Recent advances and novel approaches in deriving neurons from stem cells
Steven J.
Greco
and
Pranela
Rameshwar
*
Department of Medicine, Division of Hematology/Oncology, University of Medicine and Dentistry of New Jersey-New Jersey Medical School, Newark, NJ, USA. E-mail: rameshwa@umdnj.edu; Fax: +1 (973) 972-8854; Tel: +1 (973) 972-0625
First published on 19th October 2009
Abstract
New approaches to improve the quality and efficiency of stem cell-derived neurons are important for both research and clinical arenas. Customized subsets of differentiated neurons can be used as model systems to understand the etiology of a variety of complex neurological diseases and disorders. These same neuronal cells, or progenitors thereof, can then be tested in animal models to determine therapeutic value. Just as exciting is the potential to take somatic cells, i.e. skin cells, from patients with debilitating neurodegenerative diseases and reprogram them into stem cells for neuronal differentiation. These neurons can then be researched to learn more about underlying pathological events or used as a model to test novel therapeutics. To facilitate this potential utilization, investigators in multiple disciplines have taken their unique specialties and applied them towards generating novel induction techniques to produce functional neurons from stem cells. In this review, we highlight recent literature demonstrating cutting-edge, interdisciplinary approaches, which induce neuronal differentiation into a variety of phenotypes and discuss the potential impact of these works.
Introduction
For decades the prevailing conception of neurogenesis was that individuals were born with a finite number of central nervous system (CNS) neurons which progressively decline in numbers with age and without self-renewal. As such, neurogenesis was perceived to occur solely in utero. More recently, studies have disproven this concept and provided evidence for the existence of adult neural stem cells (NSCs), which actively replenish select neuronal populations in the CNS during adulthood. However, areas of the CNS most active in adult neurogenesis are those undergoing extensive synaptic remodeling, such as in the hippocampus, which is involved in learning and memory. Here, neurogenesis occurs at a relatively slow rate to sustain normal cognitive function. Unfortunately, many neurological insults, albeit pathological or injury-related, produce a large degree of neuronal death in a very short time, too short to be repaired by endogenous neurogenic mechanisms. Hence, exogenous stem cells, from neuronal and non-neuronal origin, were explored as a source of rapidly expandable cells which could undergo neuronal differentiation to potentially replace the dying neurons.Although NSCs seem to be the ideal source for neuronal replacement, their primary residence deep within the human brain makes them an unlikely source for harvesting. Thus, for the last decade, researchers have looked towards other stem cell populations which are more readily available or more easily harvested in humans, and with plastic functions to generate selective neuronal populations.
Embryonic stem cells (ESCs) are derived from the inner cell mass of the blastocysts that differentiate into cells of the three germ layers. Hence, in theory, ESCs could be an ideal source of stem cells for the generation of NSCs. The caveat for using ESCs, aside from the ethical dilemmas associated with ESCs, is that any cells derived from ESCs pose a potential immune challenge to the transplant recipient. Scientists have recently devised a solution to this problem by identifying how to reprogram somatic cells, such as fibroblasts, to possess the differentiation potential of ESCs without similar immunogenicity.1 These induced pluripotent stem (iPS) cells can be derived from a patient’s own cells and thus can be used as an autologous transplant. However, issues still need to be resolved regarding the potential for these cells to form tumors. Compounded with the above, is the ease in which ESCs can form tumors.
There are several other types of stem cells which have been studied for their neurogenic potential. Among these are other adult stem cells (ASCs), such as mesenchymal stem cells (MSCs) derived from the bone marrow (BM), adipose tissue-derived stem cells (ADSCs) and umbilical cord blood stem cells (UBSCs).
Each type of stem cells, ASCs or ESCs, has specific advantages and disadvantages, with some more easily differentiated into neurons than others. To this end, researchers across various spectra of biomedical science have developed unique methodologies to coax neuronal differentiation of these stem cells into defined subsets of neurons for customized therapeutic applications. This review highlights these approaches and describes the innovation and novelty of each.
Neuronal induction approaches
Induction by chemical & soluble factors
Among the initial induction methods used to spur neuronal differentiation of stem cells was the addition of soluble induction factors to the growth medium. In general, these factors are chemical entities, such as small molecules, or proteins, such as growth factors, which activate endogenous developmental pathways. Our laboratory has developed a protocol for the derivation of neurons from human MSCs through supplementation with retinoic acid (RA) and basic fibroblast growth factor (bFGF)2 (Fig. 1). These factors are endogenously present during early neural development and help define the dorsal–ventral axis of the neural tube. Interestingly, in hope of understanding how such neurons would behave if transplanted into an inflammatory microenvironment, supplementation of the growth medium with the pro-inflammatory cytokine IL-1α facilitated neurogenesis.3 These findings suggest that caution should be taken when implanting undifferentiated stem cells or partially or fully differentiated neurons derived from such stem cells into a new microenvironment, since in vivo and in vitro behavior can drastically differ.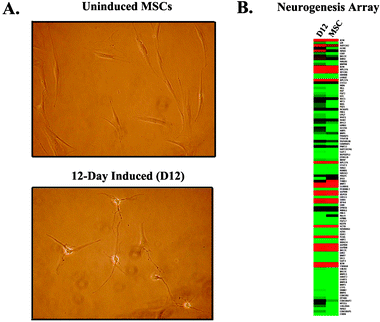 | ||
| Fig. 1 Morphological and genomic profile during neuronal differentiation of MSCs. A. Photomicrograph showing undifferentiated MSC morphology. (Top) MSCs are long, flat, asymmetrical cells, which, upon neuronal induction (Bottom), sprout extensive neurites and become refractive under phase contrast microscopy. B. This metamorphosis in appearance is coincident with a global change in neurogenic gene expression, as illustrated by the microarray heat map. | ||
Currently, many induction approaches that utilize soluble factors attempt to generate specific populations of neurons (Table 1). We have previously shown that an induction cocktail comprised of bFGF, FGF8 and sonic hedgehog (SHH) spurred the differentiation of hMSCs into a dopaminergic neuronal phenotype .4 A protocol for efficient generation of dopaminergic progenitors and neurons is clinically appealing given the degeneration of this population of cells in Parkinson’s disease. Similar findings were observed with UBSCs using a customized induction protocol incorporating a multitude of soluble inducing agents.5
| Approach | Stem cells utilized | Phenotypes | Inducing agents |
|---|---|---|---|
| Abbreviations used: RA—retinoic acid; bFGF—basic fibroblast growth factor; SHH—sonic hedgehog; FGF8—fibroblast growth factor 8; BMP4—bone morphogenic protein 4; BDNF—brain derived neurotrophic factor; EGF—epidermal growth factor; IGF—insulin-like growth factor; IBMX—isobutylmethylxanthine. | |||
| Soluble Factors | MSC, UBSC, ESC, ADSC | Functional electrophysiology; dopaminergic, peptidergic and sensory neuron phenotype | RA, bFGF, SHH, FGF8, BMP4, RA-analogues, BDNF, EGF, IGF, IBMX |
Other approaches have utilized different stem cell populations. Shi et al., showed that ESCs treated with bone morphogenic protein-4 (BMP-4) and bFGF were able to become sensory neurons,6 while another study by Christie et al., evaluated the ability of all-trans-RA analogues to induce neuronal differentiation from ESCs.7 Other studies with umbilical cord blood stem cells explored the potential for brain-derived neurotrophic factor (BDNF)8 and epidermal growth factor (EGF)9 to induce neuronal differentiation. Lastly, stem cells derived from adipose tissue were even able to undergo neuronal differentiation upon exposure to insulin growth factor (IGF) and isobutylmethylxanthine (IBMX).10
The striking point on the various approaches is the variety of stem cell sources and different types of soluble factors, from small molecules to cytokines to growth factors that can induce a neurogenic program of differentiation. These findings allude to the complexity with which soluble factors are involved in neuronal development, and demonstrate that many different types of stem cells have the potential for neuronal differentiation.
Molecular induction
One of the more recent approaches for inducing neuronal differentiation from stem cells involves reprogramming somatic cells to induce a pluripotent state (Table 2). The seminal studies in this field were performed by Takahashi et al., who were able to induce pluripotent stem cells (iPS) from adult human fibroblasts by overexpressing key developmental genes, specifically Oct3/4, Sox2, Klf4 and c-Myc.1 These iPS cells were able to differentiate into cells of all three germ layers and form teratomas. Another group was able to reprogram neural stem cells into iPS cells solely with the ectopic overexpression of Oct3/4 and either Klf4 or c-Myc.11| Approach | Cells utilized | Phenotypes | Inducing agents |
|---|---|---|---|
| Abbreviations used: REST—Re-1 silencer of transcription; NGN2—Neurogenin-2. | |||
| Molecular Induction | Fibroblasts, NSC, MSC | iPS cell generation; ability to generate tissues of all defined germ layers | Oct3/4, Sox2, Klf4, c-Myc, Notch1, REST, Nurr1, Mash1, NGN2 |
The clinical potential for such approaches is apparent in the finding that dopaminergic neurons derived from iPS cells functionally integrate into the brain of fetal mice and improve the symptoms of rats with Parkinson’s disease.12 An exciting application of this reprogramming technology is the generation of customized iPS cells derived from patients with specific diseases, which can then be differentiated into any cell of interest. An example of such an approach is the generation of motor neurons derived from the iPS cells of patients with amyotrophic lateral sclerosis (ALS).13 These neurons can be studied to provide clues for future treatments or serve a therapeutic role themselves as an autologous transplant.
A potential downside to the utilization of iPS cells is that they are reprogramming using a lentiviral-based vehicle. Thus, there is the potential for insertional mutagenesis and hence tumorigenicity. Alternate, and potentially safer, methods of reprogramming somatic stem cells include the use of plasmids or non-integrating adenoviral vectors.
Other studies have utilized ectopic manipulation of key developmental genes involved in neurogenesis to induce neuronal differentiation. Yanjie et al., demonstrated that knockdown of the stem cellgeneNotch1 facilitated differentiation of MSCs into neuron-like cells,14 while our laboratory has shown that knockdown of the neural-specific repressor, REST, enhanced differentiation of human MSCs into dopaminergic neurons.15 In contrast, overexpression of Nurr1 in NSCs derived from the adult rat brain generated functional dopamine neurons.16
Lastly, manipulation of developmental genes in stem cells can be used in a combinatorial approach whereby genetically modified cells are transplanted in vivo to spur differentiation. Yi et al., showed that when the transcription factorsMash1 and Neurogenin2 (Ngn2) were expressed in NSCs, they aided survival and engraftment as the endogenous neural tissue promoted differentiation.17 The latter result, whereby recipient cells or tissues promote differentiation of the stem cells, is the next approach to be highlighted in this review.
Cellular induction & tissue implantation
The use of soluble factors to promote neuronal differentiation of stem cells typically utilizes purified or recombinant substances produced by endogenous cells. Thus, another approach is to co-culture stem cells with cells that facilitate differentiation via production of paracrine factors or through cell–cell contact/communication.MSCs or their differentiated stromal progeny have been used in many co-culture schemes to facilitate neuronal differentiation of various stem cells. Croft and Przyborski, cultured MSCs, pre-induced to express neural antigens, with ESC-derived NSCs to induce differentiation.18 Others have utilized a similar approach for the specific generation of dopaminergic neurons from ESCs.19 Attesting to the inherent ability for MSCs and stromal cells to provide neurotrophic support, even the PA6 stromal cell line aided the generation of dopamine neurons from ESC.20 In fact, stromal cells themselves have been shown to transdifferentiate into neurons with aid of neurotrophic factors produced through co-culture with Schwann cells.21
A similar co-culture approach involves using endogenous neural subtypes, such as astrocytes, brain endothelial cells or meningeal cells as substrates for differentiation of NSCs, neural progenitor cells or ESCs, respectively.22–24 However, a variety of other cell types, including amniotic epithelial cells25 and glioblastoma,26 have been utilized in a similar respect. An interesting study by Soundararajan et al., combined the use of soluble factors, molecular engineering and cellular co-culture.27 Here, HEK293 cells were engineered to overexpress SHH, and were co-cultured with ESCs to facilitate rapid differentiation into functional motoneurons.27
Another approach for neuronal induction involves the implantation of stem cells into select tissues in vivo (Table 3). A benefit of tissue implantation over in vitro co-cultures is that the cells exist in a three-dimensional environment, which may be crucial for proper differentiation. Of course, this assumes that the transplanted cell is the desired cell for differentiation, rather than providing trophic support to facilitate endogenous neurogenesis. Several groups have demonstrated this replacement type approach, either with ESCs, NSCs or Schwann cells.28,29 Geeta et al., have shown that Parkinsonian rats transplanted with hESC-derived dopaminergic progenitors exhibited one-year survival and significant reversal of motor defects.30
| Approach | Cells utilized | Outcome | Tissue type |
|---|---|---|---|
| Transplantation | ESC, NSC, Schwann cells, dopamine progenitors, UBSC, MSC | Ability to reverse Parkinsonian motor defects; neuroprotective in stroke; BDNF production | Brain |
In contrast, the implanted stem cells may not be the effector cells undergoing differentiation. Instead they may be promoting differentiation of endogenous stem cells or providing trophic support and protection. Indeed, implantation of human umbilical cord blood stem cells in rodents exerted a neuroprotective effect for ischemic stroke,31 while MSCs produced a similar result in a progressive animal model of Parkinson’s disease.32 MSCs have also been shown to produce trophic factors, such as brain-derived neurotrophic factor (BDNF), to increase neuronal survival and synaptic stability33 or promote proliferation of endogenous NSCs.34
There are several methods which could be employed to test whether the transplanted stem cell is providing cell replacement or promoting endogenous mechanisms. In particular, tissue explants, where stem cells from a non-autologous source are injected into a cultured fragment of the tissue of interest, could shed light on this query. Here, the donor stem cells can be traced using markers, such as the Y chromosome or stably transfected GFP, to determine the fate of the transplant.
Tissue engineering
The final approach highlighted in this review entails the utilization of biomedical engineering to induce stem cell neuronal differentiation (Table 4). Principles of engineering help differentiating stem cells to form the higher order structures, such as nerve fibers or tracts, which are extremely difficult to induce in vitro or spur in vivo. For example, a patient with a severed spinal cord may need a transplant not only of stem cells, but of stem cells embedded within some form of biological matrix to aid in the joining of the severed regions and facilitate tract formation.| Approach | Cells utilized | Target tissue | Biomaterial |
|---|---|---|---|
| Bioengineering | ESC, NSC | Nerve fibers, tracts | Synthetic nanogratings, nanofibers; air–liquid interface-based culture; inkjet-printed macromolecules; hydrogels; extramedullary chitosan channels |
The primary media used by biomedical engineers in this respect are nanofibers that are electrically spun to form scaffolds for stem cell envelopment and tissue implantation. Interestingly, soluble factors may or may not be embedded within these scaffolds for release upon implantation to induce differentiation of ESCs35,36 or NSCs.37 Other engineering approaches to generate three-dimensional neuronal tissue from stem cells include the use of unique air–liquid interface-based culture,38 inkjet printing of macromolecules such as bFGF on hydrogels,39 extramedullary chitosan channels40 and synthetic nanogratings.41
Finally, in perhaps the most novel, intriguing and integrative approach employed to date for neuronal differentiation of stem cells, Piacentini et al., show that extremely low-frequency electromagnetic fields promote in vitroneurogenesis of NSCs isolated from the brain cortices of newborn mice.42
The outlook for deriving neurons from stem cells
This review details various approaches, spanning multiple scientific disciplines, which have been applied to spur neuronal differentiation of stem cells. Although these interdisciplinary methodologies are commendable, the mere fact that so many different angles have been used suggests that there is no universally accepted approach for optimal differentiation (Fig. 2). Meanwhile, stem cell therapies outside of bone marrow stem cell transplantations have begun to advance in the clinic. Specifically, bone marrow-derived stem cells such as MSCs have been explored as therapies for graft versus host disease and several autoimmune disorders, such as Crohn’s disease. In terms of neurological diseases, MSCs have been explored in humans as a treatment for multiple sclerosis; although primarily for their immunosuppressive properties rather than as facilitators of neural regeneration. | ||
| Fig. 2 Cartoon depicting various approaches utilized to induce the differentiation of neurons from stem cells. Induction schemes can range from basic approaches, such as the use of chemicals or other soluble mediators to mimic developmental differentiation signals, to more advanced methods, such as reprogramming the cell through genetic manipulation. The ultimate goal of these methods is to generate functional neurons, which can then be applied in a research or clinical setting. | ||
To date, no human clinical trials have been performed testing stem cells as a neuronal replacement. One principle reason for this lack of clinical data is that the optimal approach to promote neurogenesis, whether through stimulating endogenous cells or by replacing degenerating ones, as well as the ideal stem cell population to use are unknown. Still, the interdisciplinary differentiation approaches described in this review provide us with valuable tools to explore neuronal development as well as neuropathobiology, and this in itself may promote the development of novel therapeutics either stem cell- or non stem cell-based. In fact, the most feasible and safe approach for stem cell-based neurotherapies may involve the use of inducing agents delivered to patients for promotion of endogenous neurogenic mechanisms. However, there is considerable hope that in the future, ex vivo manipulation of a patient’s own stem cells can be used to replace their own degenerating or damaged neural tissue. This would be a significant scientific accomplishment, which would improve the lives of many patients suffering from neurological conditions around the world for which there exists no current therapy.
Acknowledgements
This work was supported by a grant from the F. M. Kirby Foundation.References
- K. Takahashi, K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda and S. Yamanaka, Cell, 2007, 131, 861–872 CrossRef CAS.
- S. J. Greco, C. Zhou, J. H. Ye and P. Rameshwar, Stem Cells Dev., 2007, 16, 811–826 CrossRef CAS.
- S. J. Greco and P. Rameshwar, J. Immunol., 2007, 179, 3342–3350 CAS.
- K. A. Trzaska, E. V. Kuzhikandathil and P. Rameshwar, Stem Cells, 2007, 25, 2797–2808 Search PubMed.
- S. Greschat, J. Schira, P. Kury, C. Rosenbaum, M. A. de Souza Silva, G. Kogler, P. Wernet and H. W. Muller, Stem Cells Dev., 2008, 17, 221–232 CrossRef CAS.
- F. Shi, C. E. Corrales, M. C. Liberman and A. S. Edge, Eur. J. Neurosci., 2007, 26, 3016–3023 CrossRef.
- V. B. Christie, J. H. Barnard, A. S. Batsanov, C. E. Bridgens, E. B. Cartmell, J. C. Collings, D. J. Maltman, C. P. Redfern, T. B. Marder, S. Przyborski and A. Whiting, Org. Biomol. Chem., 2008, 6, 3497–3507 RSC.
- J. Y. Lim, S. I. Park, J. H. Oh, S. M. Kim, C. H. Jeong, J. A. Jun, K. S. Lee, W. Oh, J. K. Lee and S. S. Jeun, J. Neurosci. Res., 2008, 86, 2168–2178 CrossRef CAS.
- W. Jin, Y. Q. Xing and A. H. Yang, In vitro Cell Dev. Biol. Anim., 2009 Search PubMed.
- H. Ning, G. Lin, T. Fandel, L. Banie, T. F. Lue and C. S. Lin, Differentiation, 2008, 76, 488–494 CrossRef CAS.
- J. B. Kim, H. Zaehres, G. Wu, L. Gentile, K. Ko, V. Sebastiano, M. J. Arauzo-Bravo, D. Ruau, D. W. Han, M. Zenke and H. R. Scholer, Nature, 2008, 454, 646–650 CrossRef CAS.
- M. Wernig, J. P. Zhao, J. Pruszak, E. Hedlund, D. Fu, F. Soldner, V. Broccoli, M. Constantine-Paton, O. Isacson and R. Jaenisch, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 5856–5861 CrossRef CAS.
- J. T. Dimos, K. T. Rodolfa, K. K. Niakan, L. M. Weisenthal, H. Mitsumoto, W. Chung, G. F. Croft, G. Saphier, R. Leibel, R. Goland, H. Wichterle, C. E. Henderson and K. Eggan, Science, 2008, 321, 1218–1221 CrossRef CAS.
- J. Yanjie, S. Jiping, Z. Yan, Z. Xiaofeng, Z. Boai and L. Yajun, NeuroReport, 2007, 18, 1443–1447 CrossRef.
- K. A. Trzaska, B. Y. Reddy, J. L. Munoz, K. Y. Li, J. H. Ye and P. Rameshwar, Mol. Cell. Neurosci., 2008, 39, 285–290 CrossRef CAS.
- J. W. Shim, C. H. Park, Y. C. Bae, J. Y. Bae, S. Chung, M. Y. Chang, H. C. Koh, H. S. Lee, S. J. Hwang, K. H. Lee, Y. S. Lee, C. Y. Choi and S. H. Lee, Stem Cells, 2007, 25, 1252–1262 Search PubMed.
- S. H. Yi, A. Y. Jo, C. H. Park, H. C. Koh, R. H. Park, H. Suh-Kim, I. Shin, Y. S. Lee, J. Kim and S. H. Lee, Mol. Ther., 2008, 16, 1873–1882 CrossRef CAS.
- A. P. Croft and S. A. Przyborski, Exp. Neurol., 2009, 216, 329–341 CrossRef CAS.
- A. Shintani, N. Nakao, K. Kakishita and T. Itakura, J. Neurosci. Res., 2008, 86, 2829–2838 CrossRef CAS.
- T. Vazin, J. Chen, C. T. Lee, R. Amable and W. J. Freed, Stem Cells, 2008, 26, 1517–1525 Search PubMed.
- M. Zurita, J. Vaquero, S. Oya, C. Bonilla and C. Aguayo, NeuroReport, 2007, 18, 1713–1717 CrossRef.
- M. M. Daadi, Methods Mol. Biol., 2008, 438, 205–212 CAS.
- M. A. Gama Sosa, R. De Gasperi, A. B. Rocher, G. M. Perez, K. Simons, D. E. Cruz, P. R. Hof and G. A. Elder, Cell Res., 2007, 17, 619–626 CrossRef CAS.
- H. Hayashi, A. Morizane, M. Koyanagi, Y. Ono, Y. Sasai, N. Hashimoto and J. Takahashi, Eur. J. Neurosci., 2008, 27, 261–268.
- X. T. Meng, D. Chen, Z. Y. Dong and J. M. Liu, Cell Biol. Int., 2007, 31, 691–698 CrossRef CAS.
- J. A. Ozolek, E. P. Jane, L. Krowsoski and P. J. Sammak, Stem Cells Dev., 2007, 16, 403–412 CrossRef CAS.
- P. Soundararajan, B. W. Lindsey, C. Leopold and V. F. Rafuse, Stem Cells, 2007, 25, 1697–1706 Search PubMed.
- X. Zhang, Y. Zeng, W. Zhang, J. Wang, J. Wu and J. Li, J. Neurotrauma, 2007, 24, 1863–1877 CrossRef.
- M. M. Daadi, A. L. Maag and G. K. Steinberg, PLoS One, 2008, 3, e1644 CrossRef.
- R. Geeta, R. L. Ramnath, H. S. Rao and V. Chandra, Biochem. Biophys. Res. Commun., 2008, 373, 258–264 CrossRef CAS.
- S. H. Koh, K. S. Kim, M. R. Choi, K. H. Jung, K. S. Park, Y. G. Chai, W. Roh, S. J. Hwang, H. J. Ko, Y. M. Huh, H. T. Kim and S. H. Kim, Brain Res., 2008, 1229, 233–248 CrossRef CAS.
- H. J. Park, P. H. Lee, O. Y. Bang, G. Lee and Y. H. Ahn, J. Neurochem., 2008, 107, 141–151 CrossRef CAS.
- R. C. Rodrigues Hell, M. M. Silva Costa, A. M. Goes and A. L. Oliveira, Neurobiol. Dis., 2009, 33, 290–300 CrossRef CAS.
- S. W. Yoo, S. S. Kim, S. Y. Lee, H. S. Lee, H. S. Kim, Y. D. Lee and H. Suh-Kim, Exp. Mol. Med., 2008, 40, 387–397 Search PubMed.
- S. M. Willerth, T. E. Faxel, D. I. Gottlieb and S. E. Sakiyama-Elbert, Stem Cells, 2007, 25, 2235–2244 Search PubMed.
- J. Xie, S. M. Willerth, X. Li, M. R. Macewan, A. Rader, S. E. Sakiyama-Elbert and Y. Xia, Biomaterials, 2009, 30, 354–362 CrossRef CAS.
- G. T. Christopherson, H. Song and H. Q. Mao, Biomaterials, 2009, 30, 556–564 CrossRef CAS.
- O. Preynat-Seauve, D. M. Suter, D. Tirefort, L. Turchi, T. Virolle, H. Chneiweiss, M. Foti, J. A. Lobrinus, L. Stoppini, A. Feki, M. Dubois-Dauphin and K. H. Krause, Stem Cells, 2009, 27, 509–520 Search PubMed.
- S. Ilkhanizadeh, A. I. Teixeira and O. Hermanson, Biomaterials, 2007, 28, 3936–3943 CrossRef CAS.
- H. Nomura, T. Zahir, H. Kim, Y. Katayama, I. Kulbatski, C. M. Morshead, M. S. Shoichet and C. H. Tator, Tissue Eng. A, 2008, 14, 649–665 Search PubMed.
- E. K. Yim, S. W. Pang and K. W. Leong, Exp. Cell Res., 2007, 313, 1820–1829 CrossRef CAS.
- R. Piacentini, C. Ripoli, D. Mezzogori, G. B. Azzena and C. Grassi, J. Cell. Physiol., 2008, 215, 129–139 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
