In vitro and in vivo release of albumin from an electrostatically crosslinked in situ-forming gel†
Ju Young
Lee
a,
Yun Mi
Kang
a,
E Sle
Kim
a,
Mi Lan
Kang
a,
Bong
Lee
b,
Jae Ho
Kim
a,
Byoung Hyun
Min
ac,
Kinam
Park
d and
Moon Suk
Kim
*a
aDepartment of Molecular Science and Technology, Ajou University, Suwon, 443-749, Korea. E-mail: moonskim@ajou.ac.kr; Fax: +82-31-219-3931; Tel: +82-31-219-2608
bDepartment of Polymer Engineering, Pukyong National University, 100, Yongdang Dong, Nam Ku, Busan, 608-739, Korea
cDepartment of Orthopedic Surgery, Ajou University, Suwon, 443-749, Korea
dDepartment of Biomedical Engineering and Pharmaceutics, Purdue University, 206 S. Intramural Drive, West Lafayette, IN 47907-1791, USA
First published on 1st March 2010
Abstract
Delivery systems capable of maintaining a sustained release of protein drugs at specific sites can potentially circumvent problems of toxicity and subtherapeutic local dosing levels associated with systemic administration. Here, we used bovine serum albumin (BSA) as a test protein to explore the potential utility of an in situ-forming gel consisting of sodium carboxymethylcellulose (CMC) and polyethyleneimine (PEI) as a depot for protein drugs. BSA-FITC-loaded CMC/PEI solutions were easily prepared and remained liquid at room temperature. When these solutions were subcutaneously injected into rats, they immediately gelled, forming an electrostatically crosslinked three-dimensional network structure that showed sustained release of BSA-FITC for 15 days in vitro and in vivo. No BSA-FITC remained in CMC/PEI gels after this time, indicating complete release of protein cargo. The sustained release of BSA-FITC was also monitored by real-time molecular imaging, which showed that BSA-FITC bioavailability in BSA-FITC-loaded CMC/PEI gels was more than twice that of BSA-FITC-only solutions. CMC/PEI gels provoked only a modest inflammatory response. Collectively, our results show that the CMC/PEI gel described here could serve as a minimally invasive therapeutics depot with numerous benefits compared to orally or intravenously administered drugs.
1. Introduction
Therapeutic protein drugs have been administered in the form of oral pills or intravenous injections.1,2 However, the administration of protein drugs via these routes presents a number of potential disadvantages, including unacceptably high or low plasma concentrations that may lead to toxicity, or failure to achieve therapeutic doses, respectively.3 Thus, there is a need for administration systems that do not suffer from these drawbacks.Considerable recent attention has focused on systems capable of local release of protein drugs as a means for delivering such drugs for predefined periods. Such a system has the potential to overcome the shortcomings of conventional protein drug formulations, improving both the therapeutic effects of administered drugs and the quality-of-life of patients.4–6 One controlled drug delivery system that may be ideal for local delivery of protein drugs is in situ-forming hydrogels.7–10 Various in situ gel-forming natural materials, including collagen, hyaluronate, cellulose, and fibrin, as well as synthetic materials such as pluronic and various block copolymers, have been proposed for this purpose.11–14
In situ-forming hydrogels form spontaneously or in response to certain biological triggers.15–17 One stimulus that lends itself to therapeutic applications is temperature. Use of in situ-forming hydrogels with a phase transition at a near-physiological temperature enables pharmaceutical formulations to be easily prepared in the solution state at low temperature by simply mixing with protein. When these protein hydrogel solutions were injected by syringe at the target location, these protein-hydrogel solutions form a protein-loaded gel that acts as a depot for sustained delivery of the protein drug.
The triggers that stimulate gelation of in situ-forming hydrogels at a certain temperature fall into two main categories: electrostatic (ionic) and hydrophobic interactions.18 There are a number of examples of in situ-forming hydrogel systems for protein drug delivery that are based on electrostatic interactions.19–21 Electrostatically mediated gelation appears to be governed by molecular interactions that occur in aqueous solutions of cationic and anionic polyelectrolytes, in which anions typically act as points of electrostatic interaction with cations. Recent studies have shown that a mixture of anionic and cationic polymers is capable of forming a gel in situ.19–21
Sodium carboxymethylcellulose (CMC), which is widely used as an ingredient in conventional drug formulation, is the most common organic polymer used for this purpose. The sources of CMC are considered virtually inexhaustible; thus, CMC is a raw material capable of satisfying the increasing demand for environmentally friendly and biocompatible products.22 CMC possesses a carboxylic acid moiety as a polyanionic group located in conformationally semi-rigid CMC chains. The anions of CMC may act as points of electrostatic interaction with cations. Polyethyleneimine (PEI), a polycation, is one of the most extensively utilized biomaterials. Like CMC, it is widely available, and is also inexpensive and easy to functionalize.23 Recently, we reported that CMC and PEI solutions exhibited phase transition in the physiological temperature range, and suggested that CMC might be crosslinked through bridges formed by electrostatic interactions between the carboxylic groups of the CMC chains and the amine groups of PEI.24 We observed a substantial change in the particle size and viscosity of PEI-containing CMC solutions as a function of temperatures and the formation of an infinite gel-network structure via the electrostatic crosslinking of CMC-PEI at body temperature (Fig. 1). In addition, the size of formed gel decreased with increasing in vivo implantation time due to the destruction of a gel-network structure via the electrostatic exchange interaction with biological cationic compounds, implying the disappearance of gel after a certain period.
 | ||
| Fig. 1 Schematic image of CMC/PEI gel formation via electrostatic crosslinking between carboxylate groups in CMC and amine groups in PEI. | ||
One of the aims of our work is to develop biocompatible materials capable of serving as depots for protein drugs. To the best of our knowledge, few previous studies have examined in situ-forming CMC/PEI gels as in vivo drug depots. In this study, we prepared bovine serum albumin-fluorescein isothiocyanate (BSA-FITC)-loaded CMC/PEI gels. Our first set of objectives was to examine the in vitro release of BSA-FITC as a model protein, and to evaluate the formation of drug depots in vivo by administering BSA-FITC-loaded CMC/PEI gels subcutaneously to rats. Our second aim was to assess the in vivo BSA-FITC release profile. Finally, we sought to characterize the host tissue response to determine whether BSA-FITC–loaded CMC/PEI gels could be used for sustained in vivo protein delivery.
2. Experimental
2.1 Preparation of CMC and PEI solutions
A 3 wt% CMC solution was prepared by dissolving CMC (low viscosity, Sigma, Steinheim, Germany) in deionized water. A 76 wt% PEI solution was prepared by dissolving 400 mg PEI (MW = 423; Sigma, Steinheim, Germany) in deionized water and then quaternarizing using a 1 N HCl solution. Experimental mixtures were prepared by adding the PEI solution to the CMC solution to yield a 10 wt% concentration of PEI in CMC. The final liquid solution was clear and homogeneous.2.2 In vitro BSA-FITC release
One millilitre solutions of CMC/PEI solutions containing BSA-FITC (0.5, 1 and 2 mg ml−1) were prepared in 5 mL vials, and then incubated at 37 °C for 1 h. Then, 4 mL of PBS incubated at 37 °C was added to each gel to start the in vitro release experiment as time 0. The vial was shaken at 100 rpm and 37 °C to determine the release amount. At specified times, a 1 mL sample of the solution was collected and 1 mL of fresh PBS at 37 °C was added to the vial. The removed sample was immediately analyzed by fluorescence spectroscopy (F-4500, Hitachi, Tokyo, Japan). The amount of cumulatively released BSA was calculated by reference to a standard calibration curve prepared by measuring the fluorescence of solutions containing known concentrations of BSA–FITC in PBS. Three independent release experiments were performed for each gel composition.2.3 In vivo gel formation and protein release
The in vivo gel formation behavior of CMC/PEI solutions was tested by injecting the CMC/PEI solutions into rats. Twenty-four 8-week-old Sprague-Dawley rats (320–350 g), divided into two groups (BSA-FITC-loaded CMC/PEI gel for twelve rats and BSA-FITC solution only for twelve rats), were used in the release tests. The rats were housed in sterilized cages, provided sterile food and water and filtered air, and handled in a laminar flow hood following aseptic techniques. All animals were treated in accordance with the Ajou University School of Medicine Animal Care Guidelines. Six formulation types of BSA-FITC-loaded CMC/PEI solution and BSA–FITC only solution (containing 0.5, 1 and 2 mg mL−1 BSA-FITC) were individually injected at four rats for each formulation for the in vivo release experiments. Within 30 min of adding BSA-FITC to CMC/PEI solutions, 1 mL of the solution was injected subcutaneously (1 cm3 syringe, 26 gauge needle) into the dorsum of a rat anesthetized with ethyl ether.For the in vivo detection of BSA-FITC, an aliquot of blood was drawn from the tail vein of each rat at specified times. A 0.3 mL aliquot of blood from the catheterized tail vein was collected into a microfuge tube, mixed with 0.2 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 499 mixture of heparin and saline, and vortexed. Plasma was obtained by centrifuging the blood solution at 10
499 mixture of heparin and saline, and vortexed. Plasma was obtained by centrifuging the blood solution at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at room temperature. Distilled water (100 μL), 66 mM EDTA (300 μL) and 50 mM HEPES, pH 7.4 (400 μL) were added to plasma, and the resulting samples were stored frozen at −20 °C until assayed. To analyze the state of the probe and to assess the reliability of the method, we recorded the fluorescence spectra of solutions of BSA-FITC in plasma containing known concentrations of BSA-FITC, using an excitation wavelength of 490 nm with a bandwidth of 2.5 nm, an emission wavelength of 525 nm with a bandwidth of 2.5 nm, and a response time of 2 s. The amount of BSA-FITC cumulatively released by BSA-FITC-loaded CMC/PEI gels was calculated by reference to standard calibration curves prepared with known concentrations of BSA-FITC. The release experiment was performed separately on each of the four rats in each group, and the results were averaged.
000 rpm for 5 min at room temperature. Distilled water (100 μL), 66 mM EDTA (300 μL) and 50 mM HEPES, pH 7.4 (400 μL) were added to plasma, and the resulting samples were stored frozen at −20 °C until assayed. To analyze the state of the probe and to assess the reliability of the method, we recorded the fluorescence spectra of solutions of BSA-FITC in plasma containing known concentrations of BSA-FITC, using an excitation wavelength of 490 nm with a bandwidth of 2.5 nm, an emission wavelength of 525 nm with a bandwidth of 2.5 nm, and a response time of 2 s. The amount of BSA-FITC cumulatively released by BSA-FITC-loaded CMC/PEI gels was calculated by reference to standard calibration curves prepared with known concentrations of BSA-FITC. The release experiment was performed separately on each of the four rats in each group, and the results were averaged.
The amount of BSA-FITC remaining at predetermined times was measured in individual gels removed from sacrificed rats. Gels were first dissolved in distilled water (5 mL), then filtered. The amount of remaining BSA-FITC was calculated by reference to standard calibration curves prepared with known concentrations of BSA-FITC in deionized water.
2.4 In vivo fluorescence imaging
A CMC/PEI solution containing 1 mg ml−1 BSA-FITC was prepared and injected subcutaneously into the left dorsum of a 6-week-old male nude mouse (anesthetized with ethyl ether) using a 26 gauge needle. At selected times, side-view images of the mouse were collected at a wavelength of 515 nm (excitation wavelength, 470 nm) using a fluorescence imaging system (FO ILLUM PL-800, Edmund Optics, NJ, USA, 150 W EKE Quartz Halogen light, glass reference number OG515 filter). After digitization using a charge-coupled device (CCD), fluorescence images were visualized with the Axiovision Rel. 4.8 software.2.5 Histological analysis
At 15 days after implantation, the rats were sacrificed and the implants were individually dissected and removed from the subcutaneous dorsum. The tissues were immediately fixed with 10% formalin and embedded in paraffin. The embedded specimens were sectioned (4 μm) along the longitudinal axis of the implant, and sections were stained with hematoxylin and eosin (H&E), DAPI (4′,6-diamino-2-phenylindole dihydrochloride, Sigma, MO, USA) and mouse anti-rat CD68 antibody (ED1; Serotec, Oxfordshire, UK). The staining procedures for DAPI and ED1 were as follows. The slides were washed with PBS-T (0.05% Tween-20 in PBS), and blocked with 5% BSA (Roche, Germany) and 5% horse serum (Gibco Invitrogen, New York, NY, USA) in PBS for 1 h at 37 °C. The sections were then incubated overnight at 4 °C with ED1 antibodies, washed with PBS-T, and then incubated with the secondary antibody (rat anti-mouse Alexa Fluor®594; Invitrogen) for 3 h at room temperature in the dark. The slides were washed again with PBS-T, counterstained with DAPI and then mounted with fluorescent mounting solution (DAKO, Glostrup, Denmark). Immunofluorescence images were visualized with an Axio Imager A1 (Carl Zeiss Microimaging GmbH, Gottingen, Germany) equipped with the Axiovision Rel. 4.8 software (Carl Zeiss Microimaging GmbH, Gottingen, Germany).2.6 Scanning electron microscopy of in vivo gels
The morphology of in vivo-formed gels was examined by scanning electron microscopy (SEM) using a Tescan VEGA II series SEM (Dortmund, Germany). Immediately after removing from the rat, the gel sample was mounted on a metal stub pre-cooled in liquid nitrogen. After mounting the gel, the metal stub was quickly immersed in a liquid nitrogen bath to minimize alterations of the gel. The stub was then freeze-dried at −75 °C using a freeze dryer, coated with a thin layer of gold using a plasma-sputtering apparatus (Emitech, K575, Kent, UK) under an argon atmosphere, and examined by SEM.2.7 Statistical analysis
The areas under individual plasma concentration–time curves for a release time period (AUC0−t) were calculated. Relative bioavailabilities were determined by dividing the AUC value for each gel-administered value by the mean AUC value for a BSA-FITC-only solution administered into the subcutaneous dorsum of rats. Statistical analyses were performed using Student's t-test.3. Results and discussion
3.1 Phase transition of CMC/PEI
In the present work, we used BSA as a test protein to examine the ability of an in situ-forming gel, created at the injection site by PEI-mediated gelation of a CMC solution containing BSA, to serve as a localized protein drug depot. Our previous work observed that the CMC solutions containing 10 wt% PEI became gels above 36 °C.24 On the basis of these results, we chose a CMC solution containing 10 wt% PEI as being most likely to form an instantaneous gel depot after subcutaneous (s.c.) injection into rats (body temperature, 38 °C). Consistent with this expectation, a CMC/PEI (90/10) solution containing 10 wt% PEI formed a clear solution with a viscosity of 1 cP at room temperature (Fig. 2a), and gelled at body temperature (Fig. 2b), exhibiting a viscosity of 3.5 × 103 cP. These results indicate that the CMC/PEI (90/10) solution can be expected to exist as a liquid at room temperature and form a gel at body temperature.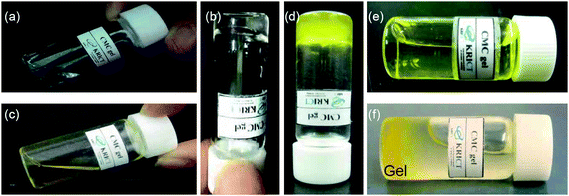 | ||
| Fig. 2 Images of CMC/PEI at room temperature without (a) and with (c) BSA-FITC; CMC/PEI gel at 37 °C without (b) and with (d) BSA-FITC; and CMC/PEI after addition of 37 °C PBS at room temperature (e) and at 37 °C (f). (c–f) The yellow colors are due to original color of the BSA-FITC. | ||
3.2 Gel maintenance and BSA release in vitro
The CMC/PEI (90/10) solution at 25 °C was immersed in a water bath at 37 °C and monitored for transition to gel by tilting. The typical time of gel formation at 37 °C was approximately ∼3 min. To examine the potential of CMC/PEI solutions as an in situ-forming gel depot, we prepared CMC/PEI (90/10) solutions containing different BSA-FITC concentrations (0.5, 1 and 2 mg mL−1). The BSA-FITC-loaded CMC/PEI solutions were easily prepared, and were liquid at room temperature (Fig. 2c for 1 mg mL−1 BSA-FITC) but gelled at body temperature (Fig. 2d). To test the persistence of the gel structure in an aqueous environment, we added a PBS solution (the temperature of PBS solution was 37 °C) to BSA-FITC-loaded CMC/PEI. As the PBS-added CMC/PEI gel kept at room temperature, the gel became a solution below 3 min (Fig. 2e). By contrast, as the PBS-added CMC/PEI gel kept at 37 °C, it maintained the gel form for 2 weeks even after the addition of PBS (Fig. 2f).If a gel is to be successfully used as a depot for a protein drug, it should exhibit a desirable drug release profile under physiological conditions. Thus, we tested BSA-FITC release from CMC/PEI gels loaded with 0.5, 1 and 2 mg mL−1 BSA-FITC in vitro, for 9 days. At each of the different BSA-FITC concentrations, the cumulative amount of BSA-FITC released from the CMC/PEI gel was greater than 90% at 9 days (Fig. 3). Importantly, there was no initial burst of BSA-FITC release at any tested BSA-FITC concentration, and the release pattern was linear in each case. This indicates that the CMC/PEI gel was able to maintain structural integrity at body temperature over an extended period, and gradually released loaded protein.
 | ||
| Fig. 3 Cumulative amount of BSA-FITC released over 9 days in vitro from CMC/PEI gels containing different concentrations of BSA-FITC (0.5, 1 and 2 mg mL−1). | ||
3.3 In vivo gelation
We next injected a CMC/PEI (90/10) solution into SD rats to test its utility as an in vivo drug depot (Fig. 4a). The CMC/PEI solution gelled in the rat almost simultaneously with s.c. injection (Fig. 4b–d). The resulting in situ-formed gel was allowed to develop in vivo and was biopsied after implantation. The injected CMC/PEI gel maintained shape at the injection site for the full experimental observation period. Following removal of the gel at 15 days (Fig. 4e), a faint distribution of fluorescence was observed around the gel (Fig. 4f). These results indicate that the BSA-FITC-loaded CMC/PEI gels maintained sufficient structural integrity to act as a drug depot in vivo, and suggest that BSA-FITC was released at the injection site. | ||
| Fig. 4 Subcutaneous injection of CMC/PEI solution (a), formed gel (b,c) and gels following removal from a rat after 1 day (d) and 15 days (e); fluorescence image at 15 days (f). | ||
3.4 Morphology of the in vivo-formed gel
To investigate the morphology of in vivo formed gels, we removed a gel formed from CMC/PEI from one rat 15 days after s.c. injection. The gel was frozen in liquid nitrogen, freeze-dried, and observed by SEM (Fig. 5). SEM micrographs of CMC/PEI gel cross-sections showed that the gel possessed an interconnected pore structure characterized by large fissures among the laminae. The CMC/PEI gel formed in vivo showed a structure sufficiently porous to not only allow biological medium to diffuse into and out of the gel, but also to enable BSA to pass out of the gel. These observations provide further support for in vivo-formed CMC/PEI gels as candidate in vivo protein drug depots. | ||
| Fig. 5 SEM micrographs showing the morphology of an in situ-formed CMC/PEI gel after 15 days in vivo. Magnification: (a) ×50, (b) ×200, (c) ×500 and (d) ×1000. | ||
3.5 In vivo release
To determine in vivo BSA-FITC release, we prepared BSA-FITC-only solutions and BSA-FITC-loaded CMC/PEI gels using different concentrations of BSA-FITC (0.5, 1 and 2 mg mL−1), and injected them into rats. BSA-FITC released from BSA-FITC-loaded CMC/PEI gels in vivo, as a function of time, was monitored by measuring plasma BSA-FITC concentrations using fluorescence spectroscopy. Fig. 6 shows a plot of the plasma BSA-FITC concentration versus time. | ||
| Fig. 6 Time course of BSA-FITC concentration in plasma over 18 days after s.c. injection of (a) BSA-FITC-only solution and (b) CMC/PEI loaded with 0.5 mg mL−1 (–■–), 1 mg ml−1 (–●–), or 2 mg ml−1 (–▲–) BSA-FITC. | ||
In rats injected with the BSA-FITC-only solution, plasma BSA-FITC concentrations reached a maximum 3–6 h after s.c. injection (depending on dosage) and then rapidly declined, approaching zero after 2 days. In contrast, plasma BSA-FITC concentrations in rats injected with a BSA-FITC-loaded CMC/PEI solution reached a maximum at 12–16 h (the levels were also dose-dependent), but exhibited a sustained-release profile, producing detectable levels of BSA-FITC in plasma for up to 16 days. A larger dose of BSA-FITC produced a greater, more sustained, release.
The Cmax and Tmax values were calculated from Fig. 6, and absolute bioavailability data are summarized in Table 1. AUC0−t values were calculated by measuring the area under the plasma BSA-FITC curves from t0 to time t using the trapezoidal rule, and the relative bioavailability of BSA-FITC was determined from the plasma concentration profiles. There were significant differences in Cmax, Tmax, and AUC between the BSA-FITC-loaded CMC/PEI gel and the BSA-FITC-only solution formulations. The Tmax and AUC0−t values of the BSA-FITC-loaded CMC/PEI gel were significantly higher than those of the BSA-FITC-only solution. In addition, the BSA-FITC-loaded CMC/PEI gel had a lower average Cmax than did the BSA-FITC-only solution. The Tmax for BSA-FITC-loaded CMC/PEI gels was more than three times greater than that of the BSA-FITC-only solution, and the Cmax was more than two times lower.
| Formulation | BSA-FITC concentration/mg ml−1 | T max/h | C max | AUC0−t (μg ml−1 day) | Relative Bioavailabilitya (%) | BSA-FITC amount remaining in gel (%) |
|---|---|---|---|---|---|---|
| a Relative bioavailability (%) = [(AUC value for each gel administration)/(mean AUC value for BSA solution only administration)] × 100. b P < 0.05 vs. BSA-FITC solution only. | ||||||
| CMC/PEI gel | 0.5 | 12 | 1.5 ± 0.2 | 4.0 ± 0.7 | 197.5 ± 0.3b | 0.047 ± 0.01 |
| 1 | 12 | 2.9 ± 0.4 | 7.5 ± 1.0 | 185.4 ± 0.3b | 0.024 ± 0.002 | |
| 2 | 18 | 5.4 ± 1.8 | 11.8 ± 1.2 | 188.1 ± 0.2b | 0.013 ± 0.001 | |
| BSA solution only | 0.5 | 3 | 3.2 ± 0.7 | 2.0 ± 0.3 | 100 | — |
| 1 | 6 | 6.4 ± 1.1 | 4.0 ± 0.8 | |||
| 2 | 6 | 8.4 ± 0.6 | 6.3 ± 0.3 |
For BSA-FITC-loaded CMC/PEI gels containing 0.5, 1, and 2 mg mL−1 BSA-FITC, the AUC0−t values were 4.0 ± 0.7, 7.5 ± 1.0 and 11.8 ± 1.2 μg mL−1, respectively. The relative bioavailability of BSA-FITC from CMC/PEI gels loaded with 0.5, 1, and 2 mg mL−1 BSA-FITC was approximately 198%, 185%, and 188% of the AUC0−t for the BSA-FITC-only solution at these same BSA-FITC concentrations (p < 0.05). An assessment of BSA-FITC content in biopsied gels after 15 days showed that only about 0.5% of the original BSA-FITC content remained inside the injected BSA-FITC-loaded CMC/PEI gels. This result indicates that BSA-FITC-loaded CMC/PEI gels were capable of sustained and effective release of BSA-FITC and, thus, increased the relative bioavailability, consistent with previous reports which demonstrated the sustained-release property of various drugs on hydrogel.25–27
3.6 In vivo fluorescence imaging
We used real-time molecular imaging, which has been shown to be effective for assessing in vivo drug release processes in live animals,28 to evaluate in vivo BSA-FITC release. Fluorescent images were acquired from a nude mouse after s.c. injection of a BSA-FITC-loaded CMC/PEI solution (Fig. 7). At the moment of s.c. injection, high levels of green fluorescence were observed at the injection site (Fig. 7a). The area of fluorescence increased by diffusion thereafter, reaching maximum plateau values 12–24 h after administration (Fig. 7b–e). The image area decreased after 24 h, at which time fluorescence could be detected only at the initial injection site. After this time, both the intensity and area of fluorescence gradually decreased (Fig. 7f–h). Negligible fluorescence was observed after 13 days, and none was evident after 15 days (Fig. 7i). This result is in good agreement with the observation that BSA-FITC release was sustained after administration of BSA-FITC-loaded CMC/PEI gels (Fig. 6) and the fact that the amount of BSA-FITC remaining in the gel after 15 days was almost negligible (Table 1). In addition to showing effective release and diffusion of loaded protein, our results lend support to the use of molecular imaging as an effective tool for non-invasive in vivo protein monitoring. | ||
| Fig. 7 In vivo fluorescent image of a nude mouse injected with BSA-FITC-loaded CMC/PEI, taken (a) 2 min, (b) 60 min, (c) 6 h, (d) 12 h, (e) 1 day, (f) 4 days, (g) 8 days, (h) 13 days and (i) 15 days after initial s.c. injection. (Line represents 1 cm). | ||
3.7 Host tissue response
BSA-FITC-loaded CMC/PEI gels injected into rats could be easily identified and isolated from the surrounding tissue. To assess the local biocompatibility of the CMC/PEI gel, we examined tissues surrounding the area into which the CMC/PEI gel had been transplanted. H&E-stained histological sections of harvested CMC/PEI gels showed that, after 15 days, the gel contained a few macrophages, neutrophils, and/or lymphocytes (Fig. 8). The extent of host cell infiltration and inflammatory cell accumulation within and near the transplanted CMC/PEI gel was characterized by staining tissue with the ED1 antibody and the nuclear stain, DAPI (Fig. 9). Positive staining by the ED1 antibody, which recognizes the macrophage marker CD68, is considered a unique in vivo indicator of an inflammatory response. DAPI staining (blue) showed many host cells surrounding the CMC/PEI gel, and ED1 staining (red) showed macrophages at the CMC/PEI gel and in surrounding tissues after 15 days. This response was less pronounced than that to the FDA-approved biomaterial, poly(lactic-co-glycolic acid) (PLGA),29 which promotes a substantial infiltration of macrophages and neutrophils.30 Collectively, these results highlight the value of in situ-formed CMC/PEI gels, showing that BSA-loaded CMC/PEI gels not only effectively release loaded protein, but are also biocompatible, and are therefore likely to successfully serve as drug depots. | ||
| Fig. 8 H&E-stained section of in situ-formed CMC/PEI gel after 15 days in vivo. Magnification: (a) ×100 and (b) ×200. Yellow, red and green dots represent macrophages, neutrophils and lymphocytes, respectively. | ||
 | ||
| Fig. 9 ED1 staining inside (a) and at the edge (b) of a CMC/PEI gel after 15 days in vivo. Magnification: ×200. | ||
4. Conclusion
Here, we explored the potential utility of in situ-forming CMC/PEI gels as a drug depot. We showed that BSA-loaded CMC/PEI solutions at room temperature gelled almost immediately upon s.c. injection into rats. We also demonstrated sustained release of BSA-FITC from the CMC/PEI gel both in vitro and in vivo over extended experimental periods. The present findings show that a CMC/PEI gel maintains structural integrity under physiological conditions and can act as an injectable drug depot. Thus, our in situ gel-forming CMC/PEI system may provide numerous benefits as a minimally invasive therapeutics depot and as a useful experimental platform for testing the sustained in vivo pharmacological performance of protein drugs.Acknowledgements
This study was supported by grants from KMOHW (A050082), National Research Foundation of Korea (NRF) pioneer grant (M10711060001-08M1106-00110), and Priority Research Centers Program through NRF funded by the Ministry of Education, Science and Technology (2009-0093826).References
- T. M. Allen and P. R. Cullis, Science, 2004, 303, 1818–1822 CrossRef CAS.
- K. Sonaje, Y. H. Lin, J. H. Juang, S. P. Wey, C. T. Chen and H. W. Sung, Biomaterials, 2009, 30, 2329–2339 CrossRef CAS.
- V. Gebbia and C. Puozzo, Expert Opin. Drug Saf., 2005, 4, 915–928 Search PubMed.
- P. P. Wang, J. Frazier and H. Brem, Adv. Drug Delivery Rev., 2002, 54, 987–1013 CrossRef CAS.
- Y. K. Joung and K. D. Park, Tissue Eng. Regen. Med., 2008, 5, 156–164 Search PubMed.
- B. Li, J. M. Davidson and S. A. Guelcher, Biomaterials, 2009, 30, 3486–3494 CrossRef CAS.
- H. Hyun, Y. H. Kim, I. B. Song, J. W. Lee, M. S. Kim, G. Khang, K. Park and H. B. Lee, Biomacromolecules, 2007, 8, 1093–1100 CrossRef CAS.
- L. Klouda, M. C. Hacker, J. D. Kretlow and A. G. Mikos, Biomaterials, 2009, 30, 4558–4566 CrossRef CAS.
- J. Wang, Z. Wang, J. Gao, L. Wang, Z. Yang, D. Kong and Z. Yang, J. Mater. Chem., 2009, 19, 7892–7896 RSC.
- S. Sakai, K. Hirose, K. Taguchi, Y. Ogushi and K. Kawakami, Biomaterials, 2009, 30, 3371–3377 CrossRef CAS.
- C. Chung, I. E. Erickson, R. L. Mauck and J. A. Burdick, Tissue Eng. A, 2008, 14, 1121–1131 Search PubMed.
- C. Chang, J. Peng, L. Zhang and D. W. Pang, J. Mater. Chem., 2009, 19, 7771–7776 RSC.
- K. S. Kim, H. H. Ahn, J. H. Lee, J. Y. Lee, B. Lee, H. B. Lee and M. S. Kim, Biomaterials, 2008, 29, 4420–4428 CrossRef CAS.
- C. J. Chun, S. M. Lee, S. Y. Kim, H. K. Yang and S. C. Song, Biomaterials, 2009, 30, 2349–2360 CrossRef CAS.
- Y. L. Chiu, S. C. Chen, C. J. Su, C. W. Hsiao, Y. M. Chen, H. L. Chen and H. W. Sung, Biomaterials, 2009, 30, 4877–4888 CrossRef CAS.
- H. Wei, S. X. Cheng, X. Z. Zhang and R. X. Zhuo, Prog. Polym. Sci., 2009, 34, 893–910 CrossRef CAS.
- A. K. Bajpai, S. K. Shukla, S. Bhanu and S. Kankane, Prog. Polym. Sci., 2008, 33, 1088–1118 CrossRef CAS.
- J. Kopeček, Biomaterials, 2007, 28, 5185–5192 CrossRef CAS.
- H. Izawa, Y. Kaneko and J. Kadokawa, J. Mater. Chem., 2009, 19, 6969–6972 RSC.
- N. Kashyap, B. Viswanad, G. Sharma, V. Bhardwaj, P. Ramarao and M. N. Ravi Kumar, Biomaterials, 2007, 28, 2051–2060 CrossRef CAS.
- M. George and T. E. Abraham, J. Controlled Release, 2006, 114, 1–14 CrossRef CAS.
- K. G. Satyanarayana, G. G. C. Arizaga and F. Wypych, Prog. Polym. Sci., 2009, 34, 982–1021 CrossRef CAS.
- B. Demeneix and J. P. Behr, Adv. Genet., 2005, 53, 217–230 Search PubMed.
- K. S. Kim, J. Y. Lee, Y. M. Kang, E. S. Kim, B. Lee, H. J. Chun, J. H. Kim, B. H. Min, H. B. Lee and M. S. Kim, Tissue Eng. A, 2009, 15, 3201–3209 Search PubMed.
- T. P. Johnston, M. A. Punjabi and C. J. Froelich, Pharm. Res., 1992, 9, 425–434 CrossRef CAS.
- J. M. Barichello, M. Morishita and K. Takayama, Int. J. Pharm., 1999, 184, 189–198 CrossRef CAS.
- Y. Liu, W. Lu, J. Wang, X. Zhang, H. Zhang, X. Wang, T. Zhou and Q. Zhang, J. Controlled Release, 2007, 117, 387–395 CrossRef CAS.
- S. Liu, B. Jia, R. Qiao, Z. Yang, Z. Yu, Z. Liu, K. Liu, J. Shi, H. Ouyang, F. Wang and M. Gao, Mol. Pharmaceutics, 2009, 6, 1074–1082 CrossRef CAS.
- H. Kranz, N. Ubrich, P. Maincent and R. Bodmeier, J. Pharm. Sci., 2000, 89, 1558–1566 CrossRef CAS.
- M. S. Kim, H. H. Ahn, M. H. Cho, Y. N. Shin, G. Khang and H. B. Lee, Biomaterials, 2007, 28, 5137–5143 CrossRef CAS.
Footnote |
| † Ju Young Lee and Yun Mi Kang are equal first authors. |
| This journal is © The Royal Society of Chemistry 2010 |
