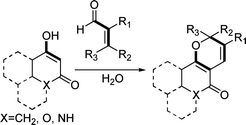
In water medium, environmentally benign, facile, and efficient synthesis of pyrans was achieved in good yields by the reactions of a variety of cyclic 1,3-dicarbonyls with several α,β-unsaturated aldehydes. The key strategy was a formal [3+3] cycloaddition by domino Knoevenagel/6π- electrocyclization. This methodology was applied to the synthesis of biologically interesting pyranocoumarin, pyranoquinolinone, and pyranonaphthoquinone derivatives along with selected natural and non-natural products.