Microfluidic fabrication of SERS-active microspheres for molecular detection†
Hyerim
Hwang‡
,
Shin-Hyun
Kim‡
and
Seung-Man
Yang
*
National Creative Research Initiative Center for Integrated Optofluidic Systems, Department of Chemical and Biomolecular Engineering, KAIST, Daejeon 305-701, Korea. E-mail: smyang@kaist.ac.kr; Fax: +82-42-350-5962; Tel: +82-42-350-3922
First published on 20th October 2010
Abstract
In this paper, we demonstrated a microfluidic system for fabricating microspheres with hierarchical surface nanopatterns for molecular detection based on surface-enhanced Raman scattering (SERS). Briefly, a photocurable silica suspension was emulsified into monodisperse droplets using a microfluidic device composed of two coaxial glass capillaries. The silica particles in each droplet protruded through the interface and spontaneously formed a hexagonal array. After polymerization of the droplets, we selectively decorated the exposed areas of the silica particles with silver nanoparticles through electroless deposition. The resulting hierarchically-structured microspheres showed high sensitivity and fast binding kinetics in molecular detection based on SERS, owing to the dense array of hot spots on each microsphere and high mobility of the microspheres, respectively. Notably, the SERS signals from molecules adsorbed on the microspheres could be detected in both the dried and suspension states. In addition, we demonstrated that the SERS-active microspheres can be functionalized into structural colored or magnetoresponsive microspheres for advanced applications.
Introduction
Chemical and biomolecular detection and analysis are of increasing importance in a wide range of industrial and research fields, including medical diagnosis, drug discovery, and bioassays, as well as in the prevention and prediction of chemical-related emergencies. Surface-enhanced Raman scattering (SERS) has been emerged as a promising strategy for molecular detection that has high sensitivity and label-free identification of molecules.1–4 The excitation of localized surface plasmons and formation of charge-transfer complexes on the surface of metal nanostructures enhance the Raman signals to levels comparable to those of the fluorescence signals that have conventionally been employed in biosensor.5,6 Numerous groups have developed new metal nanostructures to achieve higher enhancement factors of Raman signals as well as simpler and faster measurement for practical uses using two distinct approaches: (1) nanoparticles-based enhancement7–10 and (2) periodic nanopattern-based detection.11–15 However, there are still limitations hindering the practical use of SERS. Although nanoparticles are characterized by fast binding kinetics and high sensitivity, measuring signals derived from these particles is relatively difficult and expensive because such measurements require concentration of nanoparticles or a highly precise apparatus and delicate control in order to obtain sufficient intensity. On the other hand, planar substrates with a periodic metal nanostructure can produce dense arrays of hot spots enabling simple and fast measurements, but suffer from the drawbacks of slow binding kinetics and high production costs.In the present study, we developed a facile approach to generating SERS-active microspheres in order to enhance the advantages and obviate the limitations of conventional SERS systems. Microfluidic emulsification was used to produce monodisperse microspheres, and a combination of colloidal self-assembly at the droplet interface and selective deposition of silver nanostructures was employed to create hierarchical metal nanopatterns on the microspheres. The resulting microspheres exhibited both significant densities of hot spots and fast binding kinetics, which can be attributed to the periodic metal nanopatterns and high mobility of the microspheres, respectively. Recently, Han et al. has demonstrated silver nanoparticle-decorated silica microsphere with designed surface charge for favorable SERS detection of charged species.16Silver deposition was done through a layer-by-layer assembly. Detection limit up to ppt concentration has been achieved using single SERS active spheres. In this work, our different approach enables to tailor hierarchical silver nanopatterns through a facile one step deposition and give additional photonic and magnetic functionalities on SERS-active microspheres.
Experimental
A. Fabrication of monodisperse microspheres with silica particle arrays
As a first step in the fabrication of microspheres covered with hexagonal silica nanoarrays, we synthesized monodisperse silica particles through two phase-based seed preparation and subsequent seeded growth by the Stöber method.17,18 An ethanolic silica suspension of the resulting particles was mixed with ethoxylated trimethylolpropane triacrylate (ETPTA) containing 1 wt% 2-hydroxy-2-methyl-1-phenyl-1-propanone (Darocure1173, Ciba Specialty Chemicals) as a photoinitiator, and the mixture was sonicated for 30 min. After evaporating the ethanol from the mixture by heating at 70 °C overnight, a silica-ETPTA suspension was obtained. To generate monodisperse emulsion droplets, a microfluidic device composed of two coaxial glass capillaries was employed, as depicted in Fig. 1a.19 A 10 v/v% suspension of photocurable silica-ETPTA droplets of diameter 290 nm and an aqueous solution containing 1 wt/wt% Pluronic F108 (ethylene oxide–propylene oxide–ethylene oxide tri-block copolymer surfactant; BASF) as a surfactant were forced to flow through the inner and outer capillaries, respectively. Monodisperse emulsion droplets of silica-ETPTA were generated in the dripping mode20 and microspheres covered with a hexagonal silica particle array were fabricated by UV-induced photopolymerization of the droplets. Following the same procedure, 33 v/v% suspensions of silica-ETPTA composed of 145 nm, 181 nm, and 200 nm silica particles were used to make blue, green, red structural colors on the microspheres, respectively. In addition, a 10 v/v% silica-ETPTA suspension composed of 290 nm silica particles containing 0.2 wt/wt% hematite iron oxide nanoparticles (<50 nm, Aldrich) was used to create magnetoresponsive microspheres. In preparing these particles, an external magnetic field was applied to the suspension droplets with magnet of 2.78 kG for 1 min prior to photopolymerization to induce a magnetic moment in the microspheres. | ||
| Fig. 1 (a) Schematic diagram of the coaxial capillary device. The inset shows droplet formation at the end of the inner capillary. (b) Optical microscope image of monodisperse microspheres prepared using the glass capillary device. (c) Size distribution of microspheres. The mean diameter and coefficient of variation (CV) of the microspheres are 77 μm and 2.74%, respectively. | ||
B. Electroless deposition of silver nanoparticles and characterization of SERS signals
Microspheres with a silver pattern could be obtained by selective silver deposition on the exposed areas of the hexagonally arranged silica particles on the microsphere surfaces through electroless silver deposition. To achieve this, Tollens' reagent was prepared by first mixing 10 mL of 0.1 M AgNO3 solution with 180 μL of ammonia solution (Junsei, min. 28%), and then diluting the resulting solution with 90 mL of distilled water. 33 μL of 0.5 M glucose solution and 66 μL of 0.8 M KOH (Junsei, min. 85%) solution were then added to 6 mL of the diluted Tollens' reagent containing the monodisperse microspheres, causing silver nanoparticles to be selectively deposited on the exposed silica surfaces. The reaction was stopped by adding 50 mL of water to the reaction solution, and the resulting microspheres were washed with water several times. To evaluate the SERS activity of the silver-decorated microspheres, the microspheres were immersed in ethanolic solutions of analytes such as benzenethiol (BT), 2-naphthalenethiol (2-NT) and 4-aminothiophenol (ATP) for 12 h. After washing with ethanol several times, Raman spectra were measured in both of the dried and immersed states for 100 ms using a simple Raman system (Ocean Optics Inc., QE65000-RAMAN-KIT), where a 785 nm laser with a power of 350 mW was employed to illuminate a sample area of diameter 1 mm. Approximately 130 microspheres in hexagonal array were included in probing area in both dried and immersed states. In addition, we employed an elaborate Raman apparatus (Jovin Yvon, JY HR800) to evaluate the sensitivity of the microspheres, using a 785 nm laser with a power of 10 mW focused on the sample with a spot size of 1 μm in diameter for 1 s.Results and discussion
A. Preparation of hierachically-structured microspheres
We used a coaxial glass capillary device to prepare monodisperse emulsion droplets of silica-ETPTA suspension. Immediately after the droplets were generated, the silica particles migrated within each droplet such that they spontaneously formed hexagonal arrays of protruding particles at the droplet–water interface, a process driven by interfacial energy minimization.19,21,22 The droplets were solidified by UV irradiation within one second, yielding microspheres with hexagonal arrays of silica particles on their surfaces. The microspheres had a narrow size distribution, as shown in Fig. 1b and 1c (coefficient of variation ∼ 2.74%). Scanning electron microscopy (SEM) images of the microspheres were shown in Fig. 2. Fig. 2c and 2d show a Moiré fringe and a hexagonal array of 290 nm silica particles on the microsphere surfaces, respectively. The Moiré fringe, which is generated by the interaction between the hexagonal array of silica particles and the scanning line pattern of the SEM, provides evidence of the high regularity of the particle array over the entire surface.23 The particles were non-close packed due to the repulsive interparticle potential with contributions from electrostatic repulsion by the negatively charged silica surfaces of water side and the disjoining pressure associated with the solvation film on the silica surface of ETPTA side.24,25 The negative surface charge of the silica particles did, on the other hand, have the benefit that it allowed the selective deposition of silver nanoparticles on the surfaces of the particles via the silver mirror reaction.14,26 As a result, we could create silver nanoparticle-decorated hierarchical microspheres of the type shown in Fig. 2e. | ||
| Fig. 2 (a) Procedure for preparing hierarchically-structured microspheres. (b–d) SEM images of monodisperse microspheres and their surfaces with hexagonal arrays of 290 nm silica particles. (e) SEM image of silver-decorated silica arrays on microsphere surfaces. | ||
B. Morphological control of microspheres
The hierarchical surface morphology of the microspheres could be controlled by varying the deposition time of the silver nanoparticles. Such control is important for molecular detection applications because the SERS activity depends on the size and shape of the metal nanoparticles, as well as the interdistance between particles.As shown in Fig. 3a, during the initial 2 min of silver reduction, silver nanoparticles nucleated at a few points on each silica particle and grew into larger silver particles. The average size (dsilver) and number density of silver nanoparticles per silica particle (Nsilver) were increased to 47 nm and 8.3 & 68 nm and 9.4 through 1.5 and 2 min deposition, respectively. On further reduction, nanoparticles began to merge with neighbours through additional growth: dsilver increased to 80, 110, 123, 181 nm and Nsilver decreased to 9.2, 7.7, 7.2, 4.0 for deposition times of 2.5, 3, 3.5, 4 min, respectively. The insets of the SEM images display the corresponding optical microscope images, which show a gradual darkening of the microspheres as a result of silver nanoparticle generation during the first 2 min, followed by glazing as the silver particles grew. The darkening and glazing are attributed to light absorption by silver nanoparticles and light reflection by bulk silver, respectively.
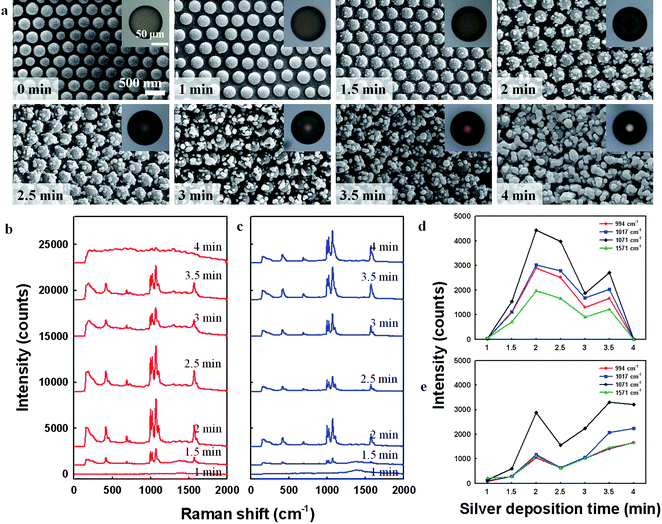 | ||
| Fig. 3 (a) SEM images showing the surface morphology evolution of silver-decorated silica arrays on microsphere surfaces for silver deposition times of 0 to 4 min. Insets show optical microscope images of the corresponding microspheres. (b,c) SERS spectra of benzenethiol (BT)-adsorbed silver-decorated microspheres prepared using various silver deposition times, measured from microspheres in (b) air and (c) ethanol. (d,e) SERS signal intensities at 994, 1017, 1071, and 1571 cm−1 as a function of silver deposition time for microspheres in (d) air and (e) ethanol. | ||
To investigate the SERS activities of microspheres with various silver morphologies, we immersed the microspheres in ethanolic solutions containing 2 mM BT for 12 h, causing BT molecules to be adsorbed on the silver arrays on the microspheres. Residual BT molecules were then removed from the microspheres by washing with ethanol. Using a simple Raman instrument (Ocean Optics Inc., QE65000-RAMAN-KIT), SERS spectra of adsorbed BT molecules were recorded, where approximately 130 microspheres in hexagonal array were included in probing area of 1 mm diameter, which gives averaged Raman signals. The spectra obtained from BT on microspheres subjected to various deposition times in air and ethanol are displayed in Fig. 3b and c, respectively. The spectra exhibit peaks at 994, 1017, 1071, and 1571 cm−1, which are characteristic peak positions of BT molecules. Fig. 3d shows the change in the intensities of these peaks according to the silver deposition time for dried microspheres, and Fig. 3e shows the corresponding data for microspheres immersed in ethanol. For the dried microspheres, the intensities of the Raman signals from the microspheres were negligible at a silver deposition time of 1 min due to a lack of silver nanoparticles, as can be seen in the second SEM image in Fig. 3a. However, the signals emerged as the deposition time increased from 1.5 to 2 min. At longer silver deposition times the intensity decreases, with the characteristic peaks of BT disappearing for microspheres subjected to 4 min of silver deposition. The increase in Raman signal intensity for deposition times of 1.5 and 2 min is attributed to an increase in the number of silver nanoparticles and the generation of nanogaps between silver particles. However, further silver deposition increased the particle size and closed the nanogaps, leading to a decrease in the signal intensity. In addition, slow burning of microspheres was observed during laser irradiation. In particular, the microspheres with heavy silver structures (4 min deposition) were burned as soon as irradiating laser on microsphere due to strong photothermal effect of silver, and hence did not display any characteristic peaks of BT.27
Based on microspheres with 2 min silver deposition, we calculated enhancement factor (EF) at 1071 cm−1 using the following equation:
When microspheres dispersed in ethanol were used to measure the Raman signal, the intensity increased with increasing deposition time until 2 min and decreased with additional deposition (2.5 min) like dried microspheres (Fig. 3e). In contrast to the decrease in signal intensity observed for the dried microspheres, however, immersed microspheres in ethanol showed the increase in Raman intensity for longer deposition time than 2.5 min. The rise can be attributed to increase of surface area of silver nanostructure while the transfer of heat from the microspheres to the surrounding medium prevented the burning. These findings indicate that the silver-decorated microspheres can be used for SERS analysis of analyte molecules in both the dried and solution states.
C. Binding kinetics and sensitivity of SERS-active microspheres
To compare the binding kinetics of the microspheres with those of substrate-based materials, we prepared planar glass substrates treated with silver nanoparticles using the same deposition procedures, yielding samples with surface morphologies similar to that shown in the inset of Fig. 4a. Fig. 4a shows the Raman spectra obtained from dried microspheres and planar substrates which were immersed in 2 μM BT solution for 60 min prior to measurement. The signal intensities are much higher for the microspheres owing to well-tailored architectures in terms of the small size of the silver nanoparticles and the nanogaps between particles. Fig. 4b shows the variation in Raman intensity for the microsphere and substrate systems according to BT immersion time, where the intensities were normalized by the intensity at 12 h of immersion to exclude the effect of silver nanoarchitectures. Immersion time was determined by time interval between microsphere injection into analyte solution and first washing step. The data show that, at all immersion times, the normalized Raman intensity is greater for the microspheres than for the planar substrate, indicating that the BT molecules bind more rapidly to the silver surface of the microspheres. Notably, the microspheres immersed for less than 1 min show considerable signal intensity, whereas the signal from the planar substrate is negligible. These results can be attributed to the high mobility of the microspheres in comparison with the solid substrate, which enhances the contact opportunity of molecules with silver surface. The higher intensity at immersion time of 0.5 min than that of 1 min is attributed to experimental error by binding of analyte on silver surface during washing steps. | ||
| Fig. 4 (a) SERS spectra of BT adsorbed on silver-decorated microspheres and silver-coated glass substrates, where both the microspheres and glass substrate were immersed in 2 μM BT solution for 60 min prior to measurement. (b) Variation of SERS signal intensity of BT adsorbed on silver-decorated microspheres (solid line) and a silver-coated glass substrate (dashed line) at 994, 1017, and 1071 cm−1 according to immersion time of microspheres in 2 μM BT solution. Abscissa is plotted on a log scale. | ||
The sensitivity of the microspheres to analyte concentration was evaluated by immersing them in BT solutions with various concentrations for 12 h. As shown in Fig. 5a and 5b, the intensity was attenuated as the BT concentration decreased, with a minimum measurable concentration of 400 nM, where Raman spectra were measured at dried state. However, by using a precise Raman system (Jovin Yvon, JY HR800) rather than the simple system, BT concentrations as low as 20 pM could be detected.
 | ||
| Fig. 5 (a) SERS spectra of BT from silver-decorated microspheres that had been immersed in BT solution of concentration 2 mM, 2 μM, and 600 nM for 12 h. (b) Variation in SERS signal intensity of BT adsorbed on silver-decorated microspheres at 994, 1017, and 1071 cm−1 according to BT concentration. | ||
D. Additional functionalities: silver-decorated photonic and magnetoresponsive microspheres
We produced structural colors on the microspheres by incorporating a high concentration of silica particles. When the highly concentrated silica-ETPTA suspensions were emulsified into droplets, the particles formed 3D crystal lattices inside the droplets, giving rise to colors by optical interference.29 Microspheres containing silica particles with diameters of 145 nm, 181 nm, and 200 nm at 33 v/v% showed blue, green, and red reflection colors, respectively. The colors and therefore the reflectance spectra were barely affected by decoration of the silver nanoparticles, as shown in Fig. 6a. Of particular interest in this context is the fact that, because the reflectance spectra do not interfere with the SERS signals, reflection colors could potentially be used as identification codes for microspheres in SERS detection. Such optically-coded SERS-active microspheres have great potential in the analysis of complex systems containing various chemicals and samples. For example, Fig. 6b shows the result of detection of 4-ATP, 2-NT, and BT molecules using blue, green, and red-colored microspheres, respectively where Raman spectra were measured at dried state. | ||
| Fig. 6 (a) Reflectance spectra of silver-decorated photonic microspheres. The insets show optical microscope images of the corresponding microspheres. (b) SERS spectra of 4-ATP (4-aminothiophenol), 2-NT (2-naphthalenethiol), and BT adsorbed on silver-decorated blue, green, and red-colored photonic microspheres, respectively. | ||
In addition, we functionalized the microspheres by incorporating magnetic nanoparticles into them. By using a silica-ETPTA suspension containing iron oxide (α-Fe2O3) nanoparticles smaller than 50 nm as the droplet phase and applying an external magnetic field to the droplets before photopolymerization, we fabricated magnetoresponsive microspheres.30 Here, the external magnetic field induces alignment of the magnetic moments of the weakly ferromagnetic nanoparticles in each droplet, thus producing a net magnetic moment on the microspheres after solidification of the droplets. The hydrophobic nature of iron oxide particles causes the particles to remain inside the ETPTA droplets while the hydrophilic silica particles protrude through the interface. Therefore, we could decorate the silica surface of the magnetoresponsive microspheres with silver nanoparticles by employing the same silver mirror reaction, without the disruption from the iron oxide nanoparticles. The motion of the magnetoresponsive microspheres could be controlled by an external magnet. In Fig. 7, we show a collection of monodisperse silver-decorated microspheres containing iron oxide nanoparticles and their concentration using an external magnet.
 | ||
| Fig. 7 (a) Optical microscope image of silver-decorated magnetoresponsive microspheres. (b) Microspheres concentrated at the wall of a vial using a magnet. | ||
Conclusions
We have prepared hierarchically-structured microspheres covered with silver nanoparticles from photocurable Pickering emulsion droplets for SERS-based molecular detection. The regular arrays of silver nanostructures were generated by the self-assembly of silica particles at the emulsion interface followed by selective silver deposition on the silica arrays. The prepared microspheres showed fast binding kinetics and high Raman signal intensity owing to the high mobility of the microspheres and the dense array of hot spots on each microsphere, respectively. It is noteworthy that our microspheres exhibited significant SERS intensity in both the dried and immersed states. In addition, the SERS-active microspheres could be functionalized optically and magnetically through simple procedures, enabling the identification of the microspheres in complex systems and control the microsphere motion, respectively.Acknowledgements
This work was supported by a grant from the Creative Research Initiative Program of the Ministry of Education, Science and Technology for “Complementary Hybridization of Optical and Fluidic Devices for Integrated Optofluidic Systems”. The authors also appreciated partial support from the Brain Korea 21 Program.Notes and references
- S. Nie and S. R. Emory, Science, 1997, 275, 1102 CrossRef CAS.
- Y. C. Cao, R. Jin and C. A. Mirkin, Science, 2002, 297, 1536 CrossRef CAS.
- S. Shanmukh, L. Jones, J. Driskell, Y. Zhao, R. Dluhy and R. A. Tripp, Nano Lett., 2006, 6, 2630 CrossRef CAS.
- D.-K. Lim, K.-S. Jeon, H. M. Kim, J.-M. Nam and Y. D. Suh, Nat. Mater., 2010, 9, 60 CrossRef CAS.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667 CrossRef CAS.
- W. E. Doering and S. Nie, J. Phys. Chem. B, 2002, 106, 311 CrossRef CAS.
- M. J. Mulvihill, X. Y. Ling, J. Henzie and P. Yang, J. Am. Chem. Soc., 2010, 132, 268 CrossRef CAS.
- W. Li, P. H. C. Camargo, L. Au, Q. Zhang, M. Rycenga and Y. Xia, Angew. Chem., Int. Ed., 2010, 49, 164 CAS.
- M. Wang, N. Jing, I.-H. Chou, G. L. Cote and J. Kameoka, Lab Chip, 2007, 7, 630 RSC.
- D. Choi, T. Kang, H. Cho, Y. Choi and L. P. Lee, Lab Chip, 2009, 9, 239 RSC.
- Q. Yu, P. Guan, D. Qin, G. Golden and P. Wallace, Nano Lett., 2008, 8, 1923 CrossRef CAS.
- T. Atay, J.-H. Song and A. V. Nurmikko, Nano Lett., 2004, 4, 1627 CrossRef CAS.
- X. Zhang, J. Zhao, A. V. Whitney, J. W. Elam and R. P. Van Duyne, J. Am. Chem. Soc., 2006, 128, 10304 CrossRef CAS.
- S. G. Jang, D.-G. Choi, C.-J. Heo, S. Y. Lee and S.-M. Yang, Adv. Mater., 2008, 20, 4862 CrossRef CAS.
- H. Ko and V. V. Tsukruk, Small, 2008, 4, 1980 CrossRef CAS.
- Y. Han, S. Sukhishvili, H. Du, J. Cefaloni and B. Smolinski, J. Nanosci. Nanotechnol., 2008, 8, 5791 CrossRef CAS.
- T. Yokoi, Y. Sakamoto, O. Terasaki, Y. Kubota, T. Okubo and T. Tatsumi, J. Am. Chem. Soc., 2006, 128, 13664 CrossRef CAS.
- W. Stober, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62 CrossRef.
- S.-H. Kim, J. W. Shim, J.-M. Lim, S. Y. Lee and S.-M. Yang, New J. Phys., 2009, 11, 075014 CrossRef.
- A. S. Utada, A. Fernandez-Nieves, H. A. Stone and D. A. Weitz, Phys. Rev. Lett., 2007, 99, 094502 CrossRef.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2000, 16, 8622 CrossRef CAS.
- K. Golemanov, S. Tcholakova, P. A. Kralchevsky, K. P. Ananthapadmanabhan and A. Lips, Langmuir, 2006, 22, 4968 CrossRef CAS.
- G. Subramania, K. Constant, R. Biswas, M. M. Sigalas and K.-M. Ho, Adv. Mater., 2001, 13, 443 CrossRef CAS.
- A. R. Bausch, M. J. Bowick, A. Cacciuto, A. D. Dinsmore, M. F. Hsu, D. R. Nelson, M. G. Nikolaides, A. Travesset and D. A. Weitz, Science, 2003, 299, 1716 CrossRef CAS.
- J. Ren, S. Song, A. Lopez-Valdivieso, J. Shen and S. Lu, J. Colloid Interface Sci., 2001, 238, 279 CrossRef CAS.
- J. Zhang, J. Liu, S. Wang, P. Zhan, Z. Wang and N. Ming, Adv. Funct. Mater., 2004, 14, 1089 CrossRef CAS.
- G. L. Liu, J. Kim, Y. Lu and L. P. Lee, Nat. Mater., 2006, 5, 27 CrossRef CAS.
- L.-J. Wan, M. Terashima, H. Noda and M. Osawa, J. Phys. Chem. B, 2000, 104, 3563 CrossRef CAS.
- S.-H. Kim, S.-J. Jeon, G.-R. Yi, C.-J. Heo, J. H. Choi and S.-M. Yang, Adv. Mater., 2008, 20, 1649 CrossRef CAS.
- S.-H. Kim, J. Y. Sim, J.-M. Lim and S.-M. Yang, Angew. Chem., Int. Ed., 2010, 49, 3786 CrossRef CAS.
Footnotes |
| † Published as part of a LOC themed issue dedicated to Korean Research: Guest Editors Professor Je-Kyun Park and Kahp-Yang Suh. |
| ‡ These authors contributed equally to this project. |
| This journal is © The Royal Society of Chemistry 2011 |

