A fast sample preparation procedure for mercury speciation in hair samples by high-performance liquid chromatography coupled to ICP-MS
Samuel S.
de Souza
,
Jairo L.
Rodrigues
,
Vanessa C.
de Oliveira Souza
and
Fernando
Barbosa Jr.
*
Laboratório de Toxicologia e Essencialidade de Metais, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, SP, Brazil. E-mail: fbarbosa@fcfrp.usp.br; Fax: +55-16-36024701; Tel: +55-16-36024701
First published on 2nd November 2009
Abstract
A simple method for mercury speciation in hair samples with a fast sample preparation procedure using high-performance liquid chromatography coupled to inductively coupled plasma mass spectrometry is proposed. Prior to analysis, 50 mg of hair samples were accurately weighed into 15 mL conical tubes. Then, an extractant solution containing mercaptoethanol, L-cysteine and HCl was added to the samples following sonication for 10 min. Quantitative mercury extraction was achieved with the proposed procedure. Separation of inorganic mercury (Ino-Hg), methylmercury (Met-Hg) and ethylmercury (Et-Hg) was accomplished in less than 8 min on a C18 reverse phase column with a mobile phase containing 0.05% v/v mercaptoethanol, 0.4% m/v L-cysteine, 0.06 mol L−1 ammonium acetate and 5% v/v methanol. The method detection limits were found to be 15 ng g−1, 10 ng g−1 and 38 ng g−1, for inorganic mercury, methylmercury and ethylmercury, respectively. Sample throughput is 4 samples h−1 (duplicate). A considerable improvement in the time of analysis was achieved when compared to other published methods. Method accuracy is traceable to Certified Reference Materials (CRMs) 85 and 86 human hair from the International Atomic Energy Agency (IAEA). Finally, the proposed method was successfully applied to the speciation of mercury in hair samples collected from fish-eating communities of the Brazilian Amazon.
Introduction
Mercury (Hg) is one of the most hazardous pollutants in the environment. Mercury exists in three basic forms: elemental mercury (Hg0), known as metallic mercury, inorganic mercury compounds (Ino-Hg), primarily mercuric chloride, and organic mercury, primarily methylmercury (Met-Hg).1 Organic forms are more toxic than inorganic.1The main source of human exposure to organic mercury, mainly in the form of methylmercury (Met-Hg), is the consumption of fish or seafood.1 On the other hand, the most common forms of exposure to inorganic forms are by inhalation of Hg vapor released from dental amalgams or from gold mining activities.
Measurements of Hg in both blood and hair are used to determine whether adverse health effects are likely to occur. The contents of mercury species in hair may represent a cumulative exposure from the daily diet and/or occupational environment exposure. The total mercury concentration (THg) in hair is often used as a proxy measure of Met-Hg exposure in individuals eating fish based on the assumption that Ino-Hg exposure, and therefore Ino-Hg concentration in hair, is much lower. However, the proportion of Met-Hg in hair may vary among individuals. This makes it essential to have analytical methods which can differentiate between chemical forms in hair to diagnose risks of toxicity.2
Speciation analysis of clinical samples is gradually becoming more widely accepted for nutritional and/or toxicological purposes. According to IUPAC, speciation analysis is defined as the analytical process of identifying and/or measuring quantities of one or more individual chemical forms in a sample, and speciation of an element is defined as the distribution of an element among defined chemical species in a system.3 However, the majority of analytical methods only distinguish between the inorganic and total Hg contents, with the arithmetical difference being assigned to “organic” mercury content.4 Such methodology is not strictly speaking speciation; it is more correctly described as fractionation.
Recommended sample preparation procedures for the speciation of mercury in hair usually include very tedious and time-consuming digestion and/or extraction procedures.5–8 Moreover, artifact formation during the sample preparation step is a potential source of error. For example, Liang and Lazoff,9 performing the analysis for Met-Hg, reported that Hg artifacts were formed in the alkaline digestion step. The transformation of Hg species during sample pre-treatment was also reported by Qvarnstrom and Frech.10 Up to 11.5% Hg2+ was methylated, and up to 6.26% Met-Hg was demethylated, after spikes were added to biological samples. According to these authors, the methylation of Hg2+ takes place mainly during and after the pH adjustment.10 On the other hand, clinical laboratories must cope with an increasing demand for trace element analysis in body fluids and tissues in response to increasing concern for occupational and environmental exposure to mercury. Thus, fast sample preparation procedures with minimal handling are extremely desirable in routine analysis.
The aim of this paper was therefore to evaluate a simple method for mercury speciation in hair by high-performance liquid chromatography coupled to inductively coupled mass spectrometry (ICP-MS) with a fast sample preparation procedure prior to analysis.
Experimental
Instruments and apparatus
All measurements were made with an ICP-MS (Elan DRC II PerkinElmer, Norwalk, CT).A Perkin Elmer model L-200 LC pump, six-port injector (Rheodyne 9725) with a reverse-phase column (C18, 5 μm, 150 mm × 4 mm) and a pre-column RP18 (7 μm, 15 × 3.2 mm) comprised the LC system. Samples were loaded with a syringe into a 100 μL sample loop. All separations were performed at room temperature under isocratic conditions (1 min equilibrium, 9 min for separation and 1 min for cleaning). The isocratic mobile phase was 0.05% v/v mercaptoethanol, 0.4% m/v L-cysteine, 0.06 mol L−1 ammonium acetate and 5% v/v methanol. The flow rate was 1.0 mL min−1. The effluent from the LC column was directly connected to the nebulizer with PEEK tubing (1.59 mm o.d.) and a low dead volume PEEK connector. Data evaluation was performed using Chromera® software supplied with the instrument, and quantification was based on peak height by external calibration.
The optimum experimental conditions for both ICP-MS and LC are given in Table 1.
| LC conditions | |
|---|---|
| Column | C18 (5 μm, 150 mm × 4 mm) |
| Pre-column | RP18 (7 μm, 15 × 3.2 mm) |
| 0.05% v/v Mercaptoethanol | |
| Mobile phase | 0.4% m/v L-cysteine |
| 0.06 mol L−1 Ammonium acetate | |
| 5% v/v Methanol | |
| Mobile phase flow rate | 1 mL min−1 |
| Sample loop | 100 μL |
| Measurement | Peak height |
| ICP-MS experimental conditions | |
|---|---|
| Radio frequency power/W | 1200 |
| Scan Mode | Peak hopping |
| Nebulizer gas flow/L min−1 | 0.58 |
| Resolution/amu | 0.7 |
| Replicates | 3 |
| Isotopes | 202Hg |
Reagents
All reagents used were of analytical grade and the solutions were prepared using high-purity water with a resistivity of 18.2 MΩ cm, obtained from a Milli-Q Plus water purification system (Millipore, Bedford, MA, USA). 37% Hydrochloric acid (Merck, Darmstadt, Germany) was doubly distilled in a quartz sub-boiling apparatus (Kürner Analysentechnik, Rosenheim, Germany).A clean laboratory and laminar-flow hood capable of producing class 100 were used for preparing solutions and samples. All solutions were stored in high-density polyethylene bottles. Plastic bottles and glassware materials were cleaned by soaking in 10% (v/v) HNO3 for 24 h, rinsed five times with Milli-Q water and dried in a class 100 laminar flow hood before use. All operations were performed on a clean bench.
A 10 mg L−1 standard solution of inorganic mercury was obtained from Perkin-Elmer (PerkinElmer, Norwalk, CT) A 1000 mg L−1 standard solution of methylmercury chloride (CH3HgCl) and a 1000 mg L−1 standard solution of ethylmercury chloride (CH3CH2HgCl) in water were obtained from Alfa Aesar. Analytical calibration standards of mercury species were prepared daily over the range of 0.0–20.0 μg L−1 for the LC-ICP-MS method by suitable serial dilutions of the stock solution in the mobile phase.
Additional chemicals were HPLC grade methanol (99.9% v/v) and mercaptoethanol (Sigma-Aldrich, USA), L-cysteine (Fluka, Japan). Ammonium acetate (99.99%) was obtained from Aldrich Chemical Company (Milwaukee, USA).
Sample preparation
The hair sample was initially cut into uniform 1.0 cm lengths, following cutting in small sizes with a clean electric hair clipper and no additional measurement of the final particle size was done. Then, ground samples (50 mg) were placed in 15 mL polypropylene test tubes with 10 mL of a solution containing 0.10% v/v HCl + 0.05% m/v L-cysteine + 0.10% v/v 2-mercaptoethanol for hair of volunteers and for CRM 085. For the CRM 086, the dilution was to 5 mL with the same diluent. The tubes were then sonicated for 10 min in an ultrasonic bath 1400 A (UNIQUE, Brazil). The resulting supernatant was then filtered through 0.20 μm Nylon® filters (Millipore, USA). Sample extraction was performed in triplicate and extraction blanks were prepared in the same manner.Standard reference materials and human hair specimens
In order to verify the accuracy and precision of the proposed method, Certified Reference Materials (CRMs) 085 and 086 human hair were purchased from the International Atomic Energy Agency (IAEA), Vienna, Austria. Human hair specimens (n = 5) collected as part of another research study involving Met-Hg exposure in the Brazilian Amazon region (fish-eating communities) were available for Hg speciation. Identifiers were removed. These specimens had been obtained with informed consent from human subjects in accordance with procedures approved by our Institutional Review Board.Results and discussion
Preliminary experiments
Different procedures have been proposed for the extraction of mercury species in biological samples for speciation purposes based on HPLC-ICP-MS or GC-ICP-MS.5,6,8,11–18 In general, protocols are based on acid6,8 or basic extractions11,18 media. However, most of these methodologies have several disadvantages when coupling with HPLC-ICP-MS. Firstly, as far as a compatible pH value for the reverse phase column is concerned, a laborious procedure usually has to be adopted to adjust an appropriate pH of the extracted solution prior to injection into the HPLC. Secondly, Hg species transformation might occur during sample preparation.9,10In order to avoid the aforementioned limitations, alternative extraction procedures have been suggested with reagents containing thiol ligands, such as mercaptoethanol,5,12L-cysteine5,16 or thiourea.19 These procedures are associated with the use of microwave energy.17 Alternatively, quantitative extractions of mercury and other elements from hair samples have been demonstrated even in low acid conditions when associated with ultrasound energy.20,21
The method of extraction evaluated here is based in part on a previous method described by Chiou et al.5 for Hg speciation in fish samples. In that method, the authors suggested the use of an extraction solution containing L-cysteine and 2-mercaptoethanol in combination with microwave radiation. Thus, our preliminary experiments were carried out to explore the efficiency of using mercaptoethanol and L-cysteine for quantitative mercury extraction from hair samples with two basic differences from the method proposed by Chiou et al.5 Firstly, we used ultrasound energy instead of microwave energy. Secondly, we also evaluated the use of a dilute solution of HCl (0.10% v/v) in the extractant to accelerate the ultrasound extraction without a major change in the pH. The use of an ultrasound bath simplifies the method, since this system is much simpler and less expensive than commercial microwave systems.
For this experiment, four different extractor solutions were evaluated for Hg extraction in the IAEA 085 Human Hair CRM: A- (0.10% v/v HCl); B- (0.05% m/v L-cysteine); C- (0.10% v/v 2-mercaptoethanol); D- (0.10% v/v HCl + 0.05% m/v L-cysteine + 0.10% v/v 2-mercaptoethanol). Ultrasound time was fixed at 10 min.
The results of this study are presented in Fig. 1. As can be observed, quantitative extraction of Hg (>95%) was observed for the 0.10% v/v HCl, 0.05% m/v L-cysteine, 0.10% v/v 2-mercaptoethanol mixture. For the subsequent experiments, therefore, this solution was used for the Hg species extraction from hair samples.
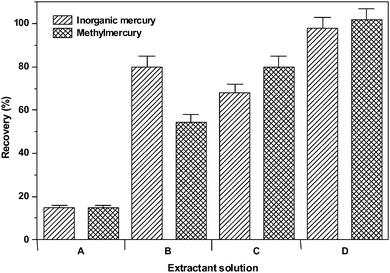 | ||
| Fig. 1 Effect of the extractant solution composition on recovery of mercury species from CRM IAEA 085. A- (0.10% v/v HCl); B- (0.05% m/v L-cysteine); C- (0.10% v/v 2-mercaptoethanol); D- (0.10% v/v HCl + 0.05% m/v L-cysteine + 0.10% v/v 2-mercaptoethanol). | ||
Optimization of LC operating conditions
After the optimization of mercury extraction from hair samples, we optimized the mobile phase composition. Different combinations of reagents in the mobile phase are usually recommended for the speciation of Hg in biological samples by HPLC-ICP-MS. Some authors recommend the use of L-cysteine and mercaptoethanol5 while others recommend methanol, mercaptoethanol and ammonium acetate6 or a mixture of L-cysteine, pyridine and methanol.14Our preliminary experiments demonstrated more promising results (time of separation, resolution, selectivity and sensitivity) for the mixture of mercaptoethanol, L-cysteine, ammonium acetate and methanol. According to Chiou et al.,5 the retention time of mercury species increases with the increase in mercaptoethanol concentration in the mobile phase. We have observed the same results (data not shown). Thus, we fixed the mercaptoethanol concentration at 0.05% v/v as a compromise between selectivity and time of analysis. Ammonium acetate was also fixed at 0.06 mol L−1. This concentration is able to maintain a favorable pH at 6.7. On the other hand, different concentrations of methanol in the mobile phase were evaluated. For this study, the concentrations of ammonium acetate, L-cysteine and mercaptoethanol were fixed at 0.06 mol L−1, 0.05% m/v and 0.05% v/v, respectively, and the concentration of methanol was varied from 0.0 to 5% v/v. A considerable increase in Hg sensitivity was observed with the increase in methanol concentration. Three mechanisms have been put forward to explain the above enhancement effect on signal intensities: (1) charge transfer reaction from C+ species to analyte atoms, (2) improvement in the nebulization transport of the sample, and (3) shift of the zone of maximum ion density.22 Concentrations of methanol higher than 5% v/v were not evaluated, since they lead to plasma instability and an increase in carbon residues on cones. Thus, the methanol concentration in the mobile phase was fixed at 5% v/v for further studies.
Subsequent experiments were carried out to optimize the concentration of L-cysteine in the mobile phase. Separation of mercury species can take place based on the cysteine-mercury complexes on the polymeric-based C18 reverse-phase column. Concentrations of L-cysteine between 0.05 and 0.4% m/v were evaluated with the concentration of mercaptoethanol, ammonium acetate and methanol fixed at 0.05% v/v, 0.06 mol L−1 and 5% v/v, respectively. Results are shown in Fig. 2a and 2b. As can be seen, the higher the concentration of L-cysteine, the lower the retention time of the three mercury species and the higher the sensitivity for all mercury species. For an L-cysteine concentration of 0.4% m/v, the separation of the three mercury species is achieved in less than 8 min (Fig. 2b) compared to 35 min when 0.05% m/v L-cysteine is used in the mobile phase (Fig. 2a). Interestingly, with an L-cysteine concentration of 0.4% m/v, the elution of inorganic mercury occurs before that of methylmercury. As a result, a solution containing 0.4% m/v L-cysteine, 0.05% v/v mercaptoethanol, 0.06 mol L−1 ammonium acetate and 5% v/v methanol was used as the mobile phase.
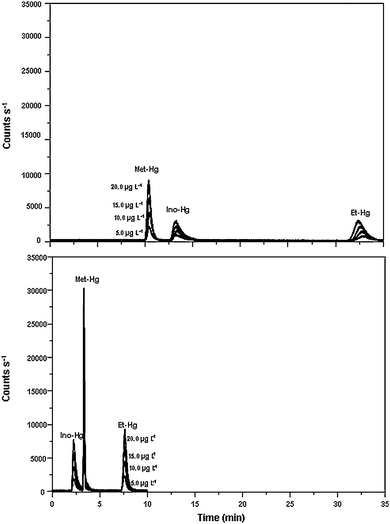 | ||
| Fig. 2 Chromatogram showing the separation of mercury species (5.0 to 20 μg L−1) with the mobile phase consisting of: a) 0.05% m/v L-cysteine, 0.05% v/v mercaptoethanol, 0.06 mol L−1 ammonium acetate and 5% v/v methanol and b) 0.4% m/v L-cysteine, 0.05% v/v mercaptoethanol, 0.06 mol L−1 ammonium acetate and 5% v/v methanol. For other conditions see Table 1. | ||
Stability of mercury species after extraction
To verify the stability of the mercury species after extraction, a time study of sample storage at room temperature was carried out. For this experiment, the CRM IAEA 86 reference material was divided into three different fractions of 50 mg. From each fraction, mercury was extracted according to the proposed procedure and left at room temperature until analysis. Then, samples were analyzed after the first hour (T0), 3 h later (T1), 8 h later (T2), 24 h later (T3) and 4 days later (T4). Our results demonstrated that mercury species present in this certified material (methylmercury and inorganic mercury) can be extracted and left at room temperature up to 4 days before analysis.Validation studies
Validation of the proposed method was accomplished using IAEA 085 and 086 Human Hair. Results for IAEA CRM samples are shown in Table 2. A paired t-test showed no significant statistical differences at a 95% confidence level between certified and found values, demonstrating the accuracy of the proposed method.| Sample | Certified values | LC-ICP-MS method | |||
|---|---|---|---|---|---|
| CRM | Met-Hg Concentration/μg g−1 | Total Concentration/μg g−1 | Ino-Hg Concentration/μg g−1 | Met-Hg Concentration/μg g−1 | Total Concentration/μg g−1 |
| a For the found values the standard deviations are based on 5 measurements. | |||||
| IAEA 85 | 22.9 ± 1.0 | 23.2 ± 0.8 | 0.110 ± 0.020 | 23.14 ± 1.65 | 23.25 |
| IAEA 86 | 0.258 ± 0.0215 | 0.513 ± 0.039 | 0.282 ± 0.035 | 0.272 ± 0.018 | 0.554 |
Method application for the speciation of mercury in hair samples collected from fish-eating communities
Human hair specimens were collected from 5 volunteers living in the Brazilian Amazon and exposed to high levels of methylmercury from fish consumption. Results are shown in Table 3. Only methylmercury and inorganic mercury were identified in these samples. Moreover, total mercury levels found with the proposed method as a sum of inorganic and methylmercury are in good agreement with Hg values found by cold vapor atomic absorption spectrometry (CV AAS).| Sample | Ino-Hg/μg g−1 | Met-Hg/μg g−1 | Et-Hg/μg g−1 | Total Hg Proposed method/μg g−1 | Total Hg [CV AAS]23/μg g−1 |
|---|---|---|---|---|---|
| 1 | 0.32 (0.01) | 3.12 (0.13) | <0.038 | 3.44 | 3.6 (0.1) |
| 2 | 0.37 (0.02) | 10.93 (0.10) | <0.038 | 11.30 | 12.5 (0.9) |
| 3 | 0.34 (0.02) | 4.54 (0.06) | <0.038 | 4.88 | 4.6 (0.2) |
| 4 | 0.39 (0.02) | 5.30 (0.02) | <0.038 | 5.69 | 5.5 (0.2) |
| 5 | 0.11 (0.01) | 2.23 (0.02) | <0.038 | 2.34 | 2.2 (0.1) |
Figures of merit
The LC-ICP-MS proposed method’s detection limit (3 SD) is 10 ng g−1, 15 ng g−1 and 38 ng g−1 for methylmercury, inorganic mercury and ethylmercury, respectively (n = 10). Typical within-day precision was always lower than 8% (CRM IAEA 086), while between-day precision was <10% RSD (CRM IAEA 086) for methylmercury and inorganic determination. Comparison of figures-of-merit between the proposed method and various published methods for Hg speciation in hair are shown in Table 4. As can be seen, considerable improvements were achieved with the proposed method.| Analytical Parameter | Proposed method | Morton et al. (2002)6 | Gibicar et al. (2007)7 | Rahman et al. (2009)8 |
|---|---|---|---|---|
| Method detection limit (ng g−1) | 10.0 (Met-Hg) | — | 5.0 (Met-Hg) | Not given |
| 15.0 (Ino-Hg) | 5.0 (Met-Hg) | 5.0 (Ino-Hg) | ||
| 38.0 (Et-Hg) | 5.0 (Ino-Hg) | 5.0 (Et-Hg) | ||
| Methodology | LC-ICP-MS | LC-ICP-MS | GC-CV-AFS | LC-ICP-MS |
| Sample mass (mg) | 50 | 100 | 20 | 200–500 |
| Dilution Factor | 100–200 | 100 | 25 | 40–100 |
| Sample preparation procedure | Sonication (10 min) | Cold digestion overnight | Alkaline dissolution for 3 h + Solvent extraction for 20 min + Derivatization for 20 min. | Microwave-assisted extraction for 10 min and mechanical shaking for 24 h. |
Conclusion
A simple method has been developed for mercury speciation in hair based on LC-ICP-MS. Sample preparation procedure is very fast and quantitative extraction of mercury is possible in 10 min. In addition, the number of handling steps, sample preparation and analysis time, as well as potential sources of analytical errors, is reduced. Finally, the method was successfully applied for the speciation of mercury in hair samples collected from volunteers exposed to methylmercury by fish consumption.Acknowledgements
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for financial support and fellowships.References
- Toxicological Profile for Mercury (update), Agency for Toxic Substances and Disease Registry (ATSDR), U.S. Department of Health and Human Services, Atlanta, GA, 1999, 617 Search PubMed.
- A. L. B. Jacob-Ferreira, C. J. S. Passos, A. A. Jordão, M. Fillion, D. Mergler, M. Lemire, R. F. Gerlach, F. Barbosa Jr and J. E. Tanus-Santos, Basic Clin. Pharmacol. Toxicol., 2009, 105, 281–288 Search PubMed.
- D. M. Templeton, F. Ariese, R. Cornelis, L. G. Danielsson, H. Muntau, H. P. Van Leeuwen and R. Lobinski, Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000), Pure Appl. Chem., 2000, 72, 1453–1470 CrossRef CAS.
- J. L. Rodrigues, D. P. Torres, V. C. de Oliveira Souza, B. L. Batista, S. S. de Souza, A. J. Curtius and F. Barbosa Jr, J. Anal. At. Spectrom., 2009, 24, 1414–1420 RSC.
- C. S. Chiou, S. J. Jiang and K. S. K. Danadurai, Spectrochim. Acta, Part B, 2001, 56, 1133–1142 CrossRef.
- J. L. Rodrigues, S. S. de Souza, V. C. O. Souza and F. Barbosa Jr, Talanta, 2009 DOI:10.1016/j.talanta.2009.09.001.
- D. Gibicar, M. Logar, N. Horvat, A. Marn-Pernat, R. Ponikvar and M. Horvat, Anal. Bioanal. Chem., 2007, 388, 329–340 CrossRef CAS.
- G. M. M. Rahman, T. Fahrenholz and H. M. S. Kingston, J. Anal. At. Spectrom., 2009, 24, 83–92 RSC.
- L. Liang and S. Lazoff, Evaluation of the procedure for alkaline digestion solvent estimation for methyl mercury artifact formation, Talanta, 1999, 48, 231–233 CrossRef CAS.
- J. Qvarnstrom and W. Frech, Mercury species transformations during sample pre-treatment of biological tissues studied by HPLC-ICP-MS, J. Anal. At. Spectrom., 2002, 17, 1486–1491 RSC.
- D. S. Vidler, R. O. Jenkins, J. F. Hall and C. F. Harrington, Appl. Organomet. Chem., 2007, 21, 303–310 CrossRef CAS.
- W. Meng, F. Weiyue, S. Junwen, Z. Fang, W. Bing, Z. Motao, L. Bai, Z. Yuliang and C. Zhifang, Talanta, 2007, 71, 2034–2039 CrossRef CAS.
- L. H. Reyes, G. M. M. Rahman, T. Fahrenholz and H. M. S. Kingston, Anal. Bioanal. Chem., 2008, 390, 2123–2132 CrossRef CAS.
- B. Vallant, R. kadnar and W. Goessler, J. Anal. At. Spectrom., 2007, 22, 322–325 RSC.
- D. S. Bushee, Analyst, 1988, 113, 1167–1170 RSC.
- S. C. Hight and J. Cheng, Anal. Chim. Acta, 2006, 567, 160–172 CrossRef CAS.
- D. Point, W. C. Davis, J. I G. Alonso, M. Monperrus, S. J. Christopher, O. F. X. Donard, P. R. Becker and S. A. Wise, Anal. Bioanal. Chem., 2007, 389, 787–798 CrossRef CAS.
- D. C. Baxter, I. Rodushkin, E. Engstrom, D. Klockare and H. Waara, Clin. Chem., 2007, 53, 111–116 CAS.
- M. V. B. krishna, M. Ranjit, D. Karunasagar and J. Arunachalam, Talanta, 2005, 67, 70 CrossRef.
- S. Rio-Segade and C. Bendicho, J. Anal. At. Spectrom., 1999, 14, 263 RSC.
- B. L. Batista, J. L. Rodrigues, V. C. O. Souza and F. Barbosa Jr, Forensic Sci. Int., 2009, 192, 88–93 CrossRef CAS.
- Z. C. Hu, S. H. Hu, S. Gao, Y. S. Liu and S. L. Lin, Spectrochim. Acta, Part B, 2004, 59, 1463 CrossRef.
- C. G. Bruhn, A. A. Rodriguez, C. Barrios, V. H. Jaramillo, J. Becerra, U. Gonzalez, N. T. Gras, O. Reyes and S. Salud, J. Anal. At. Spectrom., 1994, 9, 535–541 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
