Biorenewable polyethylene terephthalate mimics derived from lignin and acetic acid†
Laurent
Mialon
,
Alexander G.
Pemba
and
Stephen A.
Miller
*
The George and Josephine Butler Laboratory for Polymer Research, Department of Chemistry, University of Florida Gainesville, Florida, 32611-7200, USA. E-mail: miller@chem.ufl.edu; Fax: +1-352-392-9741; Tel: +1-352-392-7773
First published on 6th July 2010
Abstract
Lignin-based vanillin and acetic anhydride are subjected to the Perkin reaction and then hydrogenation to afford acetyldihydroferulic acid. Polymerization of this monomer yields poly(dihydroferulic acid), which exhibits thermal properties functionally similar to those of polyethylene terephthalate (PET).
Polyethylene terephthalate (PET) is the third most common synthetic polymer and accounts for about 18% of world polymer production.1 This aromatic/aliphatic polyester possesses very practical thermal properties that are not found in any all-aliphatic commodity thermoplastic: a glass transition temperature (Tg) near 67 °C and a melting temperature (Tm) of 265 °C.2 The key aromatic component of PET, terephthalic acid, is derived from petroleum, while ethylene glycol is derived from petroleum or natural gas (Scheme 1).
 | ||
| Scheme 1 Polyethylene terephthalate derives from fossil fuel feedstocks terephthalic acid and ethylene glycol. | ||
The design of sustainable PET mimics will probably also require aromatic components and the most abundant biorenewable source of aromatic compounds is lignin, which is found in all vascular plants, is the second most abundant naturally-occurring organic polymer, and makes up approximately 30% of wood.3 The extraction of lignin from wood is common in the paper pulping industry and can result in appreciable quantities of the aromatic aldehydes vanillin and syringaldehyde – up to 5% and 8%, respectively (Scheme 2).4
 | ||
| Scheme 2 Aromatic aldehydes vanillin and syringaldehyde are by-products of lignin extraction during the production of paper. | ||
Hence, our initial design of a fully sustainable5 aliphatic/aromatic polyester focused on incorporating aromatics derived from vanillin and syringaldehyde into the main-chain, while also including a short aliphatic component that provides necessary flexibility. All-aromatic polyesters certainly exist, but their extremely high melting temperatures usually limit their processability (e.g., poly(p-hydroxybenzoic acid) with Tm = 350 °C).6 Also, several lignin-based polyesters have been synthesized, but these generally do not match the thermal properties of PET and employ an oxidized version of vanillin, vanillic acid, as the feedstock.7 While the use of lignin-derived aromatics for building polymers appears industrially sound, such materials have not yet achieved commercial significance.8 Interestingly, the pentose-derived aromatic furan-2,5-dicarboxylic acid has been used as a successful replacement of terephthalic acid in a recent PET analogue.9
Another important design strategy is the use of a single monomer instead of two co-monomers; this avoids the necessity of stoichiometric balance to achieve high molecular weights. A final aspiration is main-chain ester functionality built from aromatic alcohols instead of aliphatic alcohols (as in PET); in this case, hydrolytic degradation should be more facile because of the superior leaving group ability of aryloxides vs. alkoxides. An ideal monomer that meets our design criteria is acetyldihydroferulic acid.10Scheme 3 depicts the two-step synthesis of this monomer (69% and 85% yields, respectively) from vanillin.
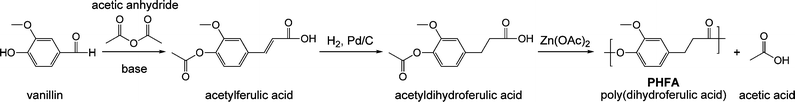 | ||
| Scheme 3 Synthetic scheme for preparing the aromatic/aliphatic polyester poly(dihydroferulic acid), PHFA, from biorenewable feedstocks vanillin and acetic anhydride. | ||
The first step employs acetic anhydride and sodium acetate—although other bases can be used—to effect a Perkin reaction on the aldehyde functional group of vanillin.11 Most acetic acid currently derives from natural gas via carbonylation of methanol but the requisite C1 feedstocks for this process can readily be prepared from wood through anaerobic distillation—first yielding wood alcohol (methanol). Alternatively, acetic acid can be derived from sustainable fermentation processes.12 Concomitant with the Perkin reaction is an acetylation of the hydroxyl group of vanillin. The obtained acetylferulic acid is conjugated and yellow, but facile hydrogenation yields a nearly colorless, aromatic/aliphatic monomer. Acetyldihydroferulic acid has been investigated as a minor component of an amorphous, liquid crystalline, random terpolymer,13 but its homopolymer, poly(dihydroferulic acid), PHFA, has not been reported.
Efficient polymerization was accomplished in the melt (200–220 °C) with Sb2O3 or Zn(OAc)2·2H2O as catalyst. Dynamic vacuum effected removal of acetic acid as the by-product of this condensation polymerization. Naturally, the evolved acetic acid could be recycled back to the anhydride form. Table 1 describes the various polymerizations as well as characterization data for the obtained polymers. Note that a catalyst is not required to achieve reasonable yields. But, a catalyst does appear to hasten the reaction, as indicated by the time transpired before viscosity impedes stirring.
| Entry | T p/°C | Catalyst (1 mol%) | Melt time/h | Vacuum time/h | Stirring stops/h | yield (%) | [η]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a/mL g−1 a/mL g−1 |
M
v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
DP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
T g/°C | T m/°C |
T
50%/°C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
a Intrinsic viscosity obtained with an Ubbelohde viscometer in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 solution of phenol/1,1,2,2-tetrachloroethane at 35 °C.
b From [η]= 1.09 × 10−2Mv0.84, using Mark–Houwink–Sakurada parameters established for PET.14
c Degree of Polymerization and Mn by 1H NMR end group analysis.
d The temperature at which 50% mass loss is observed by thermogravimetric analysis, under nitrogen.
e Gel permeation chromatography analysis. 2 solution of phenol/1,1,2,2-tetrachloroethane at 35 °C.
b From [η]= 1.09 × 10−2Mv0.84, using Mark–Houwink–Sakurada parameters established for PET.14
c Degree of Polymerization and Mn by 1H NMR end group analysis.
d The temperature at which 50% mass loss is observed by thermogravimetric analysis, under nitrogen.
e Gel permeation chromatography analysis.
|
|||||||||||||
| 1 | 200–220 | none | 2 | 2 | 1.5 | 83 | 31 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
27 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
74 | 243 | 471 |
| 2 | 200–220 | Sb2O3 | 2 | 2 | 0.5 | 67 | 29 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
23 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
73 | 234 | 462 |
| 3 | 200–220 | Zn(OAc)2 | 2 | 2 | 0.5 | 82 | 27 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
38 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
73 | 234 | 461 |
| 4 | 200–220 | Zn(OAc)2 | 2 | 6 | 0.5 | 91 | 36 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
50 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
67 | 231 | 464 |
| 5 | 220–250 | Zn(OAc)2 | 2 | 6 | 0.5 | 68 | 35 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
100 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
67 | 216 | 456 |
| 6 | 200–220 | Zn(OAc)2 | 2 | 0.17 | — | 75 | 17 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
17 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
65 | 240 | 452 |
| 7 | 200–220 | Sb2O3 | 5 | 6 | — | ∼5 | n.d. | n.d. | 3.5e | 630e | — | — | n.d. |
The insolubility of the polymers in common organic solvents precluded gel permeation chromatography analysis. Instead, molecular weight information was obtained by viscometry. The intrinsic viscosity was determined with an Ubbelohde viscometer under conditions previously reported for PET analysis.14 Since the Mark–Houwink–Sakurada parameters have not been established for novel PHFA, the parameters reported for PET ([η]= 1.09 × 10−2Mv0.84) were applied instead in order to convert intrinsic viscosity to molecular weight. While this transfer of MHS parameters from one polymer to another is not general, the associated error is typically much less than an order of magnitude when the polymeric structures are similar and the solvent and temperature are matched.15
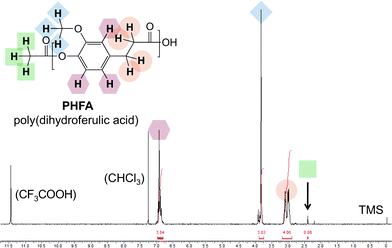
Additionally, molecular weight information was obtained by 1H NMR end group analysis. Fig. 1 shows the 1H NMR spectrum of the PHFA from Table 1, entry 3. The methyl substituent of the acetyl end group was located at 2.40 ppm. Comparative integration suggests an average degree of polymeriation of 38. Note that the 1H NMR end group analysis method provides number-average molecular weight values generally lower than the viscosity-average molecular weight values, as theoretically predicted for polydisperse samples.16
The most important factors that contribute to high molecular weight material are prolonged dynamic vacuum and the acetyl group of the monomer. When dynamic vacuum is limited to 10 min (Table 1, entry 6), comparatively low molecular weight polymer is obtained. When the acid/alcohol dihydroferulic acid is employed (Table 1, entry 7), the reaction is remarkably sluggish and yields only oligomers. Thus, the acetyl group installed by the Perkin reaction is deemed a necessity and it allows for molecular weights comparable to that of commercial PET (Mn = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 40
000 to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000).
000).
The Tm for a typical sample of PHFA is measured as 234 °C and its Tg is measured as 73 °C (Fig. 2; Table 1, entry 3). Thus, PHFA and PET have comparable glass transition temperatures, but PHFA has a melting temperature that is lower by about 30 °C. PHFA has a cooling-cycle crystallization temperature up to 17 °C higher (Tc = 207 °C for Table 1, entry 1) than that of PET (190 °C), suggesting a faster rate of crystallization.17 Additionally, PHFA typically exhibits a peak decomposition temperature near 462 °C (50% mass loss under nitrogen, as measured by thermal gravimetric analysis), which is comparable to that of PET (470 °C).18
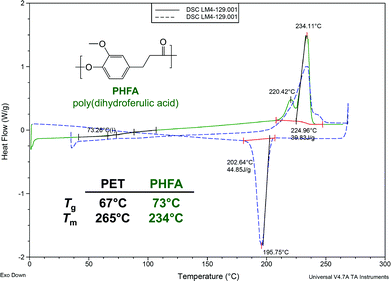 | ||
| Fig. 2 Differential scanning calorimetry of PHFA from Table 1, entry 3. | ||
The thermal properties of PHFA are arguably improved over those of PET. The limiting temperature for PET in the amorphous phase (e.g., plastic water bottles) is Tg and no compromise is made with PHFA. The limiting temperature for PET in the crystalline phase (e.g., polyester fibers) is Tm. Since most PET is rarely subjected to extremely high temperatures during routine use, the lower Tm of PHFA simply translates into more facile processability. Finally, preliminary crystallization temperature data suggests that the lamentably slow crystallization rate of PET is excelled by PHFA.
It should be noted that the cognate polymers derived from syringaldehyde (two –OCH3 groups) and 4-hydroxybenzaldehyde (zero –OCH3 groups) were seemingly heterogeneous since they were intractable even in the best solvents (e.g., trifluoracetic acid). With the present polymerization methods, it appears that the polymer derived from vanillin is synthetically optimal.
In conclusion, acetic acid and lignin-derived vanillin are readily converted into an aromatic/aliphatic A–B monomer that undergoes condensation polymerization with loss of acetic acid. The formed poly(dihydroferulic acid) (PHFA) is the first wholly biorenewable polymer that successfully mimics the thermal properties of polyethylene terephthalate.19 Future studies will determine important degradation characteristics of PHFA and target copolymers with tunable thermal properties.
Acknowledgements
This research was supported by the donors of the American Chemical Society Petroleum Research Fund (#47951-AC7) and the National Science Foundation (CHE-0848236).Notes and references
- U. K. Thiele, Chem. Fibers Int., 2010, 60, 46–48 Search PubMed.
- M. Rule, in Polymer Handbook, 4th Edition, ed. J. Brandrup, E. H. Immergut, E. A. Grulke, A. Abe, and D. R. Bloch, John Wiley & Sons, New York, 2005, pp. V/113–V/118 Search PubMed.
- S. E. Lebo, Jr., J. D. Gargulak, and T. J. McNally, Lignin, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., New York, 2001, Vol. 15, pp. 1–32 Search PubMed.
- G. Wu, M. Heitz and E. Chornet, Ind. Eng. Chem. Res., 1994, 33, 718–723 CrossRef CAS.
- The 1,3-propanediol in Dupont's Sorona® polypropylene terephthalate is biorenewable, but the aromatic terephthalic acid employed is not. J. V. Kurian, J. Polym. Environ., 2005, 13, 159–167 Search PubMed.
- (a) J. Economy, and S. G. Cottis, Hydroxybenzoic Acid Polymers, in Encyclopedia of Polymer Science and Technology, ed. H. F. Mark, N. G. Gaylord, and N. M. Bikales, Wiley, New York, 1971, Vol. 15, pp. 292–306 Search PubMed; (b) J. Economy, R. S. Storm, V. I. Matkovich, S. G. Cottis and B. E. Nowak, J. Polym. Sci., Polym. Chem. Ed., 1976, 14, 2207–2224 CrossRef CAS.
- (a) W. Lange and O. Kordsachia, Holz Roh- Werkst., 1981, 39, 107–112 CrossRef CAS; (b) L. H. Bock and J. K. Anderson, J. Polym. Sci., 1955, 17, 553–558 CrossRef CAS; (c) M. Nagata, J. Appl. Polym. Sci., 2000, 78, 2474–2481 CrossRef CAS.
- A. Gandini, Macromolecules, 2008, 41, 9491–9504 CrossRef CAS.
- (a) A. Gandini, A. J. D. Silvestre, C. P. Neto, A. F. Sousa and M. Gomes, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 295–298 CrossRef CAS; (b) A. Gandini, Polym. Chem., 2010, 1, 245–251 RSC.
- I. A. Pearl, J. Org. Chem., 1959, 24, 736–740 CrossRef CAS.
- (a) L. S. Fosdick and A. C. Starke, Jr., J. Am. Chem. Soc., 1940, 62, 3352–3355 CrossRef CAS; (b) J. R. Johnson, The Perkin Reaction and Related Reactions, in Organic Reactions, ed. R. Adams, E. W. Bachmann, L. F. Fieser, J. R. Johnson, and H. R. Snyder, John Wiley & Sons, New York, 1942, Vol. 1, pp. 210–265 Search PubMed.
- F. S. Wagner, Jr., Acetic Acid, in Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Inc., New York, 2001, Vol. 1, pp. 115–136 Search PubMed.
- H. Alanko, M. Härkönen, L. Lahtinen, and E. Pajunen, Melt-processable liquid crystalline polyesters, process for the preparation thereof and products prepared from the polyesters , PCT Int. Appl. WO 93/23451, Nov. 25th, 1993.
- K. Kamide, Y. Miyazaki and H. Kobayashi, Polym. J., 1977, 9, 317–327 CrossRef CAS.
- M. Kurata, and Y. Tsunashima, in Polymer Handbook, 4th Edition, ed. J. Brandrup, E. H. Immergut, E. A. Grulke, A. Abe, D. R. Bloch, John Wiley & Sons, New York, 2005, pp. VII/1–VII/68 Search PubMed.
- G. Odian, Principles of Polymerization, Fourth Ed., John Wiley & Sons, New York, 2004, p. 22 Search PubMed.
- M. Run, S. Wu, D. Zhang and G. Wu, Polymer, 2005, 46, 5308–5316 CrossRef CAS.
- M. E. Sánchez, A. Morán, A. Escapa, L. F. Calvo and O. Martínez, J. Therm. Anal. Calorim., 2007, 90, 209–215 CrossRef CAS.
- S. A. Miller, and L. Mialon, Poly(dihydroferulic acid): A Biorenewable Polyethylene Terephthalate Mimic Derived from Lignin and Acetic Acid, U.S. Provisional, Patent Application, Serial No. 61/334,342, filed May 13th, 2010 (University of Florida).
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details and complete polymer characterization data. See DOI: 10.1039/c0gc00150c |
| This journal is © The Royal Society of Chemistry 2010 |