM using a JEM-100CXII microscope operating at an acceleration voltage of 100 kV. The PMMA-g-DETA/DNA complexes were prepared by adding an appropriate volume of PMMA-g-DETA solution to an appropriate volume of pGL-3 DNA solution. The complexes were diluted to a total volume of 100 μL by 150 mM NaCl solution (pH 7.4) and then incubated at 37 °C for 30 min. The TEM samples were prepared by dropping the PMMA-g-DETA/DNA complex solution onto a copper grid which had been precoated with a layer of formvar film, and then stained by 0.2% (w/v) phosphotungstic acid solution before measurement.Cytotoxicity assay
The cytotoxicity of PMMA-g-oligoamines was examined by MTT assay , and the cytotoxicity of 25 kDa PEI was used as control. The 293 T cells and HeLa cells were seeded in a 96-well plate at a density of 6000 cells/well and cultured for 24 h in 200 μL DMEM containing 10% FBS. After the polymers were added for 48 h, the medium was replaced with 200 μL of fresh medium. Then 20 μL MTT (5 mg mL−1) solutions were added for 4 h. Thereafter, the medium was removed and 150 μL DMSO was added. The absorbance was measured at 570 nm using a microplate reader (BIO-RAD, Model 550, USA). The relative cell viability was calculated as: cell viability (%) = (OD570 (samples) − OD570 (DMSO)/OD570 (control) − OD570 (DMSO)) × 100, where OD570 (control) was obtained in the absence of polymers and OD570 (samples) was obtained in the presence of polymers.Luciferase assay
Transfection experiments of PMMA-g-oligoamines were performed with 293 T and HeLa cells as compared with 25 kDa PEI. PGL-3 plasmidDNA was used to evaluate the luciferase transfection activity. First, 293 T cells or HeLa cells were seeded at a density of 6 × 104 cells/well in the 24-well plate with 1 mL of DMEM containing 10% FBS and incubated at 37 °C for 24 h. Then the complexes were prepared at N/P ratios ranging from 20 to 70 by adding appropriate volumes of PMMA-g-oligoamine solution to 1 μg plasmidDNA. Before transfection, the cells were washed by PBS, and then the PMMA-g-oligoamine/pGL-3 DNA complexes were added with serum-free DMEM for 4 h at 37 °C. The serum-free DMEM was replaced by fresh DMEM containing 10% FBS, and the cells were further incubated for 48 h. After that, the medium was removed.The luciferaseassay was performed according to the manufacturer’s protocols. Relative light units (RLUs) were measured with chemiluminometer (Lumat LB9507, EG&G Berthold, Germany). The total protein was measured according to a BCA proteinassay kit (Pierce). Luciferase activity was expressed as RLU/mg protein.
Green fluorescent proteinassay
To directly detect the transfected cells expressing green fluorescent proteins (GFP), the transfection of pEGFP-C1 DNA mediated by PMMA-g-oligoamine/DNA complexes in 293 T cells was also evaluated. PMMA-g-oligoamine/DNA complexes at N/P ratios of 40 and 60 were used in this experiment. The cells expressing GFP were directly observed by a fluorescent microscope (IX 70, Olympus, Japan). The microscopy images were obtained at a magnification of 100× by using CoolSNAP-Pro (4.5.1.1) version software.Cellular trafficking of complexes
The ability of PMMA-g-oligoamines to transport plasmidDNA to the cytoplasm and nucleus was evaluated using a confocal laser scanning microscope. The cells were seeded at a density of 1.0 × 105 cells/well into biohousing chamber slide dishes loaded with a 25 mm diameter slide on cover-glass slides and cultured for 24 h. To prepare labeled DNA complexes at an N/P ratio of 60, 1 μg pGL-3 DNA was intercalated with 2.5 μL of 10 μM YOYO-1 for 15 min at 37 °C before addition of PMMA-grafted-oligoamines and then further incubated for 30 min at 37 °C. The amount of YOYO-1 used was 1 dye molecule to 60 base pairs. After complex transfection for 4 h, the complexes were removed and the cells were washed with PBS three times. Then the nucleus was stained with 20 μL (2 μg μL−1) of Hoechst 33258 for 15 min at 37 °C, after which the cells were further washed with PBS three times and incubated with 200 μL DMEM. The fluorescence was observed with a confocal laser scanning microscope (C1–Si, Nikon, Japan) equipped with a 405 nm diode for Hoechst 33258 and a 488 nm argon laser for YOYO-1.Conclusions
A series of PMMA-g-oligoamines as nonviral gene vectors were synthesized by using the aminolysis method. Gel retardation assay , particle size and zeta potential of three PMMA-g-oligoamine/DNA complexes showed that PMMA-g-oligoamines were able to condense DNA efficiently. The cytotoxicity of PMMA-g-oligoamines was significantly lower than that of 25 kDa PEI. The transfection efficiency of PMMA-g-oligoamines/DNA complexes increased with increasing length of oligoamines as well as the N% of PMMA-g-oligoamines. Importantly, the transfection efficiency of PMMA-g-TEPA/DNA complexes at certain N/P ratios was comparable to that of 25 kDa/PEI complexes at an N/P ratio of 10. These results indicated that PMMA-g-oligoamines have great potential as nonviral gene vectors, especially PMMA-g-TEPA.References
- M. A. Mintzer and E. E. Simanek, Chem. Rev., 2009, 109, 259–302 CrossRef CAS.
- W. F. Anderson, Nature, 1998, 392, 346–30 CrossRef.
- W. C. Tseng and C. M. Jong, Biomacromolecules, 2003, 4, 1277–1284 CrossRef CAS.
- T. H. Kim, S. I. Kim, T. Akaike and C. S. Cho, J. Controlled Release, 2005, 105, 354–366 CrossRef CAS.
- K. Wong, G. B. Sun, X. Q. Zhang, H. Dai, Y. Liu, C. B. He and K. W. Leong, Bioconjugate Chem., 2006, 17, 152–158 CrossRef CAS.
- M. Männistö, S. Vanderkerken, V. Toncheva, M. Elomaa, M. Ruponen, E. Schacht and A. Urtti, J. Controlled Release, 2002, 83, 169–182 CrossRef CAS.
- M. Neu, D. Fischer and T. Kissel, J. Gene Med., 2005, 7, 992–1009 CrossRef CAS.
- X. Q. Zhang, X. L. Wang, S. W. Huang, R. X. Zhuo, Z. L. Liu, H. Q. Mao and K. W. Leong, Biomacromolecules, 2005, 6, 341–350 CrossRef CAS.
- C. Dufès, I. F. Uchegbu and A. G. Schätzlein, Adv. Drug Delivery Rev., 2005, 57, 2177–2202 CrossRef CAS.
- Z. W. Mao, L. Ma, Y. Jiang, M. Yan, C. Y. Gao and J. C. Shen, Macromol. Biosci., 2007, 7, 855–863 CrossRef CAS.
- N. P. Gabrielson and D. W. Pack, J. Controlled Release, 2009, 136, 54–61 CrossRef CAS.
- S. H. Huh, H. J. Do, H. Y. Lim, D. K. Kim, S. J. Choi, H. Song, N. H. Kim, J. K. Park, W. K. Chang, H. M. Chung and J. H. Kim, Biologicals, 2007, 35, 165–171 CrossRef CAS.
- U. Lungwitz, M. Breunig, T. Blunk and A. Göpferich, Eur. J. Pharm. Biopharm., 2005, 60, 247–266 CrossRef CAS.
- Y. X. Sun, W. Xiao, S. X. Cheng, X. Z. Zhang and R. X. Zhuo, J. Controlled Release, 2008, 128, 171–178 CrossRef CAS.
- T. Azzam, A. Raskin, A. Makovitzki, H. Brem, P. Vierling, M. Lineal and A. J. Domb, Macromolecules, 2002, 35, 9947–9953 CrossRef CAS.
- M. Zhang, M. Liu, Y. N. Xue, S. W. Huang and R. X. Zhuo, Bioconjugate Chem., 2009, 20, 440–446 CrossRef CAS.
- P. van de Wetering, J. Y. Cherng, H. Talsma and W. E. Hennink, J. Controlled Release, 1997, 49, 59–69 CrossRef CAS.
- P. van de Wetering, N. M. E. Schuurmans-Nieuwenbroek, W. E. Hennink and G. Storm, J. Gene Med., 1999, 1, 156–165 CrossRef CAS.
- M. A. E. M. van der Aa, U. S. Huth, S. Y. Häfele, R. Schubert, R. S. Oosting, E. Mastrobattista, W. E. Hennink, R. Peschka-Süss, G. A. Koning and D. J. A. Crommelin, Pharm. Res., 2007, 24, 1590–1598 CrossRef CAS.
- P. van de Wetering, E. E. Moret, N. M. E. Schuurmans-Nieuwenbroek, M. J. van Steenbergen and W. E. Hennink, Bioconjugate Chem., 1999, 10, 589–597 CrossRef CAS.
- P. Dubruel, B. Christiaens, B. Vanloo, K. Bracke, M. Rosseneu, J. Vandekerckhove and E. Schacht, Eur. J. Pharm. Sci., 2003, 18, 211–220 CrossRef CAS.
- A. M. Funhoff, V. F. van Nostrum, G. A. Koning, N. M. E. Schuurmans-Nieuwenbroek, D. J. A. Crommelin and W. E. Hennink, Biomacromolecules, 2004, 5, 32–39 CrossRef CAS.
- A. M. Funhoff, V. F. van Nostrum, M. C. Lok, M. M. Fretz, D. J. A. Crommelin and W. E. Hennink, Bioconjugate Chem., 2004, 15, 1212–1220 CrossRef CAS.
- J. Luten, N. Akeroyd, A. Funhoff, M. C. Lok, H. Talsma and M. E. Hennink, Bioconjugate Chem., 2006, 17, 1077–1084 CrossRef CAS.
- Y. Z. You, D. S. Manickam, Q. H. Zhou and D. Oupicky, J. Controlled Release, 2007, 122, 217–225 CrossRef CAS.
- X. L. Jiang, M. C. Lok and W. E. Hennink, Bioconjugate Chem., 2007, 18, 2077–2084 CrossRef CAS.
- T. Azzam, H. Eliyahu, L. Shapira, M. Linial, Y. Barenholz and A. J. Domb, J. Med. Chem., 2002, 45, 1817–1824 CrossRef CAS.
- B. Lu, C. F. Wang, D. Q. Wu, C. Li, X. Z. Zhang and R. X. Zhuo, J. Controlled Release, 2009, 137, 54–62 CrossRef CAS.
- J. Kloeckner, E. Wagner and M. Ogris, Eur. J. Pharm. Sci., 2006, 29, 414–425 CrossRef CAS.
- J. H. Jeong, L. V. Christensen, J. W. Yockman, Z. Y. Zhong, J. F. J. Engbersen, W. J. Kim, J. Feijen and S. W. Kim, Biomaterials, 2007, 28, 1912–1917 CrossRef CAS.
- M. Piest, C. Lin, M. A. Mateos-Timoneda, M. C. Lok, W. E. Hennink, J. Feijen and J. F. J. Engbersen, J. Controlled Release, 2008, 130, 38–45 CrossRef CAS.
- C. Lin, C. J. Blaauboer, M. M. Timoneda, M. C. Lok, M. van Steenbergen, W. E. Hennink, Z. Y. Zhong, J. Feijen and J. F. J. Engbersen, J. Controlled Release, 2008, 126, 166–174 CrossRef CAS.
- W. X. Wang, D. J. Irvine and S. M. Howdle, Ind. Eng. Chem. Res., 2005, 44, 8654–8658 CrossRef CAS.
- Y. M. Liu and T. M. Reineke, J. Am. Chem. Soc., 2005, 127, 3004–3015 CrossRef CAS.
- J. P. A. Heuts, D. Kukulj, D. J. Forster and T. P. Davis, Macromolecules, 1998, 31, 2894–2905 CrossRef CAS.
- A. Bakač, M. E. BrynilDAon and J. H. Espenson, Inorg. Chem., 1986, 25, 4108–4114 CrossRef CAS.
- J. M. Benns, J. S. Choi, R. I. Mahato, J. S. Park and S. W. Kim, Bioconjugate Chem., 2000, 11, 637–645 CrossRef CAS.
- J. M. Benns, R. I. Mahato and S. W. Kim, J. Controlled Release, 2002, 79, 255–269 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |

