New insights into F-pilus structure, dynamics, and function†
Philip M.
Silverman
* and
Margaret B.
Clarke
Genetic Models of Disease Research Program, Oklahoma Medical Research Foundation, Oklahoma City, OK 73104. E-mail: silvermanp@omrf.org; Fax: +1 405 271-7312; Tel: +1 405 271-7663
First published on 3rd December 2009
Abstract
F-pili are thin, flexible filaments elaborated by F+ cells of Escherichia coli. They belong to the class of Gram-negative pili that function in horizontal gene transfer. F-pili are initially required to establish contacts between DNA donor and recipient cells. Beyond that, F-pilus function, and that of other conjugative pili, has remained obscure and controversial. The idea that F-pili are dynamic structures was proposed 40 years ago. Initially, F-pili were thought to remain extended until another cell bound to the filament tip, whereupon the filament retracted to bring the contacted cell to the donor cell surface. Thereafter, secure surface–surface contacts would allow efficient DNA transfer. A later variant of this hypothesis was that F-pili are inherently dynamic, elongating and retracting even in the absence of exogenous signals. A very different hypothesis, also proposed first about 40 years ago, was that F-pili are conduits, presumably passive, for the transfer of DNA from donor to recipient. In this hypothesis, DNA transfer is not obligatorily coupled to F-pilus retraction. Here, we review recent data obtained by integrating long-established facts about the biology of F-pili with modern tools of fluorescence and electron microscopy. These data suggest that one function for F-pili is to search a large volume around donor cells in liquid culture for the presence of other cells. However, this may not be the only function. We show that F-pilin is also required at a second, largely undefined step occurring after cells have been brought into direct contact by F-pilus retraction.
Insight, innovation, integrationAs mechanical objects, biological filaments participate in all fundamental life processes as the tracks, girders, and struts that contribute to cell shape, function, and motility. Conjugative pili are protein filaments that extend from the surface of certain Gram-negative bacterial cells and function in horizontal DNA transfer, or conjugation. Notwithstanding decades of research, the functions of these filaments remain largely unknown. We have explored the structural, mechanical, and dynamic properties of conjugative pili for new functional insights. To elucidate these properties, we have integrated well-established biological facts about F-pili (conjugative pili elaborated by F+ strains of Escherichia coli) with modern imaging methods. The chief insight from these studies is that the mechanical and dynamic properties of F-pili suit them to function as sensory organelles capable of searching large volumes around donor cells. |
Introduction
Type IV secretion systems (TFSS) comprise a broadly distributed group of molecular machines that function to secrete macromolecules from Gram-negative bacteria to other bacterial or eukaryotic cells. These systems play a crucial role in genetic interaction networks within bacterial communities and between bacteria and other organisms, including mammals. With their capacity to transfer DNA as well as proteins, type IV systems as a group are directly involved in such phenomena as bacterial pathogenesis,1,2biofilm formation,3,4 and the dissemination of antibiotic resistance.5,6Several TFSS classes are distinguishable by sequence comparisons. F and related F-like R (antibiotic resistance) plasmids constitute one such class;7 the Agrobacterium Ti plasmidvirB/D system is the archetype of a second class,8–10 whereas R27 and many other plasmids encode hybrid type IV systems.11,12 Given their apparent genetic diversity, these systems may have arisen more than once during bacterial evolution.
Notwithstanding the evolutionary diversity of type IV secretion systems, they appear to be functionally similar. The DNA transfer they mediate can be divided into two stages. These are: (1) formation of specific and secure contacts between donor and recipient cells (mating pair formation or Mpf) and (2) DNA transfer per se (DNA transfer replication or Dtr).7,13 Efficient formation of productive cell–cell contacts depends on surface filaments collectively designated conjugative pili.14–16 Insofar as they have been examined, conjugative pili contain one quantitatively predominant subunit.14,16,17 Conjugative pili and their component pilin subunits are generally identified by the origin, usually a plasmid, of the type IV secretion system of which they are components, e.g., F-pili(n) encoded by the plasmid F, RP4-pili(n) encoded by plasmid RP4, T-pili(n) encoded by the Ti plasmid and so on.
The present article concerns F-pili (Fig. 1). Specifically, we review recently acquired data obtained by the application of contemporaneous experimental approaches and pertinent to the structure and dynamics of these filaments. We address both the intrinsic properties of the filaments as mechanical objects (e.g., flexural rigidity) and the properties of filament assembly and disassembly. The data suggest that both sets of properties play important roles in F-pilus function.
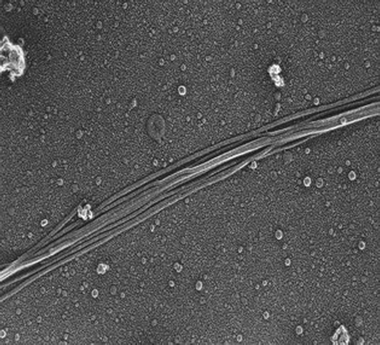 | ||
| Fig. 1 F-pili. Samples of E. coli strain DH1 containing the plasmid pTG801 were fixed in glutaraldehyde, quick frozen on a helium-cooled copper block, lyophilized, and platinum/carbon-shadowed before electron microscopy. The filaments are 8.5 nm in diameter. Photo courtesy of Robyn Roth and John Heuser. | ||
New insights into F-pilus dynamics
A long-standing hypothesis is that F-pili and related filaments retract.18,19 Up until very recently, however, the evidence for retraction has been indirect.20–26 Recently, F-pilus dynamics were directly visualized by live cell imaging.27RNA bacteriophage, such as R17, bind along the length of F-pili, and R17, made fluorescent by chemical modification, could be used to visualize F-pili on fixed cells.28 Those experiments confirmed an observation made repeatedly over the years by electron microscopy, that F+ cells in a population, though genetically identical, were heterogeneous in the number of F-pili/cell (e.g., ref. 29). The study by Clarke et al.27 provided an explanation for these observations. Live F+ cells were held under a thin layer of agarose. Fluorescent R17 penetrated the agarose layer and bound to the sides of extant F-pili. Confocal microscopy showed that fluorescent F-pili elongated at a rate of ∼40 nm s−1. R17 binding appeared to be rate-limiting with respect to the rate of F-pilus elongation, so that cell-proximal segments of elongating F-pili were lightly labeled, becoming more intensely labeled with time and distance from the cell surface. Thus, as was shown for R27 pili,30 F-pili elongate by subunit addition at the cell-proximal terminus. Numerous instances of retracting pili were also recorded.27 Interestingly, whereas elongating pili could switch to retraction, retracting pili were never observed to switch to elongation. Either the filament vanished at the cell surface or became so laden with R17 that retraction slowed and finally ceased. In any event, retraction appeared to be processive, continuing until the entire F-pilus, or at least that part of it beyond the cell surface, vanished.Thus, F-pili constantly undergo cycles of elongation and retraction. A cell with no visible F-pili at one time can extend multiple F-pili a few minutes later, and a cell with F-pili at a given time might have none a few minutes later, owing to retraction. These dynamics did not require any obvious signal, for example another cell bound at the tip. However, retraction was shown to generate enough force to bring two cells together.27
These experiments also revealed an unexpected and potentially important property of F-pilus dynamics.27 F-pili elongating under conditions where the cell-distal terminus was restrained by binding to another F-pilus or to the substratum initially buckled owing to the accumulating force of elongation, but then suddenly snapped into a supercoil originating within the buckled segment. This observation strongly suggests that F-pili rotate about their long axis as they grow. When the cell-distal terminus of an elongating F-pilus is not free to rotate, torque imposed by rotation at the cell-proximal terminus would be initially taken up by the filament. Similarly, bacterial flagella subjected to low levels of torque applied by an optical trap initially exhibited high compliance (twist angle/applied torque),31,32 apparently reflecting the mechanical properties of the hook.32,33 At higher levels, as the elastic limit of the hook was reached, compliance dropped by more than a factor of 10, reflecting the properties of the stiffer flagellar filament.31,33 Constrained F-pili too reach their elastic limit as elongation continues, at which point additional torque is taken up as bending into supercoils. One implication of this phenomenon is that F-pili must be quite flexible.
The structure and mechanical properties of F-pili
The dynamic properties of F-pili described above must be addressed at two levels. Their ability to supercoil suggests that F-pili are mechanically flexible filaments, an attribute that must be referable to filament structure. In contrast, F-pilus elongation, retraction, and rotation are expected to depend less on the properties of the filaments as such and more on the biochemical mechanism(s) by which filaments are assembled and disassembled. With the caveat that not much is known about either aspect of F-pilus dynamics, we discuss here the structural and mechanical properties of F-pili, and in the next section, their assembly and disassembly.F-pili are helical polymers of F-pilin,34–36 a 70 amino acidprotein derived from the traAgene product (121 amino acids) in the bacterial inner membrane.37–40 They are hollow cylinders 8.5 nm in diameter with a hydrophilic axial lumen of 3 nm.34,36,41,42 Beyond these few facts, little is known about F-pilus structure and less still about the relationship between the structure and function(s) of these filaments. This is now beginning to change. Recently, Egelman and co-workers combined cryo-EM with computational methods to provide important new structural insights.36,43 In this section, we discuss how these insights might be related to the mechanical properties of F-pili, and later, to F-pilus function.
The most striking new fact is that F-pili are polymorphic.36 At 13–14 Å resolution, F-pili were found to be organized into two principal helical symmetries.36 A C4 point symmetric arrangement consisted of stacked rings of four radially symmetric subunits. Adjacent rings were axially spaced at 12.8 Å. The twist between adjacent rings was quite variable. The modal value of 34° accounted for only 28% of the segments with C4 symmetry. The range of twists was ±20°, which is impressive considering the inter-ring twist cannot exceed 90°, given the four-fold symmetry.
The variable twist within C4 symmetric segments suggest that F-pili might be compliant to applied torsional strain. This could explain the behavior of elongating filaments whose cell-distal tip is restrained, as discussed above under F-pilus dynamics. Such filaments do not immediately supercoil, but continue to grow for a short time.27 During this initial interval, changes in twist within C4 symmetric segments might take up the accumulating torque.
The second grouping was characterized by a 1-start helical symmetry, with 3.6 units per turn and a pitch of 12.2 Å. This symmetry is similar to that reported earlier by fiber diffraction methods.34 Segments in this group appeared to have a broad mass/length distribution, suggesting that F-pili might be compliant to extension and compression forces as well as to torque. Such compliance could accommodate the sometimes violent forces acting on F-pili as they bring donor and recipient cells together.27
Subunits at the same axial coordinate in the C4 symmetry group interacted weakly, if at all. Instead, the principal pilin–pilin interactions occurred along steeper, multi-start helices. In the 1-start helical symmetry group, interactions along the 1-start helix were evident, but, as was true of the C4 symmetry group, the principal interactions lay along steeper, multi-start helices. Both symmetry groups gave similar filament and luminal diameters, 8.5 nm and 3 nm, respectively, and superposition of the two forms indicated modest differences in subunit packing at the available resolution.36 Given that both forms are present in individual filaments,36 these features suggest modest structural dislocations at junctions where the two forms meet; the two forms might even interconvert at such junctions.
The fact that F-pili can supercoil27 suggests that they are quite flexible. Flexibility can be represented by the persistence length, ξP, the distance over which there is a correlation of bend direction.44 We have estimated ξP for F-pili from images of negatively stained filaments acquired by electron microscopy, as described for filamentous bacteriophage M1345 (Fig. 2A). This analysis measures a static, 2-dimensional persistence length. We assume that the filament configurations represent an equilibrium, though we cannot rule out kinetic effects.46 With two different F-pili preparations, we obtained a value of 4.8 μm (Fig. 2B). This value is smaller than the persistence lengths reported for several other protein filaments, though similar to that reported for type IV pili (Table 1).
 | ||
| Fig. 2 Persistence length of F-pili. (A) F-pili purified as described by Wang et al.36 were deposited on carbon-coated electron microscope grids. Digital images were obtained with an Hitachi H-7600 transmission electron microscope. The images were projected onto a sheet of white paper and individual filaments traced. Tracings were digitized using a flat bed scanner, and the files imported into MetaMorph. The end-to-end distance, R, and contour length, L, were estimated for each filament using the Line and Trace tools, respectively. The calibration bar at the lower right is 500 nm. (B) A persistence length of 4.8 μm was estimated from plots of R2vs. L, as described,45,46 using Mathematica computational software. The data are a composite of two different experiments with two F-pili preparations. The separate values were 4.8 and 5.1 μm. | ||
We also estimated a 2-dimensional dynamic persistence length from movies of filaments labeled with fluorescent bacteriophage R17 and either stuck at one end to a glass slide (Fig. 3 and Movie 1 in the ESI† ) or cell-bound (not shown). These measurements gave higher values of ξP, in the range of 10–40 μm. However, these data refer not to F-pili alone but to F-pili–R17 complexes. Any interaction between bound R17 particles along the length of the filament would have a stiffening effect, perhaps accounting for the longer persistence length. The broad range we observe may be attributable to different levels of R17 binding. In any case, these first reported measurements of a mechanical property of F-pili confirm that they are relatively flexible.
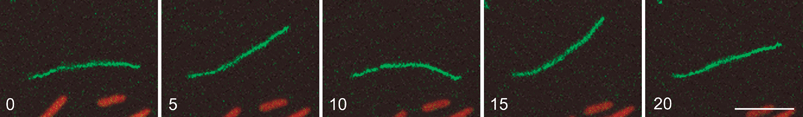 | ||
| Fig. 3 Flexibility of F-pili in liquid. An F-pilus decorated with fluorescent bacteriophage R1728 had become attached at one end to the glass surface. The movement of the filament was followed for 20 s, as described.27 The figure shows the extent of bending in the x-y plane every 5 s during this interval. The F-pilus was held close to the glass surface by a layer of agar.27 Accordingly, movement of the filament in the z dimension was minimal, as indicated by the maintenance of focus during the entire 20 s interval. The entire series can be seen in Movie 1.† Persistence lengths were calculated as described45,46 from the end-to-end distance averaged over time at 0.5 s intervals and the contour length, 8.3 μm. The calibration bar in the 20 s panel is 5 μm. | ||
The assembly and disassembly of F-pili
Though F-pili contain, in so far as is known, only a single kind of subunit, F-pilin, loss of function of any of several other Tra proteins essentially abolishes F-pilus formation.47 One of these, TraQ, is an inner membrane TraA escort.40,48 Other gene products required for F-pilus formation exist as cell envelope-associated, multi-protein assemblies.49,50 Recently, the core structure of one such assembly from a type IV secretion system other than F was solved by cryo-electron microscopy at 15 Å resolution.51 The 1.1 MDa structure, spanning the entire cell envelope, consisted of multiple copies of three different subunits in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and exhibited 14-fold radial symmetry. We have observed similar structures from F+ cells expressing the entire set of tragenes, as opposed to cells expressing only a limited set.51 The F-encoded structures were enriched from detergent-extracted outer membrane fractions by zone sedimentation through sucrose gradients. They migrated as high molecular weight material (∼2.5 MDa) and contained three Tra proteins, TraV, TraK, and TraB, known from previous yeast 2-hybrid studies to form a closed interaction group.49 Moreover, each of these is analogous to a component of the core structure analyzed by Fronzes et al.51 (Table 2). All three are required for F-pilus formation as well as for DNA transfer.47 Preliminary data suggests the presence of at least one other Tra protein also required for F-pilus formation and DNA transfer but belonging to a different interaction group.50
1 stoichiometry and exhibited 14-fold radial symmetry. We have observed similar structures from F+ cells expressing the entire set of tragenes, as opposed to cells expressing only a limited set.51 The F-encoded structures were enriched from detergent-extracted outer membrane fractions by zone sedimentation through sucrose gradients. They migrated as high molecular weight material (∼2.5 MDa) and contained three Tra proteins, TraV, TraK, and TraB, known from previous yeast 2-hybrid studies to form a closed interaction group.49 Moreover, each of these is analogous to a component of the core structure analyzed by Fronzes et al.51 (Table 2). All three are required for F-pilus formation as well as for DNA transfer.47 Preliminary data suggests the presence of at least one other Tra protein also required for F-pilus formation and DNA transfer but belonging to a different interaction group.50
| VirB homologue (KDa, localization) | F-homologue (KDa, localization) |
|---|---|
| a Data for the F-encoded system are from our unpublished data and ref. 47. Data for the VirB/D system are from Kado70, Christie71 and Fronzes et al.51 Localization assignments (OM, outer membrane; P, periplasm; IM, inner membrane) refer to the individual proteins rather than the complex as a whole. VirB7 and VirB9 are covalently linked and anchored to the outer membrane by the VirB7 lipoprotein71, whereas the outer membrane TraV lipoprotein and TraK are not covalently linked.49 See the text for further details. | |
| VirB7 (5.9, OM) | TraV (16.6, OM) |
| VirB9 (32.1, P) | TraK (23, P) |
| VirB10 (40.6, IM) | TraB (50.5, IM) |
Little is known about how these assemblies actually function in macromolecular secretion. One possibility, suggested for the type IV secretion system encoded by the Agrobacterium tumefaciens Ti plasmid,52,53 is that two, physically distinct core complexes exist, one for the secretion of conjugative (T-) pilin subunits and the other for the secretion of DNA, depending on the interactions among the core components. This proposal was based in part on the fact that certain mutations of the virB10 core component abolished the formation of visible T-pili but had little, if any, effect on DNA transfer.53 This would be consistent with the emerging picture of biological nanomachines in general as dynamic structures.54 In any case, in the Ti plasmidvirB/D system, DNA transfer and T-pilus assembly and function can be uncoupled.53 It should be noted, however, that the quantitatively major T-pilin subunit, VirB2, is required for DNA transfer.52 Hence, while extended T-pili are in some circumstances dispensable for transfer, the T-pilin subunit is not.
Extended F-pili are also not required for DNA transfer, but only after cell–cell contacts have been stabilized by the activities of two F-encoded, cell envelopeproteins, TraG and TraN.23,55–57 These activities do not significantly affect F-pilus formation, but in their absence, DNA transfer is much reduced.47 These facts are best understood by the hypothesis that, in the F-encoded system, DNA transfer is normally coupled to F-pilus-mediated retraction, bringing the cells together to where the donor cell surface components TraG and TraN can act. Harrington and Rogerson58 and Babic et al.59 reported the formation of genetic transconjugants when donor and recipient cells were separated by thin (10 μm) membranes or plated on a glass surface, respectively. If confirmed, these experiments would indicate that, under conditions where cells are immobilized on a surface, DNA transfer may not be strictly coupled to F-pilus retraction.
Whatever the relationship between F-pilus dynamics and DNA transfer, the rotation of elongating and retracting F-pili, inferred from supercoiling of restrained filaments,27 has important mechanistic implications for filament assembly and perhaps disassembly. If the F-pilus is assembled one subunit at a time along the 1-start helical path,36 which seems to be the simplest assumption, two mechanisms can be imagined. In the first, the cell envelope structure responsible for translocating F-pilin from the inner membrane to an F-pilus assembly site, perhaps similar to the structure described by Fronzes et al.,51 might rotate between subunit additions about 100° relative to the filament axis (and the cell envelope), accounting for the 3.6 subunits per turn of the 1-start helix. Alternatively, the structure could remain stationary relative to the cell envelope and the filament could rotate 100° between additions. The latter mechanism would fit better with our observations.27 Key questions are what powers subunit addition and whether an incoming F-pilin subunit is required for rotation or whether rotation allows an F-pilin molecule to enter the translocation pathway.
Putting it all together
We have documented several components of F-pilus dynamics. These filaments extend and retract; they flex; and they rotate (Fig. 4). None of these components is unique to F-pili. Bacterial type IV pili also extend and retract; bacterial flagella rotate. For these two classes of extracellular filament, dynamic properties are critical determinants of function, which in both cases is cell motility. In what way(s) might the dynamic properties of F-pili also comprise functional determinants, given that F-pili have no role in bacterial motility? | ||
| Fig. 4 F-pili as search organelles. The figure shows the different motions attributed to F-pili. Rotation, elongation, and retraction were shown in Clarke et al.27 Flexibility (bending) in terms of persistence length is described in this communication. | ||
We suggest that F-pili are sensory organelles. This may not be their only function, but we suggest it is the function most closely related to the dynamic properties we describe here. More specifically, these properties allow the F-pilus tip, which makes first contact with recipient cells prior to DNA transfer,60 to search a large volume around the donor cell. This search function would be especially important for bacteria such as F+ strains of E. coli, which conjugate efficiently in liquid media, i.e., in three dimensions, as opposed to only on surfaces. Efficient searches in liquid are critical because, whereas random walks in two dimensions will inevitably encounter every point in the search space (given enough time), random walks in three dimensions will not.61 Moreover, only one F+E. coli cell in a thousand is normally active as a donor over any short time interval. (This refers to wild-type F and F-like R factors. F itself is a derepressed mutant whose type IV secretion system is constitutively expressed.62) These facts place a premium on mechanisms that enhance the search efficiency of the F-pilus tip.
Since F-pili constantly undergo elongation/retraction cycles, F-pilus contact with a potential recipient will draw the two cells together without any sort of signal.27 Stabilization of direct cell–cell contacts, which require TraN and TraG,22,56,57 then permits DNA transfer over long intervals. During these intervals, extended F-pili are not generally required.23
Beginning with the hypothesis that F-pili function is to search the volume around donor cells for suitable recipients, we would expect that the filaments should be able to flex as they extend and retract, thereby increasing their search volume. If F-pili were as rigid as microtubules (ξP = 8 mm; Table 1), the pilus tip would sample a relatively small volume around the donor cell during each cycle of elongation and retraction. If instead F-pili were as flexible as double-stranded DNA (ξP = 50 nm46], the mean end-to-end distance would be short relative to the filament contour length, and the pilus tip would on average remain near the donor cell surface. The conditions this hypothesis imposes on F-pili, neither too rigid nor too flexible, imply that the persistence length of F-pili should be similar to their contour length when elongating filaments switch to retraction. In fact, such reversals tended to occur at filament lengths between 1.5 and 7.5 μm,27 an interval which encompasses our measured value for ξP of 5 μm (Fig. 2).
The rotation of F-pili as they extend and retract might also contribute to F-pilus bending. Specifically, rotation could cause a deflection of the filament from its axis normal to the cell surface.63 Moreover, as rotation bent the filament away from its equilibrium contour, intersubunit interactions would presumably be strained as one face of the filament was stretched and the other was compressed. These strains could produce a restoring force.
The general idea proposed here is summarized in Fig. 4. As F-pili elongate and retract, the effects of rotation and the intrinsic flexibility of the filaments cause them to precess about an axis normal to the cell surface, thereby allowing the tip to sweep a larger volume around donor cells than would occur with an especially rigid filament or an especially flexible one. This cone-shaped volume can be estimated from the fact that the volume of a cone is 1/3 the area of the base times the height. From the data in Fig. 3 and Movie 1,† we estimate that the 8.3 μm filament shown makes an excursion in the x–y plane of about 6 μm (Fig. 3, 10 and 15 s panels). If precession owing to rotation caused the tip to trace a circular path, this length would be the maximal diameter and the area would be about 28 μm.2 Using 8.3 μm for the height, the volume swept by this filament tip would be about 78 μm.3 (The volume would be somewhat smaller using filament lengths at the time when retraction begins.27) For comparison, the volume of a bacterial cell 1 μm wide by 2 μm long is about 1.6 μm.3 A search function would also explain the very slow rate of F-pilus elongation, about 40 nm s−1, compared with the 12–25-fold higher rate of type IV pilus elongation;27 the longer the search interval, the more effective the search. At 40 nm s−1, about 2 min would be required to extend a 5 μm filament and at least that long would be required to retract it.
The search function we propose for F-pili is a heuristic interpretation of what is now known about their mechanical and dynamic properties. This interpretation makes predictions about the relationship between these properties and the efficiency of conjugation in liquid media. These predictions can be tested by combining well-established genetic and physiological approaches with the biophysical approaches described here.
Can this search function be the only one assigned to F-pili? A simple experiment suggests that F-pili, or perhaps F-pilin itself, must have other functions. In this experiment, limiting donor and excess recipient cells were mixed and collected in a pellet by sedimentation. DNA transfer over a 40 min interval was then determined by plating on selective agar. About 20% of tra+ donors participated in DNA transfer (0.18 transconjugants/tra+ donor cell). However, no transconjugants at all were formed with otherwise isogenic traA1[Am] donors, setting an upper limit of 10−7 transconjugants/donor. Thus, even when conjugation occurs in a cell pellet, where the search-and-retract function of F-pili would be dispensable, F-pilin is still required. It remains to be determined if this requirement is for F-pili or F-pilin as such. For example, it has been suggested that retracted F-pili, short enough to escape detection by microscopy, might be used as a conduit for DNA transfer.64
As a coda, we note that few if any of the data underlying the hypothesis illustrated in Fig. 4 could have been obtained as recently as 10 years ago. The biological insight we present, whether or not it is confirmed by further analyses, illustrates how the application of new technologies has led to new insights into a very old phenomenon.
Acknowledgements
We are indebted to Robin Harris and Cindy Maderra for their contributions to this work. Ms Maria Vera performed the measurements of F-pilus persistence length as an OMRF Fleming Summer Scholar; these measurements were facilitated by the OMRF Imaging Facility. This work was supported by NSF grant MCB-0615583 and by the Oklahoma Medical Research Foundation. PMS acknowledges support from the Marjorie Nichlos Chair in Medical Research and MBC as the J. P. Hannigan Distinguished Research Scientist.References
- E. Cascales and P. J. Christie, Science, 2004, 304, 1170–1173 CrossRef CAS.
- M. Llosa, C. Roy and C. Dehio, Mol. Microbiol., 2009, 73, 141–151 CrossRef CAS.
- J. M. Ghigo, Nature, 2001, 412, 442–445 CrossRef CAS.
- A. Reisner, J. A. Haagensen, M. A. Schembri, E. L. Zechner and S. Molin, Mol. Microbiol., 2003, 48, 933–946 CrossRef CAS.
- M. Teuber, Cell. Mol. Life Sci., 1999, 56, 755–763 CrossRef CAS.
- M. P. Nikolich, G. Hong, N. B. Shoemaker and A. A. Salyers, Appl. Environ. Microbiol., 1994, 60, 3255–3260 CAS.
- T. D. Lawley, W. A. Klimke, M. J. Gubbins and L. S. Frost, FEMS Microbiol. Lett., 2003, 224, 1–15 CrossRef CAS.
- T. B. Cao and M. H. Saier, Jr., Microbiology, 2001, 147, 3201–3214 CAS.
- P. J. Christie, Mol. Microbiol., 2001, 40, 294–305 CrossRef CAS.
- A. Covacci, J. L. Telford, G. Del Giudice, J. Parsonnet and R. Rappuoli, Science, 1999, 284, 1328–1333 CrossRef CAS.
- E. F. Boyd, C. W. Hill, S. M. Rich and D. L. Hartl, Genetics, 1996, 143, 1091–1100 CAS.
- D. E. Taylor, P. J. Newnham, C. Sherburne, T. D. Lawley and M. M. Rooker, Plasmid, 1999, 41, 207–218 CrossRef CAS.
- M. Achtman and R. Skurray, Receptors and Recognition Series B, 1977, 3, 235–279 Search PubMed.
- W. Paranchych and L. S. Frost, Adv. Microb. Physiol., 1988, 29, 53–114 CAS.
- K. J. Fullner, J. C. Lara and E. W. Nester, Science, 1996, 273, 1107–1109 CrossRef CAS.
- R. Eisenbrandt, M. Kalkum, E. M. Lai, R. Lurz, C. I. Kado and E. Lanka, J. Biol. Chem., 1999, 274, 22548–22555 CrossRef CAS.
- E. M. Lai and C. I. Kado, J. Bacteriol., 1998, 180, 2711–2717 CAS.
- R. Curtiss, 3rd, Annu. Rev. Microbiol., 1969, 23, 69–136 CrossRef CAS.
- D. A. Marvin and B. Hohn, Bacteriol. Rev., 1969, 33, 172–209 CAS.
- C. P. Novotny and P. Fives-Taylor, J. Bacteriol., 1974, 117, 1306–1311 CAS.
- A. Jacobson, J. Virol., 1972, 10, 835–843 CAS.
- M. Achtman, J. Bacteriol., 1975, 123, 505–515 CAS.
- M. M. Panicker and E. G. Minkley, Jr., J. Bacteriol., 1985, 162, 584–590 CAS.
- M. B. Durrenberger, W. Villiger and T. Bachi, J. Struct. Biol., 1991, 107, 146–156 CrossRef CAS.
- B. A. Sowa, D. Moore and K. Ippen-Ihler, J. Bacteriol., 1983, 153, 962–968 CAS.
- T. D. Lawley, G. S. Gordon, A. Wright and D. E. Taylor, Mol. Microbiol., 2002, 44, 947–956 CrossRef CAS.
- M. Clarke, L. Maddera, R. L. Harris and P. M. Silverman, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17978–17981 CrossRef CAS.
- K. Daehnel, R. Harris, L. Maddera and P. Silverman, Microbiology, 2005, 151, 3541–3548 CrossRef CAS.
- R. Curtiss, 3rd, L. G. Caro, D. P. Allison and D. R. Stallions, J. Bacteriol., 1969, 100, 1091–1104 CAS.
- D. Maher, R. Sherburne and D. E. Taylor, J. Bacteriol., 1993, 175, 2175–2183 CAS.
- S. M. Block, D. F. Blair and H. C. Berg, Nature, 1989, 338, 514–518 CrossRef CAS.
- S. M. Block, D. F. Blair and H. C. Berg, Cytometry, 1991, 12, 492–496 CrossRef CAS.
- T. C. Flynn and J. Ma, Biophys. J., 2004, 86, 3204–3210 CrossRef CAS.
- W. Folkhard, K. R. Leonard, S. Malsey, D. A. Marvin, J. Dubochet, A. Engel, M. Achtman and R. Helmuth, J. Mol. Biol., 1979, 130, 145–160 CrossRef CAS.
- D. A. Marvin and W. Folkhard, J. Mol. Biol., 1986, 191, 299–300 CrossRef CAS.
- Y. A. Wang, X. Yu, P. M. Silverman, R. L. Harris and E. H. Egelman, J. Mol. Biol., 2009, 385, 22–29 CrossRef CAS.
- D. Moore, B. A. Sowa and K. Ippen-Ihler, J. Bacteriol., 1981, 146, 251–259 CAS.
- L. S. Frost, W. Paranchych and N. S. Willetts, J. Bacteriol., 1984, 160, 395–401 CAS.
- W. D. Paiva, T. Grossman and P. M. Silverman, J. Biol. Chem., 1992, 267, 26191–26197 CAS.
- K. Maneewannakul, S. Maneewannakul and K. Ippen-Ihler, J. Bacteriol., 1993, 175, 1384–1391 CAS.
- C. C. Brinton, Crit. Rev. Microbiol., 1971, 1, 105–160 Search PubMed.
- R. C. Valentine, P. M. Silverman, K. A. Ippen and H. Mobach, Adv. Microb. Physiol., 1969, 3, 1–52 CAS.
- E. H. Egelman, Ultramicroscopy, 2000, 85, 225–234 CrossRef CAS.
- R. Phillips, J. Kondev and J. Theriot, Physical Biology of the Cell, Garland Science, New York, 2009 Search PubMed.
- Y. A. Wang, X. Yu, S. Overman, M. Tsuboi, G. J. Thomas, Jr. and E. H. Egelman, J. Mol. Biol., 2006, 361, 209–215 CrossRef CAS.
- C. Rivetti, M. Guthold and C. Bustamante, J. Mol. Biol., 1996, 264, 919–932 CrossRef CAS.
- N. Firth, K. Ippen-Ihler and R. Skurray, in Escherichia coli and Salmonella: Cellular and Moleclar Biology, ed. F. C. Neidhardt, ASM Press, Washington DC, Editon edn, 1996, pp. 2377–2401 Search PubMed.
- R. L. Harris, K. A. Sholl, M. N. Conrad, M. E. Dresser and P. M. Silverman, Mol. Microbiol., 1999, 34, 780–791 CrossRef CAS.
- R. L. Harris, V. Hombs and P. M. Silverman, Mol. Microbiol., 2001, 42, 757–766 CrossRef CAS.
- R. L. Harris and P. M. Silverman, J. Bacteriol., 2004, 186, 5480–5485 CrossRef CAS.
- R. Fronzes, H. Remaut and G. Waksman, EMBO J., 2008, 27, 2271–2280 CrossRef CAS.
- C. I. Kado, Curr. Opin. Microbiol., 2000, 3, 643–648 CrossRef CAS.
- S. J. Jakubowski, J. E. Kerr, I. Garza, V. Krishnamoorthy, R. Bayliss, G. Waksman and P. J. Christie, Mol. Microbiol., 2009, 71, 779–794 CrossRef CAS.
- N. Delalez and J. P. Armitage, Mol. Microbiol., 2009, 71, 807–810 CrossRef CAS.
- P. A. Manning, G. Morelli and M. Achtman, Proc. Natl. Acad. Sci. U. S. A., 1981, 78, 7487–7491 CrossRef CAS.
- S. Maneewannakul, P. Kathir and K. Ippen-Ihler, J. Mol. Biol., 1992, 225, 299–311 CrossRef CAS.
- N. Firth and R. Skurray, MGG, Mol. Gen. Genet., 1992, 232, 145–153 CrossRef CAS.
- L. C. Harrington and A. C. Rogerson, J. Bacteriol., 1990, 172, 7263–7264 CAS.
- A. Babic, A. B. Lindner, M. Vulic, E. J. Stewart and M. Radman, Science, 2008, 319, 1533–1536 CrossRef CAS.
- R. Helmuth and M. Achtman, Proc. Natl. Acad. Sci. U. S. A., 1978, 75, 1237–1241 CrossRef CAS.
- M. A. McCloskey and M. M. Poo, J. Cell Biol., 1986, 102, 88–96 CrossRef CAS.
- Y. Yoshioka, H. Ohtsubo and E. Ohtsubo, J. Bacteriol., 1987, 169, 619–623 CAS.
- F. B. Hildebrand, Advanced Calculus for Applications, Prentice-Hall, Englewood Cliffs, 1964 Search PubMed.
- P. Manning and M. Achtman, in Bacterial Outer Membranes, ed. M. Inouye, Wiley Interscience, New York, Editon edn, 1979, pp. 409–447 Search PubMed.
- M. G. Van den Heuvel, M. P. de Graaff and C. Dekker, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 7941–7946 CrossRef CAS.
- F. Gittes, B. Mickey, J. Nettleton and J. Howard, J. Cell Biol., 1993, 120, 923–934 CrossRef CAS.
- S. Trachtenberg and I. Hammel, J. Struct. Biol., 1992, 109, 18–27 CrossRef CAS.
- A. Ott, M. Magnasco, A. Simon and A. Libchaber, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1993, 48, R1642–R1645 CrossRef CAS.
- J. M. Skerker and H. C. Berg, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 6901–6904 CrossRef CAS.
- C. I. Kado, Mol. Microbiol., 1994, 12, 17–22 CrossRef CAS.
- P. J. Christie, J. Bacteriol., 1997, 179, 3085–3094 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Movie 1. See DOI: 10.1039/b917761b |
| This journal is © The Royal Society of Chemistry 2010 |
