Multifunctional mesoporous material for detection, adsorption and removal of Hg2+ in aqueous solution†‡
Chan
Wang
a,
Shengyang
Tao
*a,
Wei
Wei
b,
Changgong
Meng
a,
Fengyu
Liu
a and
Mei
Han
a
aSchool of Chemical Engineering, Dalian University of Technology, Dalian, Liaoning, P. R. China. E-mail: taosy@dlut.edu.cn; Fax: +86-411-84706303; Tel: +86-411-84706303
bNational Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, P.R. China
First published on 24th March 2010
Abstract
A novel, “all-in-one”, multifunctional microsphere with a fluorescent mesoporous silica shell (Rhodamine B coordinate receptor inside) and a magnetic core (Fe3O4) has been successfully fabricated using a sol–gel method and small molecular (CTAB) surfactants as structure-directing agents. At the same time, they were examined for environmental protection applications to detect, adsorb and remove Hg2+ in aqueous solution. The prepared nanocomposite microspheres were fluorescent, mesoporous, and magnetizable, with a diameter of 300–450 nm, a surface area of 600 m2 g−1, a pore size of 2.5 nm, and a saturation magnetization of 27.5 emu g−1. These multifuctional microspheres showed excellent fluorescence sensitivity and selectivity towards Hg2+ over other metal ions (Na+, Mg2+, Mn2+, Co2+, Ni2+, Zn2+, Cd2+, Ag+, Pb2+ and Cu2+). Upon the addition of Hg2+, an overall emission change of 16-fold was observed, and the detection limit of Hg2+ was as low as 10 ppb. The adsorption process of Hg2+ on the microspheres was well described by the Langmuir equation. The equilibrium can be established within five minutes and the adsorption capacity was 21.05 mg g−1. The concentration of Hg2+ ions can be reduced to less than 0.05 ppm and the used microspheres can be easily separated from the mixture by adding an external magnetic field. These results suggest that these “all-in-one” multifunctional nanocomposites are potentially useful materials for simultaneously rapidly detecting and recovering dangerous pollutants in aqueous solution.
Introduction
Heavy metal ions are very harmful environment pollutants because they are widely distributed in the air, water and soil and cause serious human health risks.1,2 Of these ions, Hg2+ is considered to be one of the most dangerous since it can accumulate in the human body and initiate a wide variety of symptoms, such as headache, edema and anema, even at quite low concentrations,3 and is especially severe for children's growth. Inorganic mercury is released into the natural environment through a variety of artificial and mineral sources. Anthropogenic sources of mercury include waste battery, gold mining, solid waste incineration, fossil fuel combustion, and chemical manufacturing. Therefore, various kinds of methods have been developed to detect and remove the Hg2+ ion, including liquid chromatography, capillary electrophoresis, optical methods and so on, but many of the instrumental techniques are slow, hard to field in a small, low-power package and not well-suited for quick detection of Hg2+ for in vivo studies of Hg2+ biology and toxicology. Among these approaches, small-molecule ligands have been found to be good fluorescent probes for quickly detecting Hg2+ in the field.4–6 At the same time, various solid materials, like optical fibers, are used as carriers to improve the properties of small-molecule probes. Recently, mesoporous silicas have been found to be good sensing materials to anchor the sensitive probes and organic adsorbing groups for Hg2+ ions due to their large surface areas, well-defined pore structures, and tunable pore sizes.7–9Since the first discovery of M41S silica in the 1990s,10 mesoporous materials have been widely used in the techniques of adsorption, separation, and sensors.11–15 The unique structure of mesoporous materials provides possibilities to tailor the functions of materials by combining them with different components.16–21 Recently, the integration of mesoporous silica with fluorescent molecules or other coordinate groups has been proved to have high performance for the detection and adsorption of Hg2+.22–25 Meanwhile, some magnetic mesoporous microspheres consisting of a magnetic core and a mesoporous shell have also been prepared to remove the metal ions from aqueous solution in an external magnetic field.26–37 However, most of these reported mesoporous materials can only realize parts of the process in the treating of heavy metal ions due to their limited functional components. In addition, the mesoporous materials that can simultaneously detect and recover Hg2+ in aqueous solution are very rare.38,39
Recently, we have prepared a novel, “all-in-one”, core-shell, mesoporous, microspheres with a magnetic core and fluorescent coordinate receptor immobilized in the porous shell. The fabrication of these hybrid materials is very simple, and the materials used are inexpensive. Interestingly, we found that this kind of material exhibits high performance for detection, adsorption and removal of a heavy metal ion from aqueous solution. In comparison to single molecular probe-based sensors or bare mesoporous silica (without modification), these functional microspheres have three different components, the Rhodamine B derivative, the mesoporous silica shell and the Fe3O4 core, which endow the materials with three different functions, detection, adsorption and removal, at the same time. The Rhodamine B derivative acts as a selective and sensitive probe for specific detection of Hg2+ (the detection limit is 10 ppb), while the unique mesoporous shell provides the necessary conditions for facile diffusion of analytes to the sensing elements and the large surface area considerably enhances the interaction sites between analyte molecules and sensing elements, and thereby further improves the adsorptive capacity. Finally, the Fe3O4 cores of the multifunctional materials are easily pulled by an external magnetic field from a small magnet, so the microspheres with Hg2+ are quickly removed and the three functions from an organic molecule, porous silica and metal oxide are effectively combined in a single microsphere.
Experimental
Materials
Rhodamine B, trimethoxy[3-(oxiranylmethoxy)propyl]-silane, ethyl orthosilicate (TEOS), cetyltrimethylammonium bromide (CTAB), polyethylene glycol, ferric trichloride, anhydrous sodium acetate, ammonium nitrate, ammonia solution (25 wt%), hydrazine hydrate (85%), ethylene glycol, toluene and acetonitrile were purchased from Beijing Chemical Reagents Company and used without further purification.Synthesis of Fe3O4 nanoparticles
The magnetic particles were prepared by a solvothermal reaction. FeCl3·6H2O (1.35 g, 5 mmol) was dissolved in ethylene glycol (40 mL) to form a transparent, yellow solution, followed by the addition of anhydrous sodium acetate (3.6 g) and polyethylene glycol (1.00 g). The mixture was stirred vigorously for 30 min, then transferred into a Teflon-lined stainless-steel autoclave (50 mL capacity) and sealed to heat at 200 °C. After reaction for 8 h, the autoclave was allowed to cool to room temperature. The black products were washed 6 times with ethanol, and then dried under vacuum at 60 °C for 12 h.Synthesis of Fe3O4@mSiO2 magnetic mesoporous microspheres (Fe-MM)
The core-shell Fe3O4@mSiO2 magnetic mesoporous microspheres were prepared according to the previously reported method.26First, Fe3O4 nanoparticles (0.10 g) were treated with 0.1 M HCl aqueous solution (50 mL) by ultrasonication. After treatment for 10 min, the magnetite particles were separated and washed with deionised water, and then homogeneously dispersed in a mixture of ethanol (80 mL), deionised water (20 mL) and concentrated ammonia solution (1.0 mL, 25 wt%), followed by the addition of ethyl orthosilicate (TEOS, 0.03 g, 0.144 mmol). After stirring at room temperature for 6 h, the products were separated, by use of a magnet, and washed with ethanol and water.
Second, the microspheres were dispersed in a mixed solution containing cetyltrimethylammonium bromide (CTAB 0.30 g, 0.823 mmol), deionised water (80 mL), concentrated ammonia solution (1.00 g, 25 wt%) and ethanol (60 mL). The mixed solution was homogenized for 0.5 h to form a uniform dispersion. TEOS (0.40 g 1.90 mmol) was added dropwise to the dispersion with continuous stirring. After reaction for 6 h, the product was collected with a magnet and washed repeated with ethanol and water to remove non-magnetic byproducts, and then the vacuum dried at 60 °C.
Third, the exchange of surfactant molecules was carried out using an alcoholic solution of ammonium nitrate. The microspheres (1.00 g) were dispersed in ethanol (150 mL, 95%) containing NH4NO3 (0.30 g), and the mixture was stirred at 60 °C for 15 min. Solids were recovered by filtration and washed with cold ethanol, and the above treatment could be repeated twice.
Synthesis of RB-hydrazine
To a 100 mL flask, Rhodamine B (1.20 g, 2.5 mmol) was dissolved in 30 mL ethanol. 3.0 mL (excess) hydrazine hydrate (85%) was then added, dropwise with vigorous stirring at room temperature. After the addition, the stirred mixture was heated to reflux in an air bath for 2 h. The solution changed from dark purple to light orange and became transparent. Then, the mixture was cooled and the solvent was removed under reduced pressure. 1 M HCl was added to the solid in the flask to generate a transparent red solution. After that, 1 M NaOH was added slowly with stirring until the pH of the solution reached 9–10. The resulting precipitate was filtered and washed 3 times with water. After drying under an IR light, the reaction afforded 0.73 g of a pink solid.Synthesis of Fe3O4@mSiO2 microspheres epoxy silicon (Fe-MME)
To a 50 mL flask, the purified Fe-MM (1.00 g) with trimethoxy[3-(oxiranylmethoxy)propyl]-silane (0.350 g, 1.5 mmol) in dry toluene (30 mL) was refluxed for 24 h. The product was collected with a magnet and repeatedly washed with toluene, and then vacuum dried at 100 °C.Synthesis of multifunctional mesoporous microspheres (MMMs)
Fe-MME (1.00 g) was allowed to react with RB-hydrazine (1.00 g, 2.2 mmol) in toluene (15 mL) at reflux for 10 h. The product was collected with a magnet and repeatedly washed with toluene, and then vacuum dried at 100 °C.The MMMs were dissolved in a 30 mL aqueous solution of MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), the stirred mixture was heated at reflux for 2 h. The above treatment could be repeated three times in order to remove the excess RB-hydrazine. The final product was produced in 0.60 g yield.
1, v/v), the stirred mixture was heated at reflux for 2 h. The above treatment could be repeated three times in order to remove the excess RB-hydrazine. The final product was produced in 0.60 g yield.
Preparation of the fluorometric metal ion titration solution
Stock solutions (1 × 10−2 M) of the aqueous (MeCN–H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) perchlorate salts of Na+, Mg2+, Mn2+, Co2+, Ni2+, Zn2+, Cd2+, Ag+, Pb2+, Cu2+ and Hg2+ were prepared. The suspension solution of MMMs (0.02 g L−1) was prepared in acetonitrile aqueous solution (MeCN–H2O 1
1 v/v) perchlorate salts of Na+, Mg2+, Mn2+, Co2+, Ni2+, Zn2+, Cd2+, Ag+, Pb2+, Cu2+ and Hg2+ were prepared. The suspension solution of MMMs (0.02 g L−1) was prepared in acetonitrile aqueous solution (MeCN–H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). Each time, a 2 mL suspension solution of MMMs was added to a quartz cuvette of 1 cm optical path length, and different stock solutions of cations were gradually added into the quartz cuvette by micro-syringe addition.
1 v/v). Each time, a 2 mL suspension solution of MMMs was added to a quartz cuvette of 1 cm optical path length, and different stock solutions of cations were gradually added into the quartz cuvette by micro-syringe addition.
Characterization
FT-IR spectra (4000–500 cm−1) in KBr were collected on a Nicolet Avatar 360 FT-IR spectrometer. The multifunctional microspheres were mixed with KBr and pressed to a thin disc for FT-IR detection. X-Ray diffraction (XRD) patterns of the prepared magnetic, mesoporous silica were obtained at room temperature on a Rigaku D/MAX-2400 X-ray powder diffraction (Japan) using Cu Kα radiation, operating at 40 kV and 10 mA. The nitrogen adsorption and desorption isotherms were measured at 77 K using an ASAP 2010 analyzer (Micromeritics Co. Ltd.). Before measurement, samples were degassed under vacuum at 373 K for 4 h. Surface areas were calculated by the Brunauer–Emmett–Teller (BET) method, and the pore volume and pore size distributions were calculated using the Barrett–Joyner–Halenda (BJH) model. The fluorescence emission measurements were carried out using a fluorescence spectrometer (Perkin-Elmer, LS55). Both excitation and emission slit widths were 10 nm. The emission data were collected in the region of 560 to 700 nm. The scanning electron microscopy (SEM) images were taken with a JEOL JSM-6700F field emission scanning electron microscope (FESEM, 20 kV). Magnetic characterization was carried out using a VSM with fields up to 10000 Oe and at a temperature of 300 K. Related ion concentration was collected on a solaar 969 atomic absorption spectrometer. All the spectroscopic measurements were performed in at least triplicate.Results and discussion
Fabrication of multifunctional core-shell mesoporous microspheres
The synthetic strategy is shown in Scheme 1. Fe3O4 nanoparticles, which have a mean diameter of about 200 nm (Fig. 1a, 1c), were synthesized according to the reported procedure.40–42 According to the FE-SEM and HRTEM images, the final Fe-MMs show spherical shapes and the diameter increases to 300–450 nm due to the addition of the mesoporous silica shell (Fig. 1b). A typical core-shell structure with a Fe3O4 magnetic core and with two distinct silica layers is clearly shown in the TEM images (Fig. 1c, 1d). The inner shell is non-porous silica, and the outer shell is porous silica with nanoscale channels (Fig. 1d). The thickness of the mesoporous silica layer is about 90–100 nm and the inner shell is about 25 nm thick and uniform. These silica shells could also make the magnetic core stable and provide a carrier for organic recognition molecules. | ||
| Scheme 1 The synthesis scheme of multifunctional mesoporous microspheres. | ||
 | ||
| Fig. 1 The SEM images of (a) Fe3O4 nanoparticles and (b) Fe-MM and the HRTEM images (c and d) of Fe-MM. | ||
The N2 adsorption-desorption isotherms (Fig. 2) is identified as type IV according to the IUPAC classification. The pore size is 2.5 nm calculated from the desorption branch of the nitrogen isotherm with BJH model (inset of Fig. 2), indicating a uniform mesopore. The surface area and desorption cumulative volume of pores of Fe-MM are 600 m2 g−1 and 0.35 cm3 g−1 respectively calculated by the Brunauer–Emmett–Teller (BET) model. Because of the large surface area and pore size of Fe-MM, metal ions can freely diffuse and move in the larger meso-channels, combine with the fluorescent receptor and be readily adsorbed. The wide-angle XRD patterns (Fig. 3a) of MMMs show that the microspheres have similar diffraction peaks to that of the Fe3O4 nanoparticles, and therefore suggest that the magnetic cores were well retained in the silica matrix. Consistent with the BET results, an X-ray diffraction pattern shows only one diffraction peak at 2θ = 2.39° (Fig. 3b), which further confirms the existence of a mesoporous structure in MMMs, the broad diffraction peak is probably caused by the thinness and poor order of the mesoporous silica layer.
 | ||
| Fig. 2 N2 adsorption-desorption isotherms and the mesopore size distribution curve (inset) of Fe-MM. | ||
 | ||
| Fig. 3 (a) The wide-angle XRD patterns of Fe3O4 nanoparticles and MMMs, and (b) the low-angle XRD pattern of MMMs. | ||
Magnetic characterization was achieved using a magnetometer at 300 K. The saturation magnetization values are 64.1 emu g−1 and 27.5 emu g−1 for Fe3O4 nanoparticles and Fe-MM, respectively. The magnetization curves (Fig. 4a) show no hysteresis loop and no remanence is detected for all of the samples. The magnified hysteresis loops further confirmed the superparamagnetism of the particles.43 The Fe-MM dispersed in water showed a fast separating process in 30 s when a magnet was placed near the glass bottle (Fig. 4b). This suggests that the Fe-MM can be easily separated from the mixture by adding an external magnetic field. The incorporation of the epoxy silicon RB-hydrazine moieties into the synthesized magnetic mesoporous microspheres was proved by FT-IR (see the ESI, Figs S1 and S2‡) and fluorescent spectroscopy (Fig. 5). The core-shell mesoporous spheres show an obvious fluorescent increase at 580 nm, indicating that the fluorescent probe had successfully planted in the mesoporous silica shell. The content of fluorescent groups in MMMs was about 0.036 mmol g−1, estimated by elemental analysis results.
 | ||
Fig. 4 (a) The magnetic hysteresis loops of Fe3O4 nanoparticles (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ) and Fe-MM ( ) and Fe-MM (![[dash dot, graph caption]](https://www.rsc.org/images/entities/char_e090.gif) ), and (b) photographs of an aqueous suspension of MMMs (left) and after magnetic capture within 30 s (right). ), and (b) photographs of an aqueous suspension of MMMs (left) and after magnetic capture within 30 s (right). | ||
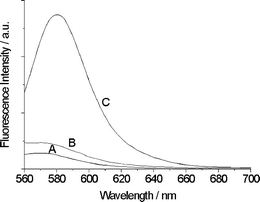 | ||
| Fig. 5 The fluorescence spectra of Fe-MM (A), MMMs (B) and MMMs + Hg2+ (C) in aqueous solution. Excitation was at 540 nm, emission was monitored at 580 nm. | ||
Sensing properties of multifunctional core-shell mesoporous microspheres
The toxicity of Hg2+, even at very low concentration, has been recognized as a problem of primary concern because it can easily pass through skin and damage the central nervous and endocrine systems. Therefore, developing new methods for analyzing Hg2+in vitro and in vivo is very important. RB and its derivants are well-known fluorescent probes in organic chemistry for detecting heavy metal ions, such as Hg2+.44–48 All spectroscopic measurements of MMMs were performed in aqueous solution (MeCN–H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). After the addition of Hg2+ ions, the fluorescence intensity increased dramatically, as shown in Fig. 6. The fluorescence intensity enhancement could be attributed to the Rhodamine group (Scheme 2). Rhodamine B is a spirolactam, which is nearly non-fluorescent. However, when Hg2+ ions were added, a delocalized xanthene moiety of the Rhodamine B group was generated, which produced a strong fluorescence.49,50
1 v/v). After the addition of Hg2+ ions, the fluorescence intensity increased dramatically, as shown in Fig. 6. The fluorescence intensity enhancement could be attributed to the Rhodamine group (Scheme 2). Rhodamine B is a spirolactam, which is nearly non-fluorescent. However, when Hg2+ ions were added, a delocalized xanthene moiety of the Rhodamine B group was generated, which produced a strong fluorescence.49,50
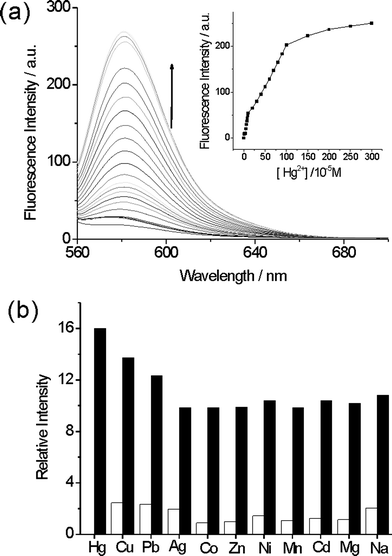 | ||
| Fig. 6 (a) Fluorescence spectra and the fluorescence titration profile (inset) of MMMs (0.02 g L−1) with increasing an concentration of Hg2+ ions in aqueous solution, and (b) the fluorescence intensity of MMMs (0.02 g L−1, 2 mL) in the presence of various interfering ions (white bars), and coexistence with Hg2+ ions (black bars) in aqueous solution. Excitation was at 540 nm, emission was monitored at 580 nm. | ||
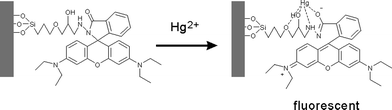 | ||
| Scheme 2 A possible proposed binding mode of MMMs with Hg2+. | ||
To obtain an excellent fluorescence sensor, high selectivity is a matter of necessity. Stock solutions (1 × 10−2 M) of the aqueous perchlorate salts of Na+, Mg2+, Mn2+, Co2+, Ni2+, Zn2+, Cd2+, Ag+, Pb2+, Cu2+ and Hg2+ ions were prepared. The suspension solutions of MMMs (0.02 g L−1) were prepared in aqueous solution. Fig. 6b illustrates the fluorescence responses of MMMs to various metal ions and its selectivity for Hg2+ ions. Interfering ions show no significant effects on fluorescence spectra. The addition of Hg2+ ions results in a prominent enhancement of the fluorescence at 580 nm, which indicates that the MMMs exhibit high selectivity for Hg2+ ions and the detection limit of Hg2+ is as low as 10 ppb. The special selectivity of sensing units to Hg2+ is probably due to several combined influences cooperating to achieve the unique selectivity for the Hg2+ ion, such as the suitable coordination geometry conformation of the receptor, the larger radius of the Hg2+ ion, the nitrogen-affinity of Hg2+ ions, and the amide deprotonation ability of the Hg2+ ion.51
The confocal fluorescence images of MMMs in solution before and after the addition of Hg2+ provide further evidence for the detection and adsorption process. MMMs (0.02 g L−1) showed a weak fluorescence in aqueous solution (Fig. 5a). After addition of Hg2+ ions, the particles exhibited a strong red fluorescence under the same test conditions (Fig. 7b). The red fluorescence MMMs with black background (Fig. 7) also proves that the fluorescent molecules, which will give a red background, didn't leak into the aqueous solution. The Hg2+-RB derivatives were firmly linked to the pore channel of the magnetic mesoporous microspheres by covalent bonds and will not create extra pollution during the sensing process. These results indicate that the MMMs could be used to detect Hg2+in vivo, especially in human tissue.
 | ||
| Fig. 7 Confocal fluorescence images of (a) MMMs before and (b) after the addition of Hg2+ ions in aqueous solution. | ||
Adsorptive capacities of multifunctional, core-shell, mesoporous microspheres
Almost all current Hg2+ sensors can only detect the heavy metal ions, but not remove them from solution. In this work, we endow the MMMs with adsorptive and magnetic separable properties to remove the Hg2+ ions from aqueous solution. The adsorption process was well described by the Langmuir adsorption isotherm equation.The adsorption equilibrium uptake capacity for MMMs was calculated according to mass balance on the Hg2+ ion expressed as
 | (1) |
In order to optimize the use of adsorbents, it is important to establish the most appropriate adsorption isotherm.52,53
 | (2) |
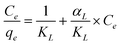 | (3) |
In a typical experiment, the MMMs (5 mg) were added to an aqueous solution containing different concentrations of Hg2+ ions and were easily and thoroughly removed by a magnet. The concentration of Hg2+ ions left in aqueous solution was determined by standard atomic absorption spectroscopy.
In addition to the selective sensory properties, the new adsorbent shows a very fast adsorption rate. The equilibrium can be established within five minutes (insert of Fig. 8a), and this may be mainly caused by the porous structure of MMMs and strong affinity of the fluorescent receptor towards Hg2+ ions. The adsorption data were analyzed according to the linear form of the Langmuir equation (Fig. 8b). The monolayer adsorption capacity was 21.05 mg Hg2+ ions per gram of adsorbent MMMs (Table 1). Since the density of fluorescent receptor (0.036 mmol g−1) is lower than the Hg2+ ions that were adsorbed (0.1 mmol g−1), the sorption may be attributed to both the fluorescent ligands and the bare silica surface. The concentration of Hg2+ ions in the solution can be reduced to less than 0.05 ppm (below the detection limit of an atomic spectrum) by repetitive treatment using MMMs. This predicts that the sensitive MMMs may be a useful and effective sorbent for the in situ removal of trace Hg2+ from aqueous environments, such as human blood and some living bio-systems.
 | ||
| Fig. 8 An adsorption isotherm, kinetics (the inset) (a) and Langmuir isotherm lines plot (b) for the adsorption of Hg2+ onto MMMs at 25 °C. | ||
| Metal ion | KL/L g−1 | αL/L mg−1 | R 2 | Q 0/mg g−1 |
|---|---|---|---|---|
| Hg2+ | 44.5236 | 2.1149 | 0.9995 | 21.0526 |
Conclusions
In summary, a novel, “all-in-one”, multifunctional mesoporous sphere was successfully fabricated. The spheres have a core-shell structure with a magnetic Fe3O4 core and two kinds of shell. One is a non-porous inner silica shell and the other is a mesoporous outer shell with a fluorescent probe in it. Because of the superparamagnetic core (Fe3O4) and fluorescent receptor (RB) immobilized by covalent bonding, the MMMs (multifunctional mesoporous microspheres) exhibit high selectivity for detecting Hg2+ in aqueous solution, and effective adsorption (adsorption capacity is 21.05 mg g−1) and fast removal of this kind of ion (in 30s). The explored method can be expected to perform the detection and recovery procedure at the same time for dangerous chemicals in aqueous, ambient systems, especially in human blood and living tissue due to the straightforward synthetic strategy, the economical starting materials and the stable metal oxide core and organic elements.Acknowledgements
This work was supported by the National Science Foundation of China (20903018) and special fund of State Key Joint Laboratory of Environment Simulation and Pollution Control. (09K10ESPCT)Notes and references
- A. Renzoni, F. Zino and E. Franchi, Environ. Res., 1998, 77, 68 CrossRef CAS.
- R. Von Burg, J. Appl. Toxicol., 1995, 15, 483 CrossRef CAS.
- Mercury Update: Impact on Fish Advisories. EPA Fact Sheet EPA-823-F-01-011; EPA, Office of Water: Washington, DC, 2001 Search PubMed.
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS and referance there in.
- A. B. Othman, J. W. Lee, J. S. Wu, J. S. Kim, R. Abidi, P. Thuéry, J. M. Strub, A. V. Dorsselaer and J. Vicens, J. Org. Chem., 2007, 72, 7634 CrossRef CAS.
- X. F. Guo, X. H. Qian and L. H. Jia, J. Am. Chem. Soc., 2004, 126, 2272 CrossRef CAS.
- X. Feng, G. E. Fryxell, L. Q. Wang, A. Y. Kim, J. Liu and K. M. Kemner, Science, 1997, 276, 923 CrossRef CAS.
- D. Y. Zhao, Q. S. Huo, J. L. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- J. L. Shi, Z. L. Hua and L. X. Zhang, J. Mater. Chem., 2004, 14, 795 RSC.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- M. Liong, S. Angelos, E. Choi, K. Patel, J. F. Stoddart and J. I. Zink, J. Mater. Chem., 2009, 19, 6251 RSC.
- M. C. Burleigh, S. Dai, E. W. Hagaman and J. S. Lin, Chem. Mater., 2001, 13, 2537 CrossRef CAS.
- S. Giri, B. G. Trewyn, M. P. Stellmaker and V. S. Y. Lin, Angew. Chem., Int. Ed., 2005, 44, 5038 CrossRef CAS.
- C. P. Tsai, C. Y. Chen, Y. Hung, F. H. Chang and C. Y. Mou, J. Mater. Chem., 2009, 19, 5737 RSC.
- B. González, M. Colilla, C. L. de Laorden and M. Vallet-Regí, J. Mater. Chem., 2009, 19, 9012 RSC.
- F. Hoffmann, M. Cornelius, J. Morell and M. Fröba, Angew. Chem., Int. Ed., 2006, 45, 3216 CrossRef.
- W. S. Han, H. Y. Lee, S. H. Jung, S. J. Lee and J. H. Jung, Chem. Soc. Rev., 2009, 38, 1904 RSC.
- B. J. Melde, B. J. Johnson and P. T. Charles, Sensors, 2008, 8, 5202 CrossRef CAS.
- L. Gao, Y. Wang, J. Q. Wang, L. Huang, L. Y. Shi, X. X. Fan, Z. G. Zou, T. Yu, M. Zhu and Z. S. Li, Inorg. Chem., 2006, 45, 6844 CrossRef CAS.
- L. L. Li, H. Sun, C. J. Fang, J. Xu, J. Y. Jin and C. H. Yan, J. Mater. Chem., 2007, 17, 4492 RSC.
- E. J. Cho, J. K. Kang and J. H. Jung, Mater. Lett., 2007, 61, 5157 CrossRef CAS.
- S. J. Lee, J. E. Lee, J. Seo, I. Y. Jeong, S. S. Lee and J. H. Jung, Adv. Funct. Mater., 2007, 17, 3441 CrossRef CAS.
- M. H. Lee, S. J. Lee, J. H. Jung, H. Lim and J. S. Kim, Tetrahedron, 2007, 63, 12087 CrossRef CAS.
- P. Zhou, Q. T. Meng, G. J. He, H. M. Wu, C. Y. Duan and X. Quan, J. Environ. Monit., 2009, 11, 648 RSC.
- L. X. Zhang, W. H. Zhang, J. L. Shi, Z. L. Hua, Y. S. Li and J. N. Yan, Chem. Commun., 2003, 210 RSC.
- Y. H. Deng, D. W. Qi, C. H. Deng, X. M. Zhang and D. Y. Zhao, J. Am. Chem. Soc., 2008, 130, 28 CrossRef CAS.
- Y. S. Lin, S. H. Wu, Y. Hung, Y. H. Chou, C. Chang, M. L. Lin, C. P. Tsai and C. Y. Mou, Chem. Mater., 2006, 18, 5170 CrossRef CAS.
- J. Kim, J. E. Lee, J. Lee, J. H. Yu, B. C. Kim, K. An, Y. Hwang, C. Shin, J. Park, J. Kim and T. Hyeon, J. Am. Chem. Soc., 2006, 128, 688 CrossRef CAS.
- X. Q. Xu, C. H. Deng, M. X. Gao, W. J. Yu, P. Y. Yang and X. M. Zhang, Adv. Mater., 2006, 18, 3289 CrossRef CAS.
- H. Y. Lee, D. R. Bae, J. C. Park, H. Song, W. S. Han and J. H. Jung, Angew. Chem., Int. Ed., 2009, 48, 1239 CrossRef CAS.
- W. R. Zhao, J. L. Gu, L. X. Zhang, H. R. Chen and J. L. Shi, J. Am. Chem. Soc., 2005, 127, 8916 CrossRef CAS.
- K. S. Lee, M. H. Woo, H. S. Kim, E. Y. Lee and I. S. Lee, Chem. Commun., 2009, 3780 RSC.
- A. Abou-Hassan, R. Bazzi and V. Cabuil, Angew. Chem., 2009, 121, 7316 CrossRef.
- X. H. Guo, Y. H. Deng, D. Gu, R. C. Che and D. Y. Zhao, J. Mater. Chem., 2009, 19, 6706 RSC.
- L. M. Guo, X. Z. Cui, Y. S. Li, Q. J. He, L. X. Zhang, W. B. Bu and J. L. Shi, Chem.–Asian J., 2009, 4, 1480 CrossRef CAS.
- C. Fang and M. Q. Zhang, J. Mater. Chem., 2009, 19, 6258 RSC.
- Z. C. Zhang, L. M. Zhang, L. Chen, L. G. Chen and Q. H. Wan, Biotechnol. Prog., 2006, 22, 514 CrossRef CAS.
- J. H. Gao, H. W. Gu and B. Xu, Acc. Chem. Res., 2009, 42, 1097 CrossRef CAS.
- J. V. Ros-Lis, R. Casasús, M. Comes, C. Coll, M. D. Marcos, R. Martínez-Máñez, F. Sancenón, J. Soto, P. Amorós, J. E. Haskouri, N. Garró and K. Rurack, Chem.–Eur. J., 2008, 14, 8267 CrossRef CAS.
- J. Liu, Z. K. Sun, Y. H. Deng, Y. Zou, C. Y. Li, X. H. Guo, L. Q. Xiong, Y. Gao, F. Y. Li and D. Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 5875 CrossRef CAS.
- H. Deng, X. L. Li, Q. Peng, X. Wang, J. P. Chen and Y. D. Li, Angew. Chem., Int. Ed., 2005, 44, 2782 CrossRef CAS.
- S. H. Sun, H. Zeng, D. B. Robinson, S. Raoux, P. M. Rice, S. X. Wang and G. X. Li, J. Am. Chem. Soc., 2004, 126, 273 CrossRef CAS.
- C. P. Bean, J. Appl. Phys., 1959, 30, 120.
- V. Dujols, F. Ford and A. W. Czarnik, J. Am. Chem. Soc., 1997, 119, 7386 CrossRef CAS.
- H. Yang, Z. G. Zhou, K. W. Huang, M. X. Yu, F. Y. Li, T. Yi and C. H. Huang, Org. Lett., 2007, 9, 4729 CrossRef CAS.
- J. Y. Kwon, Y. J. Jang, Y. J. Lee, K. M. Kim, M. S. Seo, W. Nam and J. Yoon, J. Am. Chem. Soc., 2005, 127, 10107 CrossRef CAS.
- H. Zheng, Z. H. Qian, L. Xu, F. F. Yuan, L. D. Lan and J. G. Xu, Org. Lett., 2006, 8, 859 CrossRef CAS.
- X. Zhang, Y. Shiraishi and T. Hirai, Tetrahedron Lett., 2007, 48, 5455 CrossRef CAS.
- M. J. Yuan, W. D. Zhou, X. F. Liu, M. Zhu, J. B. Li, X. D. Yin, H. Y. Zheng, Z. C. Zuo, C. B. Ouyang, H. B. Liu, Y. L. Li and D. B. Zhu, J. Org. Chem., 2008, 73, 5008 CrossRef CAS.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC.
- D. Y. Wu, W. Huang, C. Y. Duan, Z. H. Lin and Q. J. Meng, Inorg. Chem., 2007, 46, 1538 CrossRef CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361 CrossRef CAS.
- O. Redlich and D. L. Peterson, J. Phys. Chem., 1959, 63, 1024 CrossRef CAS.
Footnotes |
| † This paper is part of a Journal of Materials Chemistry themed issue on advanced materials in water treatments. Guest editors: Dongyuan Zhao, Benjamin S. Hsiao and Mietek Jaroniec. |
| ‡ Electronic Supplementary Information (ESI) available: Figs. S1 and S2. See DOI: 10.1039/c000315h/ |
| This journal is © The Royal Society of Chemistry 2010 |
