Intensification and economic and ecological assessment of a biocatalytic oxyfunctionalization process†
Daniel Kuhna, Muhammad Abdul Kholiqb, Elmar Heinzleb, Bruno Bühler*a and Andreas Schmidac
aLaboratory of Chemical Biotechnology, Department of Biochemical and Chemical Engineering, Technische Universität Dortmund, Emil-Figge-Str. 66, 44221, Dortmund, Germany. E-mail: bruno.buehler@bci.tu-dortmund.de; Fax: +49 231 7557382; Tel: +49 231 7557384
bTechnische Biochemie, Universität des Saarlandes, Campus A 1.5, 66123, Saarbrücken, Germany
cLeibniz-Institut für Analytische Wissenschaften – ISAS, Otto-Hahn-Str. 6b, 44227, Dortmund, Germany
First published on 9th April 2010
Abstract
Bio-based processes are commonly accepted to be environmentally preferable to chemical alternatives. Reasons include high selectivities, the avoidance of heavy metals, and mild reaction conditions. However, ecological benefits and economic viability have to be verified in each case. Oxygenases are a very attractive enzyme class, allowing selective oxyfunctionalization by introduction of molecular oxygen into hydrocarbons at optical purities unparalleled by traditional chemical methods. Here, styrene monooxygenase from Pseudomonas sp. strain VLB120 was used in recombinant Escherichia coli for the production of enantiopure (S)-styrene oxide from styrene. Substrate and product toxicity was attenuated using a two-liquid phase system with bis(2-ethylhexyl)phthalate as organic carrier solvent. By maintaining previously reported productivities for a longer time period, product concentrations were doubled to 36.3 g Ltot−1 making it one of the most productive biocatalytic oxyfunctionalization processes. This biotransformation was incorporated along with an appropriate upstream and downstream processing into a complete process scheme and compared with chemical process alternatives. Ecological assessment showed the bioprocess to be superior to two chemical process alternatives and slightly inferior to the third one, with solvent use being the most critical factor. However, the bioprocess performed best in terms of production costs ($10.2 kg−1). This study underlines the importance of a detailed ecological and economic assessment of bioprocesses to verify their sustainability and to identify weak points with respect to environmental and/or economical sustainability.
Introduction
Biocatalysis is emerging more and more in the fine chemical industry due to the great versatility and specificity biocatalysts can offer.1–3 Although the number of bio-based processes running on a commercial scale is steadily increasing, the majority of applications are restricted to a few enzyme classes so far.1 The implementation of oxygenases, for example, is restricted to only a few large scale processes,4,5 despite their widely-recognized attractiveness as highly regio- and enantiospecific catalysts.6,7 Such selective oxyfunctionalizations of C–H bonds are difficult to achieve by traditional chemical means. The reasons for the confined utilization of oxygenases are divers, but include cofactor dependence, uncoupling, substrate and product toxicities, substrate uptake, and instability of the biocatalyst.6 All these issues make technology transfer from a laboratory to an industrial scale a demanding and time-consuming task.A systematic assessment supports the scale-up procedure and also shortens the development time by the early identification of potential bottle-necks and disadvantages.8,9 At early stages of the planning phase, the design is still flexible and changes are thus less cost and time intensive than later on. Nevertheless, there are only a few detailed studies assessing the economic and the ecological potential of oxygenase-based processes.10–12 Next to economic parameters, environmental considerations gained attention and are of equal relevance due to the increasing public awareness and stricter legislation.3,13 For the quantification of the impact of processes on the environment, a large number of assessment tools have been developed over the past decades.14–18 Simple and flexible methodologies are required for the characterization of processes at early development stages, as, for example, the quantification of the environmental burden by so called environmental indices.16,19–21
As an example for an efficient oxyfunctionalization biocatalyst, styrene monooxygenase containing recombinant Escherichia coli has been extensively investigated (Fig. 1). This enzyme originates from Pseudomonas sp. strain VLB120 and proved to be a promising biotechnological tool for the selective epoxidation of styrene derivatives to (S)-epoxides with enantiomeric excesses over 98% (99.5% for (S)-styrene oxide).22,23 Enantiopure epoxides are attractive, high value intermediates, because of their potential and versatility as building blocks for the synthesis of many biologically active compounds in optically pure form.24,25 (S)-Styrene oxide, for instance, can serve as a precursor for the synthesis of the nematocide levamisole,26 analgestics,27,28 or the antiarrhythmic drug mexiletine.29
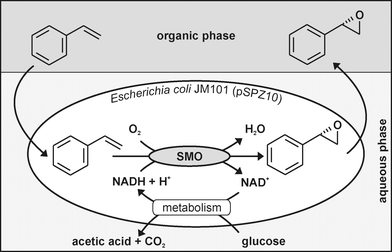 | ||
| Fig. 1 Styrene epoxidation by recombinant E. coli in a two-liquid phase system. Styrene monooxygenase (SMO) from Pseudomonas sp. strain VLB120 selectively oxidizes styrene to (S)-styrene oxide. This reaction depends on the cofactor NADH, which is regenerated by cell metabolism. Bis(2-ethylhexyl)phthalate (BEHP) was used as extraction solvent alleviating substrate and product toxicity. | ||
The dependence of monooxygenases on reduction equivalents, typically in the form of NAD(P)H, and their limited stability outside of microbial cells favor the use of whole cells as biocatalysts over isolated enzymes.30 Thus, recombinant E. coli expressing the styrene monooxygenase genes styAB have been developed and used showing promising rates for (S)-styrene oxide formation.31,32 Substrate and product toxicities were alleviated by in situ product extraction and substrate supply via a second liquid phase of bis(2-ethylhexyl)phthalate (BEHP).31 Thereby, final product concentrations of 36.7 g Lorg−1 and volumetric productivities of 4.19 g Ltot−1 h−1 have been achieved during fed-batch cultivation33 outperforming continuous cultivation by reduced medium requirements.34,35 The scalability of this process was positively evaluated at an early stage by producing 388 g of (S)-styrene oxide at a 30 L pilot scale.36 Although promising results have been achieved by using isolated styrene monooxygenase in combination with formate dehydrogenase-based37 or reductive electrochemical38,39 cofactor regeneration, the process based on growing recombinant E. coli33 proved to be superior in terms of productivity (≥9-fold higher) and achieved product concentration (≥3-fold higher).
In the present study, this process was intensified with respect to product concentration and its economic and ecological feasibility was evaluated and compared to chemical alternatives. The latter consisted of three different unselective epoxidation reactions40–42 combined with hydrolytic kinetic resolution.43,44 The direct chemical epoxidation leads to unsatisfying enantiomeric excesses.45,46
This study contributes to the field of quantitative economic and ecological evaluations comparing biocatalytic and chemical process alternatives.47,48 A greater number of such case studies will help to characterize the benefits and challenges of biotechnology and ultimately facilitate and accelerate the implementation of sustainable biocatalytic processes in fine chemical and pharmaceutical syntheses.
Results and discussion
Intensification of a biocatalytic styrene epoxidation process based on recombinant E. coli
The highest (S)-styrene oxide concentration achieved so far in biocatalytic two-liquid phase biotransformations with BEHP as extractive phase was 36.7 g Lorg−1 (Table 1).33 In order to fully exploit the economic and ecological potential of a biocatalytic process, it is necessary to identify limiting factors on the reaction level and to optimize the process performance to a maximum. To investigate possible constraining effects such as substrate limitation and product inhibition, the initial substrate concentration and the styrene feeding profile were varied. In the above mentioned biotransformation, 340 mM of styrene were added to the organic phase, an amount which led to complete substrate depletion and substrate limitation at the end of the biotransformation.33 In order to avoid such a confinement by styrene availability, its addition was increased stepwise in several experiments (results not shown). It was found that the most favorable initial styrene concentration was 700 mM, since this was the highest quantity still completely converted. Fig. 2 shows the typical course of the optimized biotransformation.| Parameter | Unit | Park et al.33 | This study |
|---|---|---|---|
| a Values estimated from figure. | |||
| Biotransformation time | h | 4.5 | 8 |
| Initial styrene concentration | mM | 340 | 695 |
| Final (S)-styrene oxide concentration | mM | 314 | 604 |
| Final 2-phenylethanol concentration | mM | 69 | |
| Cell concentration after batch | gCDW Laq−1 | 9a | 5.4 |
| Maximal cell concentration | gCDW Laq−1 | 32 | 39.3 |
| Glucose fed | g | 69a | 113.7 |
| Acetate formed | g | 10a | 3.6 |
| Average productivity | g Ltot−1 h−1 | 4.19 | 4.54 |
| Max. productivity | g Ltot−1 h−1 | 6.49 | 6.57 |
| Max. volumetric productivity | U Laq−1 | 1800 | 1821 |
| Max. specific epoxidation rate | U gCDW−1 | 60 | 56 |
 | ||
Fig. 2 Styrene epoxidation by E. coli JM101 (pSPZ10) during two-liquid phase biotransformation. After the batch phase was finished (time −2.83 h), glucose feed was started. At 0 h, the biotransformation was initiated by the addition of 1 L of BEHP containing 80 ml styrene and 10 ml octane. The phase ratio between the organic and the aqueous phase was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. During the biotransformation, the feed rate was kept constant at 12.38 gglucose h−1 and was not reduced until glucose was detected in the aqueous phase at the end of the biotransformation. 1. During the biotransformation, the feed rate was kept constant at 12.38 gglucose h−1 and was not reduced until glucose was detected in the aqueous phase at the end of the biotransformation. | ||
After addition of the organic phase, the expression of the styrene monooxygenase genes was induced by octane and epoxidation activity was already detected within the first hour. The specific epoxidation activity approached a maximum of 56 U gCDW−1 after 3 h. These activities are in accordance with previous biotransformations.33 The maximal activity was independent of the initial styrene concentration, indicating the absence of substrate inhibition. This was confirmed by experiments wherein styrene was fed stepwise, which did not improve the process performance (results not shown). During continuous cultivation at lower styrene concentrations the activities of growing E. coli JM101 (pSPZ10) have been shown to be limited by the intracellular NADH availability.34 This can also be expected for the biotransformation during fed-batch cultivation. 2-Phenylethanol accumulated as a by-product at approximately 10% of the rate of styrene oxide formation as seen in previous studies.22,36 Hydrolysis of (S)-styrene oxide was virtually prevented by its extraction to the organic phase to a level of ∼0.1% of the (S)-styrene oxide produced, which is in accordance to literature.33,37
The specific activity of the cells was maintained above 40 U gCDW−1, until growth stopped after 5 to 6 h of biotransformation. At this stage, glucose started to accumulate in the aqueous phase, despite reducing the glucose feed rate after glucose detection in the fermentation broth. This, together with the increasing dissolved oxygen concentration, indicated a reduction of the cellular metabolic activity, finally leading to cessation of growth and epoxidation. Styrene limitation can be excluded as a cause of the activity decrease, since the styrene concentration was still well above the apparent Ks value of 12.7 ± 1.9 mM in the organic phase,34 when the activities were declining between 5 and 7 h of biotransformation. The activity loss can be ascribed to the rising aqueous styrene oxide concentrations, reaching 2.2 mM and 3.1 mM after 5 and 7 h, respectively. These concentrations have been shown to heavily affect E. coli growth and metabolism.33 The toxification by the product restricts the maximal product concentration to around 600 mM (S)-styrene oxide. However, the productivity was maintained above 4 g Ltot−1 h−1 for 4 h before toxification, and therefore the final product concentration was 72.6 g Lorg−1 (= 604 mM) and thus nearly doubled compared to previous experiments (Table 1). This improvement halves the medium requirements and equipment size.
The high genetic stability of the recombinant strain is emphasized by the stable biocatalyst performance in the absence of antibiotics during four successive runs each involving batch and fed-batch cultivation including biotransformation (results not shown). Bioprocessing without antibiotics greatly improves its economic and ecological sustainability.
The avoidance of substrate limitation and the absence of substrate inhibition allowed the improvement of the biocatalytic process to an upper limit imposed by product toxicity. This optimized process meets the performance of the most powerful oxygenase-based processes described, and thus is considered to be feasible and mature for an in-depth ecological and economic assessment.
Biotechnological (S)-styrene oxide production process
An economic and ecological assessment relies on the knowledge of detailed mass-balances over the entire process (for details, see Experimental part). Therefore, the biotransformation of Fig. 2 has to be combined with suitable upstream and downstream operations. The biocatalytic (S)-styrene oxide production plant was designed and dimensioned by means of the engineering software SuperPro Designer version 6.0.8 This simulation tool allows the calculation of overall mass-balances by the generation of flow-sheets. The process scheme of the biotechnological production process is shown in Fig. 3. On the basis of the production scale of comparable chiral compounds, the production scale of such a (S)-styrene oxide production plant was estimated to be 1000 tons per year.1,2 Subsequently the implemented unit operations are addressed in detail. | ||
| Fig. 3 Flow-sheet for biotechnological (S)-styrene oxide production. The flow-sheet was generated in the SuperPro Designer software. For details, see Experimental part. Framed circles describe input mass streams and framed squares output or waste streams. | ||
The upstream processing comprises the preparation and the sterilization of the growth medium and precultivation in a seed fermenter. Sterilization is an important issue in bioprocesses using genetically modified organisms (GMOs), because contamination by other microbes and release of the GMOs have to be prevented or minimized, respectively. Prior to sterilization, the following cleaning-in-place (CIP) procedure is implemented in the process scheme. CIP included washing steps with water (5 min), 0.5 M sodium hydroxide (80 °C, 15 min) and again with water (5 min). Afterwards, the clean fermenters are sterilized in place by steam at 150 °C for 15 min.
Prior to the fermentations, the Riesenberg cultivation medium is heat-sterilized and afterwards complemented with a separately filtered solution containing glucose, magnesium sulfate, trace elements and thiamine. 90% of the medium is directly channeled to the main fermenters and 10% is used for precultivation in the seed fermenter (5.25 m3). The growth of E. coli JM101 (pSPZ10) is described by the empirical reaction eqn (1).
| 0.39 C6H12O6 + 1.30 O2 + 0.20 NH3→ 1.00 CH1.8O0.5N0.2 + 1.35 CO2 + 1.75 H2O | (1) |
Precultivation takes 12 h and stops after the depletion of the carbon source glucose at a cell concentration of approximately 5.4 gCDW L−1. In the main fermenters, the batch phase is started by the transfer of the preculture from the seed fermenter. The growth reaction is identical to the above described eqn (1), but glucose is expected to be completely consumed after 8 h of cultivation. After depletion of the carbon source, a feed, consisting of a separately filtered glucose and magnesium sulfate solution, is initiated. The biotransformation is started by organic phase addition after 4 h of fed-batch cultivation. The reaction stoichiometry during fed-batch cultivation and biotransformation is focusing on (S)-styrene oxide and the biomass is regarded as a by-product (eqn (2)).
| 0.98 C6H12O6 + 3.77 O2 + 0.46 NH3 + 1.15 C8H8→ 2.28 CH1.8O0.5N0.2 + 3.39 CO2 + 0.10 C2H4O2 + 4.18 H2O + 1.00 C8H8O + 0.11 C8H10O + 0.04 C8H8 evaporated | (2) |
Eqn (2) is based on the data shown in Table 1 and the values for oxygen, carbon dioxide, and water were calculated by atom balancing. Evaporation of styrene (C8H8) was considered as the proportion of styrene that was neither converted to styrene oxide (C8H8O) nor to 2-phenylethanol (C8H10O).
The scalability of the reaction setup was previously shown by a two-liquid phase styrene epoxidation process at a pilot-scale, reaching a similar process performance as at a laboratory scale.36 The crucial parameters for up-scaling are mass transfer rates and the avoidance of inhomogeneities. Especially, the oxygen transfer rate is of high importance, since oxygen is not only required for growth, but also for the epoxidation reaction. Oxygen transfer rates were shown to be sufficient even for 100 m3 fermenters for a similar two-liquid phase process.10 The optimal reactor size for the two main fermenters in terms of investment costs and capacity utilization is 56.4 m3, wherein the oxygen transfer achieved by aeration and stirring is expected to satisfy the oxygen demand of the biocatalyst.
The feasibility of downstream processing consisting of phase separation by centrifugation and distillation was demonstrated previously by Panke et al.36 On industrial scales a continuous downstream processing is preferred. Thus, the setup shown in Fig. 3 includes a storage tank, which enables a continuous flow towards a disk-stack centrifuge, separating the broth into a solid (cells), an aqueous, and an organic liquid phase. Legislation requires sterilization and complete inactivation of genetically engineered cells before disposal. Subsequent use, e.g. as fertilizer, is therefore not straight forward. Disposal costs for the cell paste and the aqueous phase were set to $0.15 and $0.0025 kg−1, respectively.
Work-up of the organic phase is done by continuous distillation. Due to obvious ecological and economic reasons, the recycling of the organic carrier solvent, in this case BEHP, is crucial for two-liquid phase processes on an industrial scale. This is done in the first distillation column. The quality of recycled BEHP was ensured by a purge flow of 5%. Volatile impurities such as remaining traces of styrene and octane are separated from the product stream in the second distillation step, before (S)-styrene oxide is separated from 2-phenylethanol in the last column. Due to its rose-like odor, 2-phenylethanol is considered to be industrially relevant in the food and flavor industries.49 The quality of this output stream would allow additional marketing, which was not considered here.
The process model as represented by the flow-sheet in Fig. 3 sets the basis for an in-depth ecological and economic assessment of the feasibility of biocatalytic (S)-styrene oxide production on industrial scale.
Alternative chemical processes for (S)-styrene oxide production
In order to compare the biocatalytic epoxidation and chemical process alternatives with respect to economic and environmental performance, chemical routes towards (S)-styrene oxide with an enantiomeric excess of >98% were analyzed.The chemical equivalent to the styrene monooxygenase catalyzed reaction is the selective epoxidation by means of salen transition metal catalysts discovered by the Jacobsen group, often referred to as Jacobsen catalysts.46 However, the direct epoxidation of terminal double bonds, as in styrene, suffers from insufficient selectivities (e.e. of around 36%)50 and/or impractical reaction temperatures of −78 °C (e.e. of 86%).51
Hydrolytic kinetic resolution of racemic styrene oxide is an attractive alternative leading to e.e. values comparable to the styrene monooxygenase catalyzed epoxidation.43 The dependence on racemic styrene oxide as a substrate requires a preceding unselective epoxidation of styrene. For this reaction step, three different methods with high productivities have been published. These are based on a ferric phenanthroline,40 a titanium silicate41 and a manganese sulfate52 catalyst. For the design of the chemical (S)-styrene oxide production processes, these unselective epoxidation reactions were coupled to the hydrolytic kinetic resolution to obtain the product with high optical purity (e.e. 98%) from styrene. In the following, the hydrolytic kinetic resolution and then the three epoxidation methods are discussed in the context of process modeling and their flow-sheets (Fig. 4).
 | ||
| Fig. 4 Flow-charts of the chemical epoxidation processes. The flow-sheets were generated by means of the SuperPro Designer software. Processes based on ferric phenanthroline (a), titanium silicate (b), and manganese sulfate (c) catalysts are depicted. Framed circles describe input mass streams and framed squares output or waste streams. Abbreviations: acetonitrile, ACN; ferric phenanthroline catalyst, Fe-catalyst; titanium silicate catalyst, TS-1 catalyst. | ||
The hydrolytic kinetic resolution reported by Tokunaga et al. includes the selective conversion of one enantiomer of racemic styrene oxide to the corresponding 1-phenyl-1,2-ethanediol by the attack of water.43 In the procedure described, (S)-styrene oxide is isolated in an e.e. of 98% and a yield of 38% by applying 0.008 equivalents of (S,S)-(salen)CoIII(OAc) catalyst and 0.7 equivalents of water. Water-miscible solvents such as tetrahydrofuran would enhance the epoxide yield to around 44%, but simultaneously increase the complexity of the process.44
(S,S)-(salen)CoIII(OAc) is inactivated during the reaction, but the inactive (S,S)-(salen)CoII can be completely regenerated by dissolving the catalyst in an appropriate solvent, e.g. toluene, followed by treatment with acetic acid in the presence of oxygen.44
Due to its complex synthesis44,53 and hence its high price, recycling of the chiral catalyst is desirable and also technically feasible, as it can be recovered by filtration54 or in the retentate of a distillation column.43 No reduction of the performance was observed with reused catalyst for three consecutive runs.43,44 Therefore, a recycling stream with a recovery of 90% is implemented in the process models (Fig. 4).
Separation of (S)-styrene oxide from (R)-1-phenyl-1,2-ethanediol was achieved by vacuum distillation.44 The enantiopure (R)-1-phenyl-1,2-ethanediol constitutes a potential building block for the synthesis of (R)-mandelic acid, β-lactam antibiotics, and analytical reagents.55 As in the case of 2-phenylethanol, separate marketing was not considered.
For the epoxidation of double bonds, the ferric phenanthroline catalyst of Dubois et al. is promising (Fig. 4A).40 This catalyst efficiently converts a wide range of alkenes including terminal alkenes such as styrene. The oxidant is peracetic acid, which has to be added in excess (2 equimolar). The selectivity of the reaction is 63%, because low pH and the presence of acetic acid cause the formation of the by-product phenyl acetaldehyde (37%). Two distillation columns assure the quality of the acetonitrile recycling stream and separate the volatile acids from the product stream, respectively. The second column, where acetic acid and peracetic acid are evaporated and collected in the distillate, is critical with respect to safety and corrosion. The third column separates styrene oxide from phenyl acetaldehyde. Separation of these two components is difficult, because their chemical characteristics, including their boiling points, are highly similar. Thus, isolation of the racemic styrene oxide to high purity might be difficult to realize on a large scale.
Titanium silicate catalysts (TS-1) were used for the epoxidation of terminal double bonds by several groups (Fig. 4B).41,56–58 This catalyst often leads to the formation of by-products such as benzaldehyde or phenyl acetaldehyde. Rode et al. almost completely avoided by-product formation by maintaining the pH at 7.5–8.0.41 Styrene is epoxidized by hydrogen peroxide to styrene oxide (selectivity: 96.5%), while benzaldehyde is only formed to a small extent as a by-product (selectivity: 3.1%).41 The heterogeneous nature of the titanium silicate catalyst allows catalyst recovery by simple filtration of the reaction mixture.41 The synthesis of the catalyst is rather simple, but requires expensive tetra-tert-butyl orthotitanate.59 Since titanium silicate structures are prone to substantial deactivation due to titanium leaking from active sites and deterioration of the catalyst surface in the presence of aqueous hydrogen peroxide and methanol,60 its reuse does not make sense making recycling obsolete. The solvents for the epoxidation, methanol and acetonitrile, can easily be separated from the product stream by distillation. Their consumption is minimized by recycling. The quality is assured by replacing 5% of this stream by fresh solvents per cycle. A smaller column is used to separate benzaldehyde from styrene oxide, before channeling the product stream to the kinetic resolution.
In a simple method published by Lane et al., manganese salts, such as manganese sulfate, in combination with bicarbonate catalyze the epoxidation of alkenes using aqueous hydrogen peroxide as oxidant (Fig. 4C).61 Buffering of the reaction system by urea improves the reaction.42 The epoxidation tank contains a two-phase reaction mixture consisting of an organic styrene phase and an aqueous phase containing urea, sodium bicarbonate, manganese sulfate, and hydrogen peroxide. After the reaction, the two phases are separated by a decanter centrifuge and the organic phase is distilled to generate a purified styrene oxide stream to the hydrolytic kinetic resolution tank. Inexpensive manganese sulfate is used in small amounts. Thus, in contrast to the previously presented catalysts, its reuse is economically irrelevant and its recycling is not considered in this study. Unfortunately, the by-products formed to 5% during the reaction have not been identified. Here, it is assumed that these impurities can be removed via the volatile fraction of a distillation column as in the cases of benzaldehyde and phenyl acetaldehyde.
The incorporation of the presented process models in the SuperPro Designer software allowed the economic and ecological assessment including the calculations of the mass-balances.
Process assessment: resource consumption and waste generation
Balancing all of the involved chemical compounds is typically the starting point of any economic and ecological assessment. Quantification of resource consumption and waste generation serves as the basis for the assessment procedure and allows first insights in the sustainability of a process. Lower input streams reduce the environmental burden by definition, since the resources are utilized more effectively. Resource consumption and waste generation of the aforementioned process variants in terms of total mass are displayed in Fig. 5 and Table 2.| Ferric phenanthroline | Titanium silicate | Manganese sulfate | Biotechnol. process | |||||
|---|---|---|---|---|---|---|---|---|
| Input/kg kgSO−1 | Output/kg kgSO−1 | Input/kg kgSO−1 | Output/kg kgSO−1 | Input/kg kgSO−1 | Output/kg kgSO−1 | Input/kg kgSO−1 | Output/kg kgSO−1 | |
| Substances contributing less than 1 kg kg(S)-styrene oxide−1 to the mass-balances are summarized in the category “miscellaneous”. | ||||||||
| (R)-1-Phenyl-1,2-ethanediol | 0 | 1.08 | 0 | 1.13 | 0 | 1.12 | 0 | 0 |
| (S)-Styrene oxide | 0 | 1.00 | 0 | 1.00 | 0 | 1.00 | 0 | 1.00 |
| 50% Hydrogen peroxide | 0 | 0 | 0.31 | 0.05 | 1.33 | 0.76 | 0 | 0 |
| Acetic acid | 0.01 | 1.60 | 0.01 | 0.01 | 0.01 | 0.01 | 0 | 0.05 |
| Glucose | 0 | 0 | 0 | 0 | 0 | 0 | 1.69 | 0 |
| Peracetic acid | 4.03 | 2.01 | 0 | 0 | 0 | 0 | 0 | 0 |
| Phenyl acetaldehyde | 0 | 1.18 | 0 | 0 | 0 | 0 | 0 | 0 |
| Styrene | 2.76 | 0 | 1.89 | 0.09 | 1.85 | 0.11 | 1.00 | 0.03 |
| Urea | 0 | 0 | 0 | 0 | 2.83 | 2.83 | 0 | 0 |
| Water | 8.72 | 8.57 | 0.48 | 0.33 | 5.92 | 6.07 | 15.92 | 16.65 |
| Miscellaneous | 0.64 | 0.71 | 0.35 | 0.44 | 0.87 | 0.90 | 2.46 | 3.34 |
| Sum | 16.15 | 16.15 | 3.04 | 3.04 | 12.80 | 12.80 | 21.07 | 21.07 |
 | ||
| Fig. 5 Mass-balances of resource consumption and waste generation. Resource consumption is displayed in (a), whereas product and waste formation is shown in (b). Substances contributing less than 1 kg kg(S)-styrene oxide−1 to the mass-balances are summarized in the category “miscellaneous”. Abbreviations: Fe-cat, ferric phenanthroline; TS-1, titanium silicate; MnSO4, manganese sulfate; Biol, biotechnological process. | ||
The titanium silicate process excels in the low resource demand and waste generation, consuming only 3.04 kg resources for the production of 1 kg of (S)-styrene oxide (subsequently kgSO). The recycling of the epoxidation solvents and the efficient use of hydrogen peroxide are the main reasons for this outstanding performance.
The manganese sulfate and the ferric phenanthroline processes are inferior with larger resource demands of 12.80 and 16.15 kg kgSO−1, respectively. The dependence on an excess of oxidants is a disadvantage of these reactions. Furthermore, the oxidants are employed in aqueous solutions, hydrogen peroxide in a 50% and peracetic acid in a 32% solution, increasing the mass balances by the contained water fraction, which, however, is only of minor ecological and economic significance. Apart from the ecological impact of the oxidants, their handling may cause additional safety costs, which were not included in the economical assessment.
As expected from the typically high water requirement of biocatalytic processes (here 76%), the biotechnological setup requires the highest amount of resources (21.07 kg kgSO−1). The cleaning-in-place procedures for the fermenters adding another 4.91 kg of water and 0.04 kg of sodium hydroxide for the production of 1 kg (S)-styrene oxide are not included. The reuse of the medium is hampered by toxic concentrations of (S)-styrene oxide and 2-phenylethanol and the accumulation of acetic acid. Other substances added in prominent amounts are the carbon source glucose and the organic phase BEHP. Since the styrene monooxygenase is highly enantioselective, the lowest amount of styrene needs to be employed and the only by-product, 2-phenylethanol, is formed to a minor extent (116 g kgSO−1).
E-factors, defined as the mass ratio of waste to desired product, are an environmental metric concept addressing the problem of waste generation in synthetic industry.14,62 All involved compounds are considered with the sole exception of water. This neglect of water utilization uprates the biotechnological setup, which has with 3.42 a lower E-factor than the ferric phenanthroline (6.58) and manganese sulfate (5.72) based processes. Titanium silicate remains the most favorable epoxidation catalyst regarding the waste generation (E-factor: 1.71). It is noteworthy that all presented processes are below or at the lower limit of the E-factors of 5–50 typically encountered in fine chemical processes of 100–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t a−1 scale.62
000 t a−1 scale.62
In conclusion, the titanium silicate catalyst based process is clearly the most favorable procedure regarding resource demand (3.04 kg kgSO−1), followed by the manganese sulfate and the iron phenanthroline catalyst based processes with resource demands of 12.80 and 16.15 kg kgSO−1, respectively. Although the biocatalytic setup depends on 21.07 kg kgSO−1, it can be considered economically and environmentally interesting, as the dominating proportion is water with 15.92 kg kgSO−1, which is cheap and environmentally harmless. In order to take the differing environmental impact of the involved chemicals into account, environmental indices are introduced in the next section for the quantitative assessment of the overall impact of the processes.
Process assessment: environmental impact potential
The environmental burden of a process is not only defined by the amount of resources consumed and waste formed, but also by their harmfulness. It is self-explanatory that 1 kg of heavy metal or solvent waste imposes a bigger ecological problem than 1 kg of water. Quantification and balancing of the multi-faceted impacts on the environment was performed by the assignment of so-called environmental indices to all compounds involved.16,20,21 The results are presented in Fig. 6 and Table 3.| Ferric phenanthroline | Titanium silicate | Manganese sulfate | Biotechnol. process | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| input | output | total | input | output | total | input | output | total | input | output | total | |
Input and output indices were added in a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 ratio to obtain the overall environmental indices (referred to as total index in the text). Substances contributing to a minor extent are summarized in the category “miscellaneous”. 60 ratio to obtain the overall environmental indices (referred to as total index in the text). Substances contributing to a minor extent are summarized in the category “miscellaneous”. | ||||||||||||
| (S)-styrene oxide | 0 | 46.2 | 27.7 | 0 | 46.2 | 27.7 | 0 | 46.2 | 27.7 | 0 | 46.2 | 27.7 |
| 50% hydrogen peroxide | 0 | 0 | 0 | 10.9 | 1.2 | 5.1 | 46.0 | 19.2 | 29.9 | 0 | 0 | 0 |
| acetic acid | 0.1 | 29.2 | 17.6 | 0.2 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 0 | 0.9 | 0.5 |
| BEHP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31.1 | 22.9 | 26.2 |
| peracetic acid | 136.6 | 58.1 | 89.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| styrene | 60.3 | 0 | 24.1 | 41.4 | 1.9 | 17.7 | 40.3 | 2.2 | 17.5 | 21.8 | 0.6 | 9.1 |
| urea | 0 | 0 | 0 | 0 | 0 | 0 | 2.8 | 27.5 | 17.6 | 0 | 0 | 0 |
| water | 8.7 | 8.6 | 8.6 | 0.5 | 0.3 | 0.4 | 5.9 | 6.1 | 6.0 | 15.9 | 16.6 | 16.4 |
| miscellaneous | 5.7 | 21.8 | 15.3 | 4.9 | 11.6 | 8.9 | 2.3 | 8.2 | 5.8 | 9.7 | 14.6 | 12.7 |
| sum | 211.5 | 163.9 | 182.9 | 57.7 | 61.4 | 59.9 | 97.5 | 109.5 | 104.7 | 78.6 | 102.0 | 92.6 |
 | ||
Fig. 6 Environmental indices for the input (a) and the output (b) side of the process and overall environmental indices (c). Input and output indices were added in a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 ratio to obtain the overall environmental indices (referred to as total index in the text). Substances contributing to a minor extent are summarized in the category “miscellaneous”. Abbreviations: Fe-cat, ferric phenanthroline; TS-1, titanium silicate; MnSO4, manganese sulfate; Biol, biotechnological process. 60 ratio to obtain the overall environmental indices (referred to as total index in the text). Substances contributing to a minor extent are summarized in the category “miscellaneous”. Abbreviations: Fe-cat, ferric phenanthroline; TS-1, titanium silicate; MnSO4, manganese sulfate; Biol, biotechnological process. | ||
In terms of sustainability, the ferric phenanthroline process clearly performed the worst (total environmental index: 183). This poor performance is mainly due to safety and corrosion risks by high amounts of peracetic and acetic acids. Furthermore, significant by-product formation leads to a high consumption of all resources and contributes to the environmental index on the output side (acetic acid, phenyl acetaldehyde, and (R)-1-phenyl-1,2-ethanediol).
The titanium silicate process exhibits the lowest environmental index (60) due to its minimal resource demand as exemplified by the almost 100% utilization of hydrogen peroxide as oxidant. In contrast, the manganese sulfate process requires an excess of hydrogen peroxide, which, together with the high amount of urea waste, leads to an intermediate environmental index (105).
The biotechnological process performs second in terms of sustainability (total environmental index: 93), despite showing the highest input and output mass-balances. The biotechnological approach profits from the avoidance of harmful substances, e.g. by using molecular oxygen from air as oxidant. The organic solvent BEHP is the most problematic factor and is negatively judged due to its origin from fossil resources and its toxicity to humans (environmental index: 26). Although replacement of the organic phase is obviously favored from an environmental perspective, it is difficult to find an alternative solvent with similar extraction and superior ecological characteristics. BEHP was chosen as it provides a high organic/aqueous partition coefficient for styrene oxide, considering also other important criteria for solvent selection such as low water solubility, low toxicity to microbes, chemical and thermal stability, non-biodegradability, low foam formation in bioreactors, and availability at low prices.63,64 A lower extraction efficiency for styrene oxide would increase its aqueous concentration, resulting in an earlier inactivation of the biocatalyst and finally in a reduced final product concentration.
From an ecological perspective, this study clearly identifies the carrier solvent to be the most critical factor in two-liquid phase biotransformations. Thus, environmental sustainability is proposed to be taken up as a key parameter for solvent selection.
Process assessment: economic potential
Economic success and profitability are the driving forces for the development of all new processes in industry. A process can be beneficial in terms of ecology, but will never be implemented if the economic parameters are unfavorable. Therefore the economic viability of the four processes was evaluated on the basis of the SuperPro Designer flow-sheets as they contain all necessary equipment, material streams, and labor-requiring operations for a reliable estimation of the fixed and variable production costs. The approach for the economic assessment is described in detail in the Experimental section. The resulting key economic parameters for plants designed for an annual production of 1000 t (S)-styrene oxide are summarized in Table 4.| Parameter | Unit | Ferric phenanthroline | Titanium silicate | Manganese sulfate | Biotechnol. process |
|---|---|---|---|---|---|
| Data sources are listed in the assessment methods part. | |||||
| Capital investment | million $ | 15.1 | 10.1 | 9.6 | 20.4 |
| Direct fixed capital | million $ | 10.9 | 8.4 | 8.2 | 18.9 |
| Working capital | million $ | 3.6 | 1.4 | 1.0 | 0.6 |
| Start-up costs | million $ | 0.6 | 0.4 | 0.4 | 0.9 |
| Annual operating costs | million $ a−1 | 41.6 | 16.9 | 12.8 | 10.2 |
The investment costs for the biotechnological plant were found to be 1.4 to 2.1 times higher as compared to the chemical alternatives. Biocatalytic processes typically are capital intensive, because large and complex fermenters are required. Main reasons for this are usually dilute reaction systems and complex requirements for process control to guarantee optimal growth of microorganisms.10,65 Additionally, sterilization and biocontainment entail extra measures. In the evaluated example, the seed and main fermenters make up 55% of the equipment costs, also because the downstream processing is straightforward and relatively cost saving. The purchase costs of the fermenters might be reduced by modulating and rebuilding second-hand fermenters or leasing equipment.65 In the latter case, recurring costs have to be weighed with investment costs. The comparably dilute product concentration in the fermenter is another reason for the high investment costs. As a consequence, the equipment has to be designed larger as compared to chemical plants to achieve similar product amounts.10,47,66 This fact stresses the importance of the process intensification presented in the first part of this study.
In contrast to the investment costs, the biotechnological process outperforms all chemical processes with respect to operational costs (Table 5).
| Category | Fe-cat. [$ kg−1] | TS-1 [$ kg−1] | MnSO4 [$ kg−1] | Biol. [$ kg−1] |
|---|---|---|---|---|
| Data sources are listed in the assessment method part. | ||||
| Facility dependent costs | 1.96 | 1.57 | 1.55 | 3.16 |
| depreciation | 1.04 | 0.79 | 0.78 | 1.80 |
| insurance | 0.11 | 0.08 | 0.08 | 0.19 |
| local taxes (property) | 0.22 | 0.17 | 0.16 | 0.38 |
| factory expenses | 0.30 | 0.30 | 0.30 | 0.30 |
| maintenance | 0.30 | 0.23 | 0.22 | 0.50 |
| Raw materials | 34.95 | 13.02 | 7.77 | 2.86 |
| (salen)CoII catalyst | 2.48 | 2.48 | 2.48 | — |
| 50% hydrogen peroxide | — | 0.69 | 2.96 | — |
| acetic acid | 0.01 | 0.01 | 0.01 | — |
| acetonitrile | 0.74 | 0.18 | — | — |
| ammonium | — | — | — | 0.02 |
| bis(2-ethylhexyl)phthalate | — | — | — | 1.17 |
| ferric phenanthroline catalyst | 23.25 | — | — | — |
| glucose | — | — | — | 0.86 |
| manganese sulfate | — | — | 0.01 | — |
| methanol | — | 0.03 | — | — |
| octane | — | — | — | 0.04 |
| peracetic acid | 6.59 | — | — | — |
| riesenberg medium salts | — | — | — | 0.12 |
| sodium bicarbonate | — | — | 0.47 | — |
| styrene | 1.87 | 1.22 | 1.21 | 0.63 |
| thiamine hydrochloride | — | — | — | 0.01 |
| titanium silicate catalyst | — | 8.40 | — | — |
| toluene | 0.01 | 0.01 | 0.01 | — |
| urea | — | — | 0.63 | — |
| trace elements | — | — | — | 0.00 |
| Total labor expenses | 1.62 | 1.66 | 1.56 | 2.86 |
| Laboratory/quality control | 0.24 | 0.25 | 0.23 | 0.43 |
| Consumables | — | 0.12 | — | 0.25 |
| Waste treatment | 2.31 | 0.15 | 1.70 | 0.26 |
| Utilities | 0.50 | 0.11 | 0.04 | 0.37 |
| Total unit production costs | 41.59 | 16.89 | 12.85 | 10.19 |
The discrepancy among the different processes is mainly caused by differing raw material costs. As expected, the biocatalytic process benefits from the absence of expensive chemical catalysts, which dominate the material costs of all chemical processes. The integration of biocatalytic synthesis into the (S)-styrene oxide production plant is advantageous, because the catalyst does not have to be purchased or produced in a separate process. The medium costs for the growth of the biocatalyst ($1.0 kgSO−1) contribute only to a minor extent to the overall costs, although a high amount of biomass is formed during the fed-batch cultivation. Thus, the total expenses for raw materials, i.e. $2.9 kgSO−1, play a subordinate role (28% of the operational costs) for the bioprocess, with BEHP ($1.2 kgSO−1), the growth medium ($1.0 kgSO−1) and styrene ($0.6 kgSO−1) as major factors. This contrasts the significant costs of the epoxidation catalysts ferric phenanthroline ($23.3 kgSO−1) and titanium silicate ($8.4 kgSO−1). The inexpensive manganese sulfate is the exception profiting from its simplicity ($0.01 kgSO−1).
For the bioprocess, the factory-related fixed costs are higher than the raw material costs. Especially, the depreciation of the expensive equipment has a pronounced impact. In this study, the depreciation period was assumed to be 10 years for all equipment. In the case of the fermenters, one can argue for longer depreciation periods of 12 or 15 years.10,67 This will, however, only slightly reduce the depreciation costs by $0.09 and $0.18 kgSO−1, respectively.
Labor-related expenses belong to the major cost factors in the biocatalytic plant. Compared to the chemical processes, especially the upstream processing of the biocatalytic setup is more complex and more labor-intensive. Furthermore, the cultivation and biotransformation steps have to be monitored carefully. Thus, less operational effort is required in all chemical plants despite the fact that two reaction steps have to be employed.
The high fixed and the low variable production costs of the biocatalytic process would clearly favor larger annual production volumes, as the contribution of the fixed costs with respect to total costs decreases with increasing scale. For example, in an economic study on a comparable two-liquid phase process for the annual production of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t 1-octanol, variable expenses accounted for 70% of the production costs,10 whereas (S)-styrene oxide production at a 1000 t a−1 scale was dominated by fixed costs accounting for 63% of the total costs.
000 t 1-octanol, variable expenses accounted for 70% of the production costs,10 whereas (S)-styrene oxide production at a 1000 t a−1 scale was dominated by fixed costs accounting for 63% of the total costs.
From an economic perspective, only the manganese sulfate-based plant can compete with the biotechnological process. This is visualized in Fig. 7 showing the net profit and the payback time as a function of the selling price.
 | ||
| Fig. 7 Net profit and payback time as a function of the selling price. Net profits (a) and payback time (b) are shown for selling prices between $8 and $30 kg−1. | ||
While the net profit only depends on the revenues and the operational costs, the calculation of the payback time also considers the capital investment. At low selling prices, the biotechnological process outcompetes the chemical alternatives in both categories. Above prices of $15.4 kgSO−1, the payback time favors the manganese sulfate process due to low investment costs. The best process method from a environmental point of view, the titanium silicate based process, reaches similar payback times only at a relatively high selling price of $24 kgSO−1. The ferric phenanthroline process is not competitive with unit production costs of $41 kgSO−1.
Borole et al. performed a similar economic analysis of an enzymatic (S)-styrene oxide production plant based on chloroperoxidase of the fungus Caldariomyces fumago (scale: 200 tSO a−1).11 The high costs for the enzyme resulted in non-competitive (S)-styrene oxide production costs of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153 $ kgSO−1. This is clearly inferior to all epoxidation processes evaluated here, but might be improved by gene expression and enzyme production in recombinant Aspergillus niger as proposed in the respective study. According to the authors, a selling price of $25 kg−1 could be assumed for enantiopure styrene oxide. Except for the ferric phenanthroline process, all evaluated processes are highly profitable at this selling price. However, with increasing amounts of (S)-styrene oxide produced, the market price might decrease favoring processes already profitable at lower selling prices as the styrene monooxygenase- and the manganese sulfate-based reaction setups.
153 $ kgSO−1. This is clearly inferior to all epoxidation processes evaluated here, but might be improved by gene expression and enzyme production in recombinant Aspergillus niger as proposed in the respective study. According to the authors, a selling price of $25 kg−1 could be assumed for enantiopure styrene oxide. Except for the ferric phenanthroline process, all evaluated processes are highly profitable at this selling price. However, with increasing amounts of (S)-styrene oxide produced, the market price might decrease favoring processes already profitable at lower selling prices as the styrene monooxygenase- and the manganese sulfate-based reaction setups.
Conclusion
The process intensification presented in the first part of this study shows that two-liquid phase biotransformations allow the production of toxic compounds such as (S)-styrene oxide from cheap, but toxic, substrates such as styrene by whole-cell biocatalysis to high final product concentrations (72.6 g Lorg−1). The process performance is comparable to the most powerful oxygenase-based processes in terms of product concentration and average productivity (4.19 g Ltot−1 h−1), proving a basis for the industrial feasibility of this remarkably attractive enzyme class.6,7 In an in-depth environmental and economic assessment of this optimized process for a 1000 t a−1 scale, the organic solvent BEHP was identified as the most critical factor. Its origin from fossil resources and its human toxicity were the main reasons for the negative judgment resulting in a contribution of 28% to the overall environmental index of the bioprocess. Thus, the environmental compatibility of the organic extractive phase has to be addressed foremost in the bioprocess to become ecologically superior to the best chemical (S)-styrene oxide production method, which is based on titanium silicate as epoxidation catalyst (Table 6). Nevertheless, the biotechnological approach was found to be highly interesting, because it outcompeted all evaluated chemical alternatives in terms of economics (Table 6). Whereas the production costs for 1 kg (S)-styrene oxide by the environmentally friendliest method amount to $16.9, the biocatalytic process produced the same quantity for $10.2 or 60% of the expenses. Solving the problem of the environmental burden of the organic phase would ultimately guarantee whole-cell two-liquid phase biotransformations and oxygenase-based biocatalysis an important role in the development of greener syntheses on industrial scale.| Parameter | Unit | Ferric phenanthroline | Titanium silicate | Manganese sulfate | Biotechnol. process |
|---|---|---|---|---|---|
| a Average annual instalment payment over 10 years include instalment of direct fixed capital (interest rate: 9% over a period of 10 years) and instalment of working capital (interest rate: 12%; period: 6 years). | |||||
| Resource consumption | kg kgSO−1 | 16.15 | 3.04 | 12.80 | 21.07 |
| Environmental index | 182.9 | 59.9 | 104.7 | 92.6 | |
| Capital investment | million $ | 15.1 | 10.1 | 9.6 | 20.4 |
| Production costs | $ kgSO−1 | 41.6 | 16.9 | 12.8 | 10.2 |
| Production costs including instalment paymenta | $ kgSO−1 | 44.3 | 18.7 | 14.6 | 13.9 |
Experimental
Strain, cultivation and two-liquid-phase biotransformation
E. coli JM101 (supE thi-1Δ(lac-proAB) F’[traD36 proAB+lacIqlacZΔM15]), a derivative of E. coli K-12, was used as recombinant host strain.68 The expression plasmid pSPZ10 is based on the pBR322 vector and contains the styrene monooxygenase genes styAB from Pseudomonas sp. strain VLB120, under the control of the alk regulatory system from Pseudomonas putida GPo1.31Transformants were selected on Luria Bertani (LB) agar plates containing 50 μg ml−1 kanamycin.69 A single colony was picked and used to inoculate 5 ml liquid LB medium. After reaching the stationary growth phase, 1 ml was diluted in 96 ml M9 medium69 complemented with 1 ml L−1 US* trace elements,70 1 ml L−1 1% (w/v) thiamine solution, and 2 ml L−1 1 M magnesium sulfate (MgSO4) solution. After overnight growth, this culture was used to inoculate a KLF 2000 reactor (Bioengineering, Wald, Switzerland) containing 900 ml Riesenberg medium.71 15 g L−1 glucose were used as carbon and energy source. The pH was maintained at 7.20 by adding 30% phosphoric acid and 25% NH4OH solutions. The latter additionally served as a nitrogen feed. The batch phase lasted approximately 12 h (overnight). Aeration and stirring rate were maintained constant on 1 L min−1 and 1500 rpm, respectively. After depletion of the carbon source, a feed consisting of 730 g L−1 glucose and 19.6 g L−1 MgSO4·7 H2O was started and increased stepwise until a biomass concentration of approximately 18 g L−1 was reached. Then, the biotransformation was started by the addition of the organic phase consisting of 910 ml BEHP, 80 ml styrene, and 10 ml octane serving as the inducer of styAB expression. The phase ratio of the organic to the aqueous phase was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 during the biotransformation. The dissolved oxygen concentration was kept above 20% by increasing the stirring speed (to 2800 rpm) and the aeration rate (up to 2.5 L min−1). Antifoam A (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany) was added only in cases of excessive foaming. The biotransformation was monitored by taking samples every hour. Sampling was performed as described previously.70 For two-liquid phase biotransformations, volumetric rates and concentrations are given per litre of aqueous phase (Laq), organic phase (Lorg), or total volume (Ltot).
1 during the biotransformation. The dissolved oxygen concentration was kept above 20% by increasing the stirring speed (to 2800 rpm) and the aeration rate (up to 2.5 L min−1). Antifoam A (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany) was added only in cases of excessive foaming. The biotransformation was monitored by taking samples every hour. Sampling was performed as described previously.70 For two-liquid phase biotransformations, volumetric rates and concentrations are given per litre of aqueous phase (Laq), organic phase (Lorg), or total volume (Ltot).
Analytics
Concentrations of styrene, styrene oxide, and 2-phenylethanol were measured by a TRACE GC Ultra (Thermo Fisher Scientific Inc., Waltham, MA, USA) equipped with a FactorFour VF-5ms column (Varian, Inc., Palo Alto, CA, USA). The oven temperature was raised from 50 °C to 140 °C at a heating rate of 15 °C min−1, followed by heating at a rate of 100 °C min−1 to 300 °C, which was maintained for 3.5 min. Detection was achieved by a flame ionization detector (FID). Specific epoxidation activities are given in units (U), whereby 1 U is defined as 1 μmol product formed in 1 min.Glucose and acetate were separated on an Aminex HPX-87-H column (Bio-Rad Laboratories, Hercules, CA, USA) in a LaChrom Elite HPLC system (Hitachi High Technologies America, Inc., Pleasanton, CA, USA), after extraction of aqueous styrene by BEHP. The flow rate of 2.5 mM sulfuric acid as mobile phase was set to 1.0 ml min−1. The temperature was kept constant at 60 °C. Detection of the analytes was done by a L-2420 UV-Vis and a L-2490 refractive index module.
Cell concentrations were determined spectrometrically on a Libra S11 spectrophotometer (Biochrom Ltd., Cambridge, UK) at a wavelength of 450 nm (OD450), whereby one OD450 unit corresponded to a cell dry weight (CDW) of 0.166 g L−1.72
Assessment method
Economic and ecological process efficiencies were assessed by means of a method designed for utilization at early development stages of fine chemical processes.8,16,20,21 Mass-balances were generated on the basis of process flow-sheets created with the simulation tool, SuperPro Designer version 6.0 (Intelligen, Inc., Scotch Plains, NY, United States).For the calculation of the environmental indices, all substances are characterized in different categories according to their impact on human health and on the environment. These categories are land use, raw material availability, complexity of synthesis, thermal risk, acute toxicity, chronic toxicity, biological risk, ecotoxicity, global warming potential, ozone depletion potential, photochemical ozone creation potential, odor, and eutrophication potential. For each category, the substances are classified in three rating levels (ABC analysis), where “A” stands for high, “B” for medium and “C” for low risk in the respective category. The criteria for the classification have been published elsewhere.19 Weighing factors are used to account for the differing significance of the impact categories, allowing the calculation of an environmental index for the input and the output materials. The total environmental index of a process is obtained by balancing the input and the output indices in a 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 ratio.
60 ratio.
SuperPro Designer comprises comprehensive databases for the calculation of numerous economic parameters. These values were used for the economic assessment unless otherwise stated. Purchase prices for the equipment were taken from the SuperPro Designer database. The direct investment costs are the sum of the equipment purchase costs (PC) and charges for the installation (40% of PC), the instrumentation (15% of PC), piping (46% of PC), electronic facilities (10% of PC), buildings (16% of PC), yard improvements (14% of PC), and service facilities 60% of PC) as suggested by Roffler et al.73 Additionally, indirect costs such as expenses for engineering and the contractor's fee (16% of direct costs) and construction (10% of direct costs) have to be taken into account for the total capital investment. Raw material costs were complemented with industrial market prices from the ICIS webpage (www.ICIS.com) or, if necessary, by divison of the lab-scale prices from Sigma Aldrich (www.sigmaaldrich.com) by a factor of 10. The depreciation period was assumed to be 10 years with a salvage value of 5%. Insurance and local taxes were estimated to account for 1% and 2% of the investment costs, respectively, whereas the factory expenses were fixed to $300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 a−1 in all processes. For the maintenance costs, unit-specific default values of SuperPro Designer were used. Basic labor costs (BLC) are estimated from the labor requirements of each operational step in the factory and assuming a standard wage of $30 h−1 for an operator. The total labor expenses additionally include charges for fringe benefits (40% of BLC), supervision (20% of BLC), operational supplies (10% of BLC), and administration (60% of BLC). Laboratory costs for quality checks were 15% of the total labor costs.
000 a−1 in all processes. For the maintenance costs, unit-specific default values of SuperPro Designer were used. Basic labor costs (BLC) are estimated from the labor requirements of each operational step in the factory and assuming a standard wage of $30 h−1 for an operator. The total labor expenses additionally include charges for fringe benefits (40% of BLC), supervision (20% of BLC), operational supplies (10% of BLC), and administration (60% of BLC). Laboratory costs for quality checks were 15% of the total labor costs.
Acknowledgements
We thank Jin Byung Park for sharing his excellent experience in cultivation and valuable discussions. The financial support from the Deutsche Bundesstiftung Umwelt (DBU; project AZ13145) is gratefully acknowledged.References
- A. J. J. Straathof, S. Panke and A. Schmid, Curr. Opin. Biotechnol., 2002, 13, 548–556 CrossRef CAS.
- A. Schmid, J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts and B. Witholt, Nature, 2001, 409, 258–268 CrossRef CAS.
- A. Bull, R. Kurane and B. Marrs, Biotechnology for clean industrial products and processes – Towards industrial sustainability, Organisation for Economic Co-operation and Development (OECD), Paris, France, 1998, p. 194 Search PubMed.
- A. Kiener, Chem. Tech., 1995, 25, 31–35 CAS.
- C. Dingler, W. Ladner, G. A. Krei, B. Cooper and B. Hauer, Pestic. Sci., 1996, 46, 33–35 CrossRef CAS.
- B. Bühler and A. Schmid, J. Biotechnol., 2004, 113, 183–210 CrossRef.
- J. B. van Beilen, W. A. Duetz, A. Schmid and B. Witholt, Trends Biotechnol., 2003, 21, 170–177 CrossRef CAS.
- E. Heinzle, A. Biwer and C. L. Cooney, Development of sustainable bioprocesses: Modeling and assessment, John Wiley & Sons Ltd, Chichester, United Kingdom, 2007 Search PubMed.
- E. Heinzle and K. Hungerbühler, Chimia, 1997, 51, 176–183 CAS.
- R. G. Mathys, A. Schmid and B. Witholt, Biotechnol. Bioeng., 1999, 64, 459–477 CrossRef CAS.
- A. P. Borole and B. H. Davison, Appl. Biochem. Biotechnol., 2007, 137–140, 437–449 CrossRef CAS.
- M. Eissen, G. Geisler, B. Bühler, C. Fischer, K. Hungerbühler, A. Schmid and E. M. Carreira, in Green chemistry metrics – Measuring and monitoring sustainable processes, ed. A. Lapkin and D. J. C. Constable, Blackwell Publishing Ltd, Chichester, United Kingdom, 2008, pp. 200–227 Search PubMed.
- S. Hellweg, U. Fischer, M. Scheringer and K. Hungerbühler, Green Chem., 2004, 6, 418–427 RSC.
- R. A. Sheldon, Chem. Tech., 1994, 24, 38–47 CAS.
- P. Saling, A. Kicherer, B. Dittrich-Krämer, R. Wittlinger, W. Zombik, I. Schmidt, W. Schrott and S. Schmidt, Int. J. LCA, 2002, 7, 203–218 Search PubMed.
- E. Heinzle, D. Weirich, F. Brogli, V. H. Hoffmann, G. Koller, M. A. Verduyn and K. Hungerbühler, Ind. Eng. Chem. Res., 1998, 37, 3395–3407 CrossRef CAS.
- A. D. Curzons, D. J. C. Constable, D. N. Mortimer and V. L. Cunningham, Green Chem., 2001, 3, 1–6 RSC.
- M. Eissen and J. O. Metzger, Chem. Eur. J., 2002, 8, 3580–3585 CrossRef CAS.
- E. Heinzle, A. Biwer, M. Eissen and M. A. Kholiq, Chem. Ing. Tech., 2006, 78, 301–305 CrossRef CAS.
- A. Biwer and E. Heinzle, J. Chem. Technol. Biotechnol., 2004, 79, 597–609 CrossRef CAS.
- G. Koller, D. Weirich, F. Brogli, E. Heinzle, V. H. Hoffmann, M. A. Verduyn and K. Hungerbühler, Ind. Eng. Chem. Res., 1998, 37, 3408–3413 CrossRef CAS.
- S. Panke, B. Witholt, A. Schmid and M. G. Wubbolts, Appl. Environ. Microbiol., 1998, 64, 2032–2043 CAS.
- A. Schmid, K. Hofstetter, H. J. Feiten, F. Hollmann and B. Witholt, Adv. Synth. Catal., 2001, 343, 732–737 CrossRef CAS.
- P. Besse and H. Veschambre, Tetrahedron, 1994, 50, 8885–8927 CrossRef CAS.
- A. Archelas and R. Furstoss, Top. Curr. Chem., 1999, 200, 159–191 CAS.
- A. V. R. Rao, M. K. Gurjar and V. Kaiwar, Tetrahedron: Asymmetry, 1992, 3, 859–862 CrossRef.
- A. Datla, P. A. Walavalker, A. Konda and S. B. Trivikram, WO Pat. 2007/135463, 2007 Search PubMed.
- M. Couturier, J. L. Tucker, B. M. Andresen, K. M. DeVries, B. C. Vanderplas and F. Ito, Tetrahedron: Asymmetry, 2003, 14, 3517–3523 CrossRef CAS.
- C. Franchini, A. Carocci, A. Catalano, M. M. Cavalluzzi, F. Corbo, G. Lentini, A. Scilimati, P. Tortorella, D. C. Camerino and A. de Luca, J. Med. Chem., 2003, 46, 5238–5248 CrossRef CAS.
- D. Meyer, B. Bühler and A. Schmid, Adv. Appl. Microbiol., 2006, 59, 53–91 Search PubMed.
- S. Panke, M. G. Wubbolts, A. Schmid and B. Witholt, Biotechnol. Bioeng., 2000, 69, 91–100 CrossRef.
- S. Panke, J. M. Sanchez-Romero and V. de Lorenzo, Appl. Environ. Microbiol., 1998, 64, 748–751 CAS.
- J. B. Park, B. Bühler, T. Habicher, B. Hauer, S. Panke, B. Witholt and A. Schmid, Biotechnol. Bioeng., 2006, 95, 501–512 CrossRef CAS.
- B. Bühler, J. B. Park, L. M. Blank and A. Schmid, Appl. Environ. Microbiol., 2008, 74, 1436–1446 CrossRef.
- J. B. Park, B. Bühler, S. Panke, B. Witholt and A. Schmid, Biotechnol. Bioeng., 2007, 98, 1219–1229 CrossRef CAS.
- S. Panke, M. Held, M. G. Wubbolts, B. Witholt and A. Schmid, Biotechnol. Bioeng., 2002, 80, 33–41 CrossRef CAS.
- K. Hofstetter, J. Lutz, I. Lang, B. Witholt and A. Schmid, Angew. Chem., Int. Ed., 2004, 43, 2163–2166 CrossRef CAS.
- R. Ruinatscha, C. Dusny, K. Buehler and A. Schmid, Adv. Synth. Catal., 2009, 351, 2505–2515 CrossRef CAS.
- F. Hollmann, K. Hofstetter, T. Habicher, B. Hauer and A. Schmid, J. Am. Chem. Soc., 2005, 127, 6540–6541 CrossRef CAS.
- G. Dubois, A. Murphy and T. D. P. Stack, Org. Lett., 2003, 5, 2469–2472 CrossRef CAS.
- C. V. Rode, U. N. Nehete and M. K. Dongare, Catal. Commun., 2003, 4, 365–369 CrossRef CAS.
- N. H. Khan, S. H. R. Abdi, R. I. Kureshy, S. Singh, I. Ahmad, R. V. Jasra and P. K. Ghosh, US Pat. 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 235
235![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676, 2007 Search PubMed.
676, 2007 Search PubMed. - M. Tokunaga, J. F. Larrow, F. Kakiuchi and E. N. Jacobsen, Science, 1997, 277, 936–938 CrossRef CAS.
- S. E. Schaus, B. D. Brandes, J. F. Larrow, M. Tokunaga, K. B. Hansen, A. E. Gould, M. E. Furrow and E. N. Jacobsen, J. Am. Chem. Soc., 2002, 124, 1307–1315 CrossRef CAS.
- M. Palucki, P. J. Pospisil, W. Zhang and E. N. Jacobsen, J. Am. Chem. Soc., 1994, 116, 9333–9334 CrossRef CAS.
- W. Zhang, J. L. Loebach, S. R. Wilson and E. N. Jacobsen, J. Am. Chem. Soc., 1990, 112, 2801–2803 CrossRef CAS.
- J. M. Woodley, Trends Biotechnol., 2008, 26, 321–327 CrossRef CAS.
- D. J. Pollard and J. M. Woodley, Trends Biotechnol., 2007, 25, 66–73 CrossRef CAS.
- C. J. Huang, S. L. Lee and C. C. Chou, J. Biosci. Bioeng., 2000, 90, 142–147 CrossRef CAS.
- B. D. Brandes and E. N. Jacobsen, Tetrahedron: Asymmetry, 1997, 8, 3927–3933 CrossRef CAS.
- M. Palucki, G. J. McCormick and E. N. Jacobsen, Tetrahedron Lett., 1995, 36, 5457–5460 CrossRef CAS.
- B. S. Lane, M. Vogt, V. J. de Rose and K. Burgess, J. Am. Chem. Soc., 2002, 124, 11946–11954 CrossRef CAS.
- J. F. Larrow and E. N. Jacobsen, Org. Synth., 1998, 75, 1–11 CAS.
- L. Aouni, K. E. Hemberger, S. Jasmin, H. Kabir, J. F. Larrow, I. Le-Fur, P. Morel and T. Schlama, in Asymmetric catalysis on industrial scale: challenges, approaches and solutions, ed. H. U. Blaser and E. Schmidt, Wiley-VCH, 2004 Search PubMed.
- L. Cao, J. Lee, W. Chen and T. K. Wood, Biotechnol. Bioeng., 2006, 94, 522–529 CrossRef CAS.
- I. W. C. E. Arends, R. A. Sheldon, M. Wallau and U. Schuchardt, Angew. Chem., Int. Ed. Engl., 1997, 36, 1144–1163 CrossRef.
- S. B. Kumar, S. P. Mirajkar, G. C. G. Pais, P. Kumar and R. Kumar, J. Catal., 1995, 156, 163–166 CrossRef.
- S. C. Laha and R. Kumar, J. Catal., 2001, 204, 64–70 CrossRef CAS.
- M. Taramasso, G. Perego and B. Notari, US Pat. 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410
410![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 501, 1983 Search PubMed.
501, 1983 Search PubMed. - O. A. Kholdeeva and N. N. Trukhan, Russ. Chem. Rev., 2006, 75, 411–432 Search PubMed.
- B. S. Lane and K. Burgess, J. Am. Chem. Soc., 2001, 123, 2933–2934 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- L. J. Bruce and A. J. Daugulis, Biotechnol. Prog., 1991, 7, 116–124 CrossRef CAS.
- A. D. Curzons, D. C. Constable and V. L. Cunningham, Clean Products and Processes, 1999, 1, 82–90.
- P. S. J. Cheetham, in Applied biocatalysis, ed. A. J. J. Straathof and P. Adlercreutz, Taylor & Francis Group, London, United Kingdom, 2000 Search PubMed.
- G. Chotani, T. Dodge, A. Hsu, M. Kumar, R. LaDuca, D. Trimbur, W. Weyler and K. Sanford, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 2000, 1543, 434–455 Search PubMed.
- J. E. Bailey and D. F. Ollis, Biochemical engineering fundamentals, McGraw-Hill, Inc., New York, United States, 1986 Search PubMed.
- J. Messing, Recomb. DNA Tech. Bull., 1979, 79-99, 43–48 Search PubMed.
- J. Sambrook and D. W. Russell, Molecular cloning – A laboratory manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, 2001 Search PubMed.
- S. Panke, A. Meyer, C. M. Huber, B. Witholt and M. G. Wubbolts, Appl. Environ. Microbiol., 1999, 65, 2324–2332 CAS.
- D. Riesenberg, V. Schulz, W. A. Knorre, H. D. Pohl, D. Korz, E. A. Sanders, A. Ross and W. D. Deckwer, J. Biotechnol., 1991, 20, 17–28 CrossRef CAS.
- L. M. Blank, B. E. Ebert, B. Bühler and A. Schmid, Biotechnol. Bioeng., 2008, 100, 1050–1065 CrossRef CAS.
- S. Roffler, H. W. Blanch and C. R. Wilke, Biotechnol. Prog., 1987, 3, 131–140 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary Table A: Criteria for ABC classification; Supplementary Table B: ABC classification of all compounds; Supplementary Table C: Detailed mass-balances for all (S)-styrene oxide production processes; Supplementary Table D: Environmental indices for all (S)-styrene oxide production processes. See DOI: 10.1039/b921896c |
| This journal is © The Royal Society of Chemistry 2010 |
