Liquid phase oxidation of p-xylene to terephthalic acid at medium-high temperatures: multiple benefits of CO2-expanded liquids
Xiaobin Zuoab, Fenghui Niua, Kirk Snavelyac, Bala Subramaniam*ac and Daryle H. Busch*ab
aCenter for Environmentally Beneficial Catalysis, University of Kansas, Lawrence, KS 66047, USA. E-mail: bsubramaniam@ku.edu; Fax: 785-864-6051; Tel: 785-864-2903
bDepartment of Chemistry, University of Kansas, Lawrence, KS 66047, USA. E-mail: busch@ku.edu; Fax: 785-864-6051; Tel: 785-864-1644
cDepartment of Chemical and Petroleum Engineering, University of Kansas, Lawrence, KS 66045, USA
First published on 15th January 2010
Abstract
The Co/Mn/Br catalyzed oxidation of p-xylene to terephthalic acid (TPA) is demonstrated in CO2-expanded solvents at temperatures lower than those of the traditional Mid-Century (MC) process. As compared with the traditional air (N2/O2) oxidation system, the reaction with CO2/O2 mixture at 160 °C and using an additional inert gas (N2 or CO2) pressure of 100 bar increases both the yield of TPA and the purity of solid TPA via a more efficient conversion of the intermediates, 4-carboxybenzaldehyde and p-toluic acid. At the same time, the amount of yellow colored by-products in the solid TPA product is also lessened, as determined by spectroscopic analysis. Equally important, the decomposition or burning of the solvent, acetic acid, monitored in terms of the yield of the gaseous products, CO and CO2, is reduced by ca. 20% based on labeled CO2 experiments. These findings broaden the versatility of this new class of reaction media in homogeneous catalytic oxidations by maximizing the utilization of feedstock carbon for desired products while simultaneously reducing carbon emissions.
1. Introduction
The Mid-Century (MC) process is well known for the production of terephthalic acid (TPA), a very important intermediate in the synthesis of polyethylene terephthalate and related polymers that can be fabricated into excellent fibers, resins and films.1,2 In this process, p-xylene is oxidized on a large scale by air at 180–225 °C and 15–30 bar in the presence of a catalyst composed of cobalt acetate, manganese acetate and hydrogen bromide. The optimized yield of TPA is greater than 95%, along with excellent product purity as shown by various analytical techniques.3 This process, despite successful on-stream operation for over 50 years, has the following distinct disadvantages. (1) High temperature operation decreases the solubility of O2, while increasing solvent and product destruction and causing safety concerns. (2) Air is used as the primary oxidant instead of pure O2 for safety reasons. This produces large amounts of contaminated vent gas and the abundant unreacted N2 must be cleansed before venting into the environment. (3) The combination of corrosive bromide as a catalyst component and a high operating temperature requires the use of highly resistant but expensive titanium reactors. A recent breakthrough made by Poliakoff et al.,4 Savage et al.5 and Fulton et al.6 has shown that this reaction can be effectively catalyzed by MnBr2 with pure O2 in a new reaction medium—high temperature and supercritical water, thus circumventing the use of acetic acid. However, the scope of such reaction systems is limited by the even harsher reaction conditions (T = 300–400 °C, P > 200 bar).CO2-based media have attracted much attention in recent years because they provide the possibility of using pure O2 under safe conditions while overcoming most of the aforementioned limitations of air oxidation systems, as follows.7-10 (1) The use of CO2 in a mixed solvent increases both the O2 solubility and the mass transport of the reactants in the liquid phase, the former because of the significant solubility of O2 in liquid CO2 and the latter because of improved diffusivities in CO2 media.11 This is especially the case in carbon dioxide expanded liquids (CXLs). A rule of thumb is the nearly ten fold increase in oxygen solubility when the volume of solvent is expanded by a factor of two, depending on the properties of solvent. (2) The presence of CO2 in the vapor phase reduces flammability hazards because of the great abundance of inert CO2 and its large heat capacity. (3) Replacing the N2 from air with a much greater amount of CO2 eliminates the contaminated vent gas because the CO2 is more easily separated and recycled in the oxidation process. However, this is counterbalanced by the required separation of O2 from N2.
The first successful application of CXLs in oxidation reactions was reported in 2002.12,13 The Co(salen) catalyzed oxidation of 2,6-di-tert-butyl phenol to 2,6-di-tert-butyl-1,4-benzoquinone in CO2-expanded acetonitrile was 1–2 orders of magnitude more active than in either the neat organic solvent or supercritical CO2. We recently described the synergistic effect of co-catalyst zirconium and promoter ketone in the oxidation of toluene from 50 to 100 °C.14 It was found that CO2 has a strong activation effect on the reaction, especially by shortening the induction period. Under certain conditions, the Co/Zr(acac)4 catalyzed oxidation did not work at all with N2/O2 but proceeded effectively with CO2/O2, affording high yields of benzoic acid. In this work, we extend the CO2 effect to this important industrial TPA process, which is extremely challenging because of much higher temperatures and the complexity of intermediates. More specifically, the TPA precipitates during the reaction, occluding with it 4-carboxybenzaldehyde and other by-products. In addition, the decomposition, or burning of solvent, is more significant at higher temperatures. To this end, in the present work, we first measured the expansion of acetic acid, containing dissolved catalysts and substrate, by dense CO2. Then, based on these results, we investigated the semi-continuous oxidation of p-xylene at lower temperatures as compared with the MC process for a more significant CO2 effect, with the expectation that CXLs could bring about some specific advantages for product selectivity and purity in this complex reaction system.
2. Experimental
2.1 Materials
All the catalysts, additives, substrates and solvents were commercially available and used without further treatment. Industrial grade (≥ 99.9% purity, < 32 ppm H2O, < 20 ppm THC) liquid CO2 and ultra high purity grade oxygen were purchased from Linweld. Isotopic gas 13CO2 (99.5 atom% 13C) was purchased from Aldrich Chemical Co., Inc.2.2 Volumetric expansion of acetic acid + catalyst mixtures by dense CO2
The volumetric expansion by CO2 of acetic acid containing dissolved catalysts was measured in a high-pressure Jerguson view cell that was placed in a high temperature oven (Yamato DKN-400, part of a Cambridge Viscosity ViscoPro 2000 system, Fig. 1). For these expansion studies, a measured volume of acetic acid containing a known amount of catalysts was loaded into the view cell and heated to the experimental temperature, which was followed by the addition of 20 bar N2 as the substitute for O2. Then CO2 was gradually introduced into the cell until an expansion ratio (ΔV/Vo, eqn 1) of ca. 1.0 was obtained. | (1) |
 | ||
| Fig. 1 Jerguson cell in oven for high temperature studies of CXLs. | ||
2.3 Oxidation experiments and product analysis
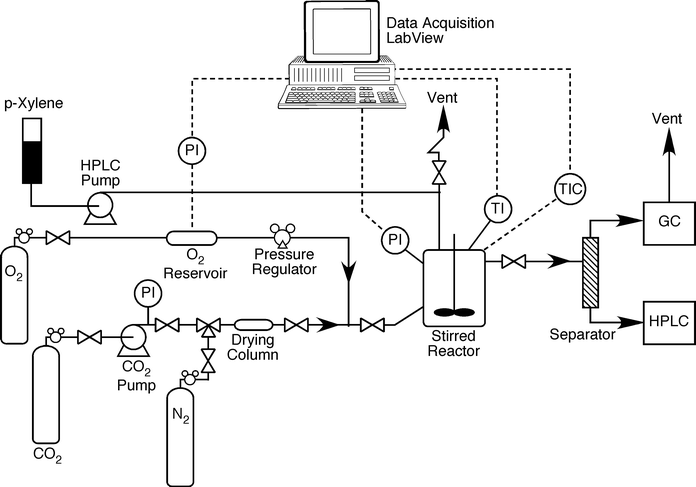 | ||
| Fig. 2 Schematic diagram of semi-continuous oxidation p-xylene to terephthalic acid. | ||
The gas mixture was analyzed by GC (Shin Carbon ST 100/120 mesh) to determine the yield of CO and CO2 produced by solvent burning. For isotopic experiments, the 12CO2/13CO2 ratio was measured by mass spectrometry.
The insoluble terephthalic acid was separated from the liquid mixture by filtration and the solid was washed with methanol to remove most of the soluble impurities. The resulting white solid was dried at 100 °C for 2 hrs to remove absorbed solvent, after which a 10 mg sample was dissolved in 50 mL of methanol by sonication and analyzed by HPLC (C18 ODS-2 column). The reactor was washed with methanol and DMF to scavenge the residual TPA solid, which was combined with the filtrate that was retained after isolation of the solid TPA and analyzed by HPLC to determine the composition of liquids. The yields of products were based on the sums of those determined for the solid and liquid components.
The presence of yellow colored by-products in solid TPA was determined from optical density measurements using a UV-Vis spectrometer at 340 nm.15 Typically, 0.3 g solid sample was dissolved in 5 mL 4N NH4OH. The optical density was calculated as in eqn 2:
| ODλ = Aλ/L | (2) |
2.4 Safety
The amount of substrate used in the reaction studies was such that the maximum adiabatic temperature rise for total combustion of the substrate (taking into account the heat capacities of the reaction mixture and the solid reactor) was 25 °C. The actual temperature rise observed during reactions was a few °C, at most. In addition, the reactor was equipped with a safety release valve that exhausts the reactor contents safely to the building vent by means of a rupture disk in the event the set safe pressure (200 bar) is exceeded.3. Results and discussion
3.1 CO2 expansion measurements at 80 and 120 °C
Since supercritical CO2 (TC = 31 °C) expands liquids most effectively at temperatures up to 1.2 times the absolute critical temperature, our previous work was focused on reactions performed at 60 °C or less wherein it was possible to measure the solvent expansion in a water bath.12,16,17 Here, to measure the catalyst solubility in CO2-expanded solvent at much higher temperatures, in experiments that are the first of their kind for p-xylene oxidation, we placed the high pressure Jerguson view cell in a high temperature oven that was originally designed for viscosity measurement (Fig. 1). Though the oven temperature can be maintained as high as 200 °C, these studies did not go beyond 120 °C because of the corroding capability of acetic acid and hydrogen bromide toward the stainless steel Jerguson cell.The catalyst solution, containing 60 mM cobalt acetate, 1.8 mM manganese acetate and 60 mM hydrogen bromide in acetic acid, has a slight pink tint at room temperature (Fig. 3a) which turns blue when heated to 120 °C (Fig. 3b). The solution is expanded significantly at the much higher CO2 pressure of ca. 160 bar (Fig. 3c).
![Catalyst solution and its expansion with increasing CO2 pressure. (a) room temperature, (b) 120 °C with 20 bar N2 and 18 bar CO2, (c) 120 °C with 20 bar N2 and 159 bar CO2; [Co] = 60 mM, [Mn] = 1.8 mM, [Br] = 60 mM prior to the expansion.](/image/article/2010/GC/b920262e/b920262e-f3.gif) | ||
| Fig. 3 Catalyst solution and its expansion with increasing CO2 pressure. (a) room temperature, (b) 120 °C with 20 bar N2 and 18 bar CO2, (c) 120 °C with 20 bar N2 and 159 bar CO2; [Co] = 60 mM, [Mn] = 1.8 mM, [Br] = 60 mM prior to the expansion. | ||
Fig. 4a shows the dependence of the expansion ratio on CO2 pressure at 120 °C. The expansion ratio increases gradually and reaches a value of 0.85 when CO2 partial pressure is elevated to 160 bar (total pressure 180 bar). Accordingly, the concentrations of cobalt, manganese and bromide are diluted to 33 mM, 1.0 mM and 33 mM, respectively. It is noteworthy that no catalyst precipitates during the CO2 expansion. This implies the possibility of replacing up to at least 50 vol% of the organic solvent by CO2 while maintaining the catalyst in solution.
![Volumetric expansion of acetic acid by CO2 with Co/Mn/Br catalysts. (a) T = 120 °C, (b) T = 80 °C; [Co] = 60 mM, [Mn] = 1.8 mM, [Br] = 60 mM prior to the expansion.](/image/article/2010/GC/b920262e/b920262e-f4.gif) | ||
| Fig. 4 Volumetric expansion of acetic acid by CO2 with Co/Mn/Br catalysts. (a) T = 120 °C, (b) T = 80 °C; [Co] = 60 mM, [Mn] = 1.8 mM, [Br] = 60 mM prior to the expansion. | ||
Fig. 4b shows the expansion result at 80 °C. As compared with 120 °C, the expansion ratio at 80 °C is much higher, reaching an approximate value of one (1) when the CO2 partial pressure is 130 bar. However, the catalysts precipitate upon further increases in CO2 pressure; e.g., approaching 140 bar. Further, the expansion behavior is different from that at 120 °C, displaying a sharp enhancement when CO2 pressure exceeds 100 bar. This is attributed to the higher compressibility of the CO2 with pressure at the lower temperature resulting in enhanced dissolution into and volumetric expansion of the liquid phase.
3.2 Semi-continuous oxidation of p-xylene
We started the reaction at 80 °C, much lower than the temperature used in the p-xylene oxidation process, to ensure a substantial expansion at relatively low CO2 pressure. Accordingly, a preliminary reaction was performed at a CO2 pressure of 111 bar, where the expansion ratio was 0.75. However, as shown in Table 1, the process was very sluggish, generating only 1% TPA after 22 h (Entry 1). We rationalize this result on the basis of our understanding that the temperature was too low to overcome the activation energy of the reaction.
| Entry | Inert gas | P inert (bar) | PO2 (bar) | T (°C) | Expansion Ratio (ΔV/Vo) | Reaction Time (h) | Y TPA (%) | Y 4-CBA (%) | Y PTA (%) | TPA (s)b (wt%) | 4-CBA (s)c ppm | OD340 | CO/p-x (mol/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Reaction conditions: [Co] = 33 mM, [Mn] = 1.0 mM, [Br] = 33 mM, VL (volume of liquid phase) = 35 mL (with or without CO2 expansion), 1.6 mL p-xylene added at 0.08 mL/min, n (stirrer speed) = 1200 rpm.b TPA in dry isolated solid.c 4-CBA in dry isolated solid. The conversion of p-xylene is above 99% in all the reactions except entry 1. In addition to 4-CBA and PTA, a few other byproducts were detected in small amounts during the HPLC analysis. These compounds were not identified. | |||||||||||||
| 1 | CO2 | 111 | 28 | 80 | 0.75 | 22 | 1.0 | 0.5 | 15.5 | —— | —— | —— | —— |
| 2 | N2 | 45 | 45 | 120 | 0 | 0.5 | 78.6 | 10.5 | 4.7 | 85.7 | 104000 | 0.69 | 0.011 |
| 3 | CO2 | 45 | 45 | 120 | 0.17 | 0.5 | 79.0 | 10.0 | 4.3 | 86.2 | 101000 | 0.58 | 0.0065 |
| 4 | CO2 | 107 | 45 | 120 | 0.42 | 2.0 | 77.6 | 6.9 | 4.2 | 89.1 | 72000 | 0.66 | 0 |
| 5 | CO2 | 145 | 20 | 120 | 0.69 | 2.0 | 73.4 | 8.6 | 5.7 | 85.4 | 94000 | 1.30 | 0 |
| 6 | N2 | 30 | 30 | 160 | 0 | 0.5 | 94.6 | 1.3 | 0.7 | 98.4 | 11700 | 0.086 | 0.040 |
| 7 | CO2 | 30 | 30 | 160 | 0 | 0.5 | 94.6 | 1.3 | 0.7 | 98.4 | 11500 | 0.087 | 0.038 |
| 8 | N2 | 100 | 30 | 160 | 0 | 0.5 | 93.8 | 1.2 | 0.7 | 98.5 | 10500 | 0.069 | 0.035 |
| 9 | CO2 | 100 | 30 | 160 | 0.15 | 0.5 | 95.1 | 0.8 | 0.6 | 99.1 | 6400 | 0.063 | 0.019 |
In the next reaction, the temperature was raised to 120 °C to accelerate the oxidation. As shown in Table 1, under a low inert gas pressure of 45 bar, there is no obvious difference between N2 and CO2 in the yield of TPA (ca. 80% in 0.5 h) as well as those of the by-products 4-carboxybenzaldehyde (4-CBA) and p-toluic acid (PTA) (Entries 2 and 3). HPLC analysis of the solid TPA quantified the expected major impurities, 4-CBA and PTA. As compared with p-xylene, PTA is much less reactive than the starting material due to the deactivating effect of the electron withdrawing carboxyl group. During the reaction, 4-CBA tends to co-precipitate with TPA and is very difficult to remove. Since 4-CBA is an inhibitor in the subsequent polymerization reaction, its concentration should be kept as low as possible. In addition, the solid also contains trace amounts of yellow colored by-products such as the derivatives of fluorenone and anthraquinone that can affect the quality of polymers.21 The presence of these compounds is detected by the optical density at 340 nm.15 The lower that optical density, the lesser the amounts of these yellow colored by-products. As shown in Table 1, when the inert gas pressure is 45 bar, the amount of 4-CBA in the TPA solid is quite similar for N2 and CO2, whereas the optical density is lower for CO2. As CO2 pressure is increased to 107 bar, the corresponding expansion ratio is 0.42, and the yield of TPA approaches 80% (Entry 4), however, a relatively long reaction time of 2 h is required. Further increase of CO2 pressure to 145 bar (expansion ratio = 0.69) and decrease of O2 pressure to 21 bar produces a lower yield of TPA (Entry 5). On the other hand, the optical density is much higher, which indicates higher concentrations of yellow colored by-products. The reason for this phenomenon remains unclear. Based on HPLC analysis, the yields of TPA and the common by-products (4-CBA and PTA) at a CO2 pressure of 145 bar are 73.4%, 8.6% and 5.7% respectively (Table 1, entry 5). Since the p-xylene conversion is above 99%, the unknown by-products (peaks seen in the HPLC but not positively identified) account for more than 10% of the p-xylene converted. The TPA precipitate recovered from the reaction mixture has a very dense yellow color (the optical density is much higher as compared with that of the TPA solid produced at lower CO2 pressure), which indicates significant formation of derivatives of fluorenone and anthraquinone. Based on these results, it is reasonable to assume that at 120 °C, regardless of the use of CXLs at various O2 partial pressures, the kinetic rates of the consecutive oxidation reactions of p-xylene to yield TPA are not fast enough to avoid the intermediate oxidation products and the other byproducts that adversely affect the TPA purity.
At 120 °C, the only positive effect of CO2-based media is therefore the lower yield of the gaseous product CO, which falls to zero when CO2 pressure is above 100 bar. This suggests that CO2 might inhibit solvent burning. However, at this point we have not determined the yield of the second gaseous product, CO2 produced by burning, although such a differentiation should be possible using labeled CO2 (vide infra).
The catalytic performance is greatly improved as the temperature is further increased to 160 °C, where two sets of reactions were carried out to provide additional comparisons between the behaviors in N2 and CO2 at even higher yields of TPA. As shown in Table 1, under a low inert gas (N2 or CO2) pressure of 30 bar, the reaction results are almost identical for N2 and CO2 (Entries 6 and 7). When N2 pressure is increased to 100 bar, there is little difference in the reaction results except a modest decrease of optical density (Entries 6 and 8). The results are completely different, however, when CO2 pressure is increased to 100 bar. The expansion ratio is estimated to be 0.15, as extrapolated from the expansion data for 80–120 °C. In addition to a minor enhancement in TPA yield as compared to that for 100 bar of N2, the oxidation process in CXL benefits a great deal in several aspects (Entries 6–9). (1) The yield of by-products, especially that of 4-CBA, is markedly decreased. This indicates the more complete oxidation of the intermediates to TPA in CO2-expanded solvent that is possibly associated with increased oxygen availability. (2) The purity of solid TPA is enhanced by the reduction of 4-CBA concentration from 11000 ppm to 6400 ppm. (3) The optical density is the lowest among the four sets of reaction conditions. (4) The yield of CO is significantly decreased; i.e., by about 50%. In general, the concentration of 4-CBA in TPA solid is inversely related to the burning of solvent, i.e. lower 4-CBA concentrations are always accompanied by higher burning rate.22 In sharp contrast, our finding demonstrates that it is possible to reduce the production of both these solid and gaseous by-products simultaneously by employing high pressure CO2. In conclusion, a multi-beneficial effect of CXL has been exhibited in the Co/Mn/Br catalyzed oxidation of p-xylene to terephthalic acid.
As shown in Fig. 5a, the yield of TPA is significantly elevated by doubling the concentration of cobalt from 33 mM to 66 mM and adding zirconium, in the form of the acetate (Zr/Co = 1/6) at lower temperatures. However, this promoting effect diminishes when the temperature is above 150 °C. The highest TPA yield of 97.5% is achieved with the combination of cobalt and zirconium at 170 °C.
![Effects of cobalt concentration and zirconium on p-xylene oxidation. Reaction conditions: PCO2 = PO2 = 30 bar, [Mn] = 1.0 mM, [Br] = 33 mM, VL = 35 mL, 1.6 mL p-xylene added at 0.08 mL/min, t = 0.5 hr, n = 1200 rpm.](/image/article/2010/GC/b920262e/b920262e-f5.gif) | ||
| Fig. 5 Effects of cobalt concentration and zirconium on p-xylene oxidation. Reaction conditions: PCO2 = PO2 = 30 bar, [Mn] = 1.0 mM, [Br] = 33 mM, VL = 35 mL, 1.6 mL p-xylene added at 0.08 mL/min, t = 0.5 hr, n = 1200 rpm. | ||
The effect on the purity of solid TPA parallels that on TPA yield. As shown in Fig. 5b, increasing the cobalt concentration and the use of zirconium enhances the purity of solid TPA, an effect that is more pronounced at lower temperatures. Also the highest purity is achieved with the combination of cobalt and zirconium at 170 °C. This is not surprising because the more complete conversion of the dissolved intermediates will definitely decrease their concentration in solid TPA.
The effect on the optical density at 340 nm is somewhat intriguing. As shown in Fig. 5c, an increase in cobalt concentration always helps lower the yield of yellow colored by-products within the studied temperature ranges. However, the incorporation of zirconium works well only at lower temperatures. The quality of the optical density no longer benefits from zirconium when the temperature is greater than 160 °C.
The effect on the yield of CO is more complicated. As shown in Fig. 5d, the production of CO can be inhibited by doubling the cobalt concentration and adding zirconium at temperatures below 135 °C. This might be unexpected since higher cobalt concentrations are considered to favor solvent burning.30 In contrast, there is a surge in CO yield when the temperature rises above 150 °C, especially with the use of zirconium.
Fig. 6 shows the effect of manganese. The reactions were carried out with a higher cobalt concentration of 66 mM at 170 °C. No zirconium was used because of the much higher solvent burning at this temperature.
![Effects of manganese concentration on p-xylene oxidation. Reaction conditions: PCO2 = PO2 = 30 bar, T = 170 °C, [Co] = 66 mM, [Br] = 33 mM, VL = 35 mL, 1.6 mL p-xylene added at 0.08 mL/min, t = 0.5 hr, n = 1200 rpm.](/image/article/2010/GC/b920262e/b920262e-f6.gif) | ||
| Fig. 6 Effects of manganese concentration on p-xylene oxidation. Reaction conditions: PCO2 = PO2 = 30 bar, T = 170 °C, [Co] = 66 mM, [Br] = 33 mM, VL = 35 mL, 1.6 mL p-xylene added at 0.08 mL/min, t = 0.5 hr, n = 1200 rpm. | ||
As shown in Fig. 6a and 6b, the yield of TPA and solid TPA purity decrease monotonically with the increase of manganese concentration. In the absence of manganese, the yield and purity can be as high as 96.9% and 99.5%, and these are decreased to 93.3% and 98.5% respectively when the manganese concentration is increased to 8.0 mM.
Too much manganese is also disadvantageous from the standpoint of the optical density. As shown in Fig. 6c, in the complete absence of manganese, the TPA solid has a very low optical density of 0.025. In comparison, this value quadruples to ca. 0.1 when the manganese concentration is 8.0 mM.
In spite of all these negative effects, manganese still plays a crucial role. As shown in Fig. 6d, the yield of CO is decreased by ca. 30% at a very low manganese concentration of 1.0 mM. At the same time, the yield of TPA is only slightly decreased from 96.9% to 96.6%. Thus, manganese is very effective in reducing solvent burning.
From the results described above, the optimized parameters for the medium-high temperature oxidation of p-xylene are: [Co] ≥ 60 mM, [Mn] ≤ 1.0 mM, [Zr] = 0 mM, T = 150–160 °C.
For this purpose, we chose the labeled compound 13CO2 as the inert gas. By mass spectrometry, the CO2 produced by burning can be differentiated from the CO2 added to the system by chemical reaction(s). To compare the different behavior of N2 and CO2 in solvent burning, three sets of reactions have been carried out, among which two sets are based on a lower temperature of 160 °C and a higher inert gas pressure of 45 bar, while the other set is based on a higher temperature of 191 °C and a lower inert gas pressure of 23 bar. The O2 pressure for the latter case is controlled at 7 bar to mimic an MC process as operated by industry. As the data in Table 2 reveal, the reactions yield 2–3 times as much CO2 as CO, regardless of the kind of inert gas present during reaction. This is in good agreement with the previous report that the CO2/CO mole ratio is around 3.32 Remarkably, the use of CO2 can suppress not only the production of CO, but also the production of CO2 at 160 °C, and it is interesting to note that the yield of CO is reduced to a larger extent. As a result, the burn rate is 0.14 for N2 and 0.11 for CO2 when O2 pressure is 45 bar, and 0.095 for N2 and 0.075 for CO2 when O2 pressure is 12 bar. In both cases, the burning is reduced by ca. 20% when compared to N2 with similar yields (ca. 95%) of TPA. Also, the yield of TPA is much better with CO2 at 191 °C, suggesting the positive effect of overcoming the kinetic barrier. While the yield of CO is nearly indistinguishable, the yield of CO2 is increased markedly at the higher temperature. Consequently, the reactions should be performed at lower temperature and higher CO2 pressure to inhibit solvent burning.
| Inert gas | T (°C) | P inert (bar) | PO2 (bar) | Y TPA (%) | 12CO (mmol) | 12CO2 (mmol) | (12CO2+12CO)/p-x (mol/mol) |
|---|---|---|---|---|---|---|---|
| Reaction conditions: for 160 °C reaction [Co] = 33 mM, [Mn] = 1.0 mM, [Br] = 33 mM; for 191 °C reaction [Co] = [Mn] = [Br] = 5.5 mM; VL = 35 mL, 1.6 mL p-xylene added at 0.08 mL/min, t = 0.5 hr, n = 1200 rpm. The conversion of p-xylene is above 99% in all the reactions. In addition to 4-CBA and PTA, a few other byproducts were detected in small amounts during the HPLC analysis. These compounds were not identified. | |||||||
| N2 | 160 | 45 | 45 | 94.6 | 0.52 | 1.30 | 0.14 |
| 13CO2 | 160 | 45 | 45 | 95.1 | 0.32 | 1.11 | 0.11 |
| N2 | 160 | 45 | 12 | 94.1 | 0.37 | 0.89 | 0.095 |
| 13CO2 | 160 | 45 | 12 | 94.3 | 0.23 | 0.76 | 0.075 |
| N2 | 191 | 23 | 7 | 84.7 | 0.45 | 0.68 | 0.085 |
| 13CO2 | 191 | 23 | 7 | 94.1 | 0.46 | 1.13 | 0.12 |
The formation of COx (CO plus CO2) during the MC process involves a very complicated reaction mechanism. Apart from the solvent burning that is the major source of COx, COx can also be produced by the over-oxidation of p-xylene and the decomposition of the TPA product. These mechanisms are currently under investigation. From the point of view of thermodynamics, it seems plausible that the equilibrium shifts to the left upon the addition of a large excess of CO2. In addition, we also observed that the CO2 yield is decreased by adding an appropriate amount of water, another by-product of burning. This also suggests the leftward shift of the equilibrium.
4. Conclusion
In summary, we have studied the Co/Mn/Br catalyzed medium-high temperature oxidation of p-xylene to terephthalic acid in CO2-expanded acetic acid based on the measurement of solvent expansion in a Jerguson cell that is equipped with a high temperature oven. The reactions have been optimized by varying the reaction temperature, the concentration of cobalt and manganese and the use of co-catalyst zirconium. As compared with N2/O2, the use of CO2/O2 at sufficiently high inert gas pressure and 160 °C significantly improved the catalytic performance by decreasing the yield of 4-carboxybenzaldehyde, p-toluic acid and other sources of yellow colored by-products. In addition, the quality of solid TPA is also greatly increased due to the reduction of the concentration of the by-products, especially 4-carboxybenzaldehyde. More worthy of mention is that solvent decomposition can also be effectively inhibited with CO2, as confirmed by isotopic experiments, which adds greater value to the CO2-expanded solvents. These results, as an extension of our previous work on the remarkable CO2 effect on Co/Zr catalytic system, further support the feasible and promising industrial application of CXLs in this kind of important oxidation reaction. The use of high pressure CO2 as reaction medium requires compression power and this could nullify the energy gain made by operating at lower temperatures. However, with the ever-growing global sentiment towards reduction of carbon emissions, novel solutions such as the one discussed here would be needed to meet government regulations on such emissions and thereby make carbon-emitting processes economically viable.Acknowledgements
This work was supported by the National Science Foundation Engineering Research Centers Program, Grant EEC-0310689. We are particularly grateful for the valuable discussions with Drs. Peter Metelski, Wayne Schammel and David Sikkenga of BP, Naperville, IL. We would also like to thank Dr. Todd Williams of the University of Kansas for his kind help with MS analysis.Notes and references
- W. Partenheimer, Catal. Today, 1995, 23, 69–158 CrossRef CAS.
- P. Raghavendrachar and S. Ramachandran, Ind. Eng. Chem. Res., 1992, 31, 453–462 CrossRef CAS.
- H. Milan, A. G. Hussain and R. Roberto, US Pat., 20080293964.
- P. A. Hamley, T. Ilkenhans, J. M. Webster, E. Garcia-Verdugo, E. Vernardou, M. J. Clarke, R. Auerbach, W. B. Thomas, K. Whiston and M. Poliakoff, Green Chem., 2002, 4, 235–238 RSC.
- J. B. Dunn and P. E. Savage, Environ. Sci. Technol., 2005, 39, 5427–5435 CrossRef CAS.
- Y. S. Chen, J. L. Fulton and W. Partenheimer, J. Am. Chem. Soc., 2005, 127, 14085–14093 CrossRef CAS.
- P. Jessop and B. Subramaniam, Chem. Rev., 2006, 107, 2666–2694.
- T. Seki and A. Baiker, Chem. Rev., 2009, 109, 2409–2454 CrossRef CAS.
- C. A. Eckert, D. Bush, J. S. Brown and C. L. Liotta, Ind. Eng. Chem. Res., 2000, 39, 4615–4621 CrossRef CAS.
- C. A. Eckert, C. L. Liotta, D. Bush, J. S. Brown and J. P. Hallett, J. Phys. Chem. B, 2004, 108, 18108–18118 CrossRef CAS.
- G. T. Musie, M. Wei, B. Subramaniam and D. H. Busch, Coord. Chem. Rev., 2001, 219, 789–820 CrossRef.
- M. Wei, G. T. Musie, D. H. Busch and B. Subramaniam, J. Am. Chem. Soc., 2002, 124, 2513–2517 CrossRef CAS.
- M. Wei, G. T. Musie, D. H. Busch and B. Subramaniam, Green Chem., 2004, 6, 387–394 RSC.
- X. Zuo, B. Subramaniam and D. H. Busch, Ind. Eng. Chem. Res., 2008, 47, 546–552 CrossRef CAS.
- M. A. Zeitlin and D. Wilger-Nowicki, US Pat., 5 095 146, 1992.
- H. Jin, B. Subramaniam, A. Ghosh and J. Tunge, AIChE J., 2006, 52, 2575–2581 CrossRef CAS.
- B. Rajagopalan, M. Wei, G. T. Musie, B. Subramaniam and D. H. Busch, Ind. Eng. Chem. Res., 2003, 42, 6505–6510 CrossRef CAS.
- D. R. Burri, K. W. Jun, J. S. Yoo, C. W. Lee and S. E. Park, Catal. Lett., 2002, 81, 169–173 CrossRef.
- J. S. Yoo, S. H. Jhung, K. H. Lee and Y. S. Park, Appl. Catal. A-Gen., 2002, 223, 239–251 CrossRef CAS.
- S. E. Park, J. S. Chang and K. W. Lee, Stud. Surf. Sci. Catal., 2004, 153, 303–314 CAS.
- P. Roffia, P. Calini and L. Motta, Ind. Eng. Chem. Prod. Res. Dev., 1984, 23, 629–634 CrossRef CAS.
- Personal Communication, BP Personnel (names provided in the acknowledgement section), 2007.
- G. G. Lavoie, R. T. Hembre, C. E. J. Sumner, J. N. Bays, D. B. Compton, B. A. Tennant, B. W. Davenport, D. Lange and T. R. Floyd, PCT Int. Appl. 2006, WO 2006096312.
- S. H. Jhung and Y. S. Park, Bull. Korean Chem. Soc., 2002, 23, 369–373 CAS.
- A. W. Chester, E. J. Y. Scott and P. S. Landis, J. Catal., 1977, 46, 308–319 CrossRef CAS.
- R. L. June, M. W. Potter, E. J. Simpson and C. L. Edwards, US Pat., 6 153 790, 2000.
- W. Partenheimer, J. Mol. Catal. A-Chem., 2003, 206, 105–119 CrossRef CAS.
- W. Partenheimer, US Pat., 4 992 580, 1991.
- B. Rajagopalan, Ph.D. Thesis, University of Kansas, 2007.
- W. Partenheimer, in Catalysis of Organic Reactions, ed. D. W. Blackburn, Marcel Dekker, New York, 1990, pp. 321-346 Search PubMed.
- P. D. Metelski and J. H. Espenson, PCT Int. Appl. 2005, WO 2005000779.
- Y. Cheng, L. Zhang, G. Xie and X. Li, Chem. React. Eng. Technol. (China), 2003, 19, 182–187 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
