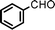
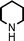
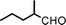
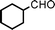
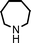
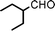
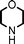
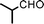
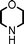
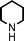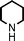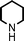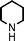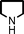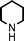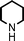Fe3O4 nanoparticles: a robust and magnetically recoverable catalyst for three-component coupling of aldehyde, alkyne and amine
Tieqiang Zengab, Wen-Wen Chena, Ciprian M. Cirtiua, Audrey Mooresa, Gonghua Song*b and Chao-Jun Li*a
aDepartment of Chemistry, McGill University, 801 Sherbrooke St. West, Montreal, Quebec, Canada H3A 2K6. E-mail: cj.li@mcgill.ca; Fax: (+1)-514-398-3797; Tel: (+1)-514-398-8457
bShanghai Key Laboratory of Chemical Biology, Institute of Pesticides and Pharmaceuticals, East China University of Science and Technology, Shanghai, 200237, P.R. China. E-mail: ghsong@ecust.edu.cn; Fax: +86-21-64252603
First published on 13th January 2010
Abstract
A robust, safe and magnetically recoverable Fe3O4 nanoparticle catalyzed three-component coupling of aldehyde, alkyne, and amine (A3-coupling) was developed. A diverse range of propargylamines were obtained in moderate to high yield under mild conditions in air. The separation and reuse of the magnetic Fe3O4 nanoparticles were very simple, effective and economical.
Environmentally benign, economical, practical, and efficient processes for catalyst separation and reuse have been increasingly important goals in the chemical community from economic, safety, and environmental points of view.1 The strategy of magnetic separation, taking advantage of magnetic nanoparticles, is typically more effective than filtration or centrifugation as it prevents loss of the catalyst.2 Magnetic separation of the magnetic nanoparticles is simple, economical and promising for industrial applications.3 In recent years, Fe3O4 nanoparticles (magnetite nanoparticle) have attracted worldwide attention.3 Various strategies have successfully demonstrated the applications of Fe3O4 nanoparticle-immobilized or -supported catalysts.4 However, the direct use of Fe3O4 nanoparticles without modification as magnetically recoverable catalysts for organic reactions is very rare.5
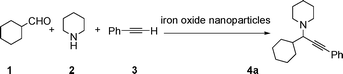
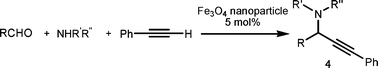
Propargylic amines, products of the three-component aldehyde–alkyne–amine coupling (A3-coupling), are useful building blocks and important skeletons of biologically active compounds.6 During the past decade, significant efforts have been made in order to develop one-pot multi-component reactions to make new carbon–carbon bonds.7 In the past few years, we8 and others9 have reported highly efficient couplings catalyzed by various metals such as copper, silver, gold, iridium, and indium to afford various propargylamines. Recently, we10 and others11 also reported that such a coupling can also be catalyzed by iron salts. In these studies, we also observed that iron powder could catalyze the reaction. To rationalize this result, we speculated that the iron-powder reaction was due to the catalytic activities of iron oxides on the surface of the powder. Consequently, we were intrigued by the possibility of using Fe3O4 nanoparticles as catalysts for the A3-coupling reaction, featuring both a much greater surface area than iron powder and magnetic recoverability. Herein, we wish to report the robust and magnetically recoverable Fe3O4 and Fe2O3 nanoparticles catalyzed three-component coupling of aldehyde, alkyne and amine (Fig. 1). It was found that good yields were obtained and the magnetic recovery of Fe3O4 nanoparticles was simple and efficient. The catalyst was directly reused 12 times without the need for activation. Fe2O3 nanoparticles were also effective as catalysts.
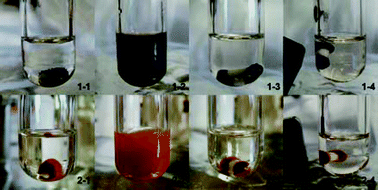 | ||
| Fig. 1 Photo of 1-1: Fe3O4 nanoparticles in THF; 1-2: Fe3O4 nanoparticle dispersion in THF; 1-3: Fe3O4 nanoparticles adsorbed on the magnetic stirring bar; 1-4: a magnet attracted the magnetic stirring bar and Fe3O4 nanoparticles; 2-1: Fe2O3 nanoparticles in THF; 2-2: Fe2O3 nanoparticle dispersion in THF; 2-3: Fe2O3 nanoparticles adsorbed on the magnetic stirring bar; 2-4: a magnet attracted the magnetic stirring bar and Fe2O3 nanoparticles. | ||
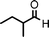
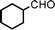
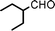
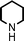
We used cyclohexanecarbaldehyde, piperidine, and phenylacetylene as standard substrates to search for a suitable solvent for the Fe3O4 nanoparticle (<50 nm) catalyzed A3-coupling (Table 1). Among the solvents tested, tetrahydrofuran was the most effective reaction medium for this three-component coupling reaction (Table 1, entry 1). The use of tetrahydrofuran effected not only the coupling reaction of aldehyde, alkyne, and amine in good yield, but also performed well in the process of magnetic separation of nanoparticle catalysts, by reducing the viscosity of the reaction mixture and facilitating the congregation of magnetic catalyst, when the reaction was complete. Slightly lower yields were obtained when using acetonitrile or ethyl acetate as the solvent (Table 1, entries 2 and 3). Ethanol, dichloromethane, water, acetone and dimethyl sulfoxide (DMSO) afforded the products in only low or moderate yields (Table 1, entries 4–8). No desired product was detected by NMR when the reaction was carried out in N,N-dimethylformamide (DMF) (Table 1, entry 9). The corresponding product was also obtained in good yield under neat conditions (Table 1, entry 10). However, the mixture was viscous in the absence of a solvent and made the separation of catalyst from products difficult magnetically unless an extraction solvent such as ether was added. No significant difference was observed when slightly increasing the catalyst loading (Table 1, entry 11). It is worth noting that γ-Fe2O3 nanoparticles (<50 nm) also catalyzed the reaction, affording the corresponding propargylamine products in a lower yield (Table 1, entry 12). The gamma-form iron oxide nanoparticles can also be separated easily by magnetic method and reused. No desired product was obtained in the absence of Fe3O4 nanoparticles or Fe2O3 nanoparticles. The optimized reaction conditions include 1.0 equiv of aldehyde, 1.2 equiv of amine, 1.5 equiv of alkyne, and 5 mol % of Fe3O4 nanoparticles in THF at 80 °C in air. Buchwald and Bolm recently found that the results of FeCl3-catalyzed reactions may be due to trace quantities of copper.12 To preclude such a possibility in the present case, we tested 99.99% Fe3O4 powder (from Aldrich) to catalyze the three-component coupling of Cu-free cyclohexanecarbaldehyde, piperidine, and phenylacetylene under the optimized reaction conditions, which gave 57% NMR yield.13 In comparison, a 54% NMR yield was obtained when 600 ppm Cu2O was added into this 99.99% Fe3O4 powder, which indicated that trace quantity of copper has no obvious effect on this reaction. Considering Fe3O4 nanoparticles have a much larger surface area than their powder form, we conclude that the reaction was catalyzed by Fe3O4 nanoparticles rather than by trace copper impurities.
| ||
|---|---|---|
| Entry | Solvent | NMR Yield(%) |
| a All reactions were carried out with cyclohexanecarbaldehyde (0.5 mmol), piperidine (0.6 mmol), phenylacetylene (0.75 mmol) and Fe3O4 nanoparticles (0.025 mmol) at 80 °C (oil bath) at 24 h.b (0.05 mmol) Fe3O4 nanoparticles was used as catalyst.c (0.025 mmol) Fe2O3 nanoparticles was used as catalyst. | ||
| 1 | THF | 89 |
| 2 | CH3CN | 86 |
| 3 | Ethyl acetate | 84 |
| 4 | CH3CH2OH | 69 |
| 5 | CH2Cl2 | 42 |
| 6 | H2O | 39 |
| 7 | Acetone | 29 |
| 8 | DMSO | 10 |
| 9 | DMF | 0 |
| 10 | — | 89 |
| 11 | — | 92b |
| 12 | THF | 70c |
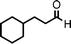
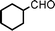
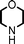
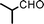
To expand the scope of this A3-coupling, various aldehydes and amines were used as substrates under the optimized reaction conditions, and the results are summarized in Table 2. In general, both aromatic and aliphatic aldehydes underwent the addition reaction smoothly to provide the desired product in moderate to good yields (Table 2, entries 1–14). However, similar to the FeCl3-catalyzed A3-coupling,10 the reaction was found to be strongly influenced by the nature of the aldehyde. As shown in Table 2, aliphatic aldehydes were more reactive than the aromatic aldehyde. The reactions involving aliphatic aldehydes such as cyclohexanecarboxaldehyde, valeraldehyde, isobutyraldehyde, 2-methylbutanal, and 3-phenylpropanal all provided higher yields than benzaldehyde (Table 2, entries 1, 2, 4, 5, 6 and 10). Formaldehyde (37 wt% in water) afforded the desired products also in good yield (Table 2, entry 3). Moderate to good yields were observed when cyclic dialkylamines such as pyrrolidine, morpholine and azepane were used (Table 2, entries 7, 8, 12, 13 and 14).
The magnetic Fe3O4 or Fe2O3 nanoparticles were adsorbed onto the magnetic stirring bar when the magnetic stirring was stopped. The nanoparticles were then washed with ethyl acetate, air-dried and used directly for the next round of reactions without further purification. It was shown that the Fe3O4 nanoparticle catalyst could be recovered and reused 12 times without significant loss of catalytic activity (Table 3).
| Cycle | NMR Yield(%) | Cycle | NMR Yield(%) |
|---|---|---|---|
| a All reactions were carried out with 0.5 mmol cyclohexanecarbaldehyde, 0.6 mmol piperidine, 0.75 mmol phenylacetylene and 0.025 mmol Fe3O4 nanoparticles (for cycle 1) or recovered Fe3O4 nanoparticles (for other cycles) at 80 °C (oil bath) in THF for 24 h. | |||
| 1 | 89 | 7 | 73 |
| 2 | 85 | 8 | 74 |
| 3 | 82 | 9 | 79 |
| 4 | 83 | 10 | 78 |
| 5 | 80 | 11 | 80 |
| 6 | 77 | 12 | 82 |
In summary, we have demonstrated a robust and magnetically recoverable Fe3O4 nanoparticle catalyzed three-component coupling of aldehyde, alkyne, and amine (A3-coupling). A diverse range of propargylamines were obtained in moderate to high yield under mild conditions in air. The separation and reuse of the magnetic Fe3O4 nanoparticles were very simple, effective and economical. In addition, the use of iron oxides as catalysts is also more environmentally friendly and safer than other transition-metal catalysts. We also showed that the efficiency of the catalytic activity is also affected by the different forms of iron oxides. The direct use and recycling of magnetic Fe3O4 and Fe2O3 nanoparticles to catalyze other reactions by the same strategy are under investigation in our laboratory.
Experimental section
Fe3O4 (<50 nm particle size (TEM), ≥98%) and Fe2O3 (<50 nm particle size) were purchased from Sigma-Aldrich. To a reaction tube charged with a magnetic stir bar and Fe3O4 nanoparticles (0.05 mmol, 5 mol %) in air, aldehyde (0.5 mmol), amine (0.6 mmol), and alkyne (0.75 mmol) were added. The tube was then stoppered. The reaction mixture was stirred at 80 °C (oil bath temperature) for 24 h. The Fe3O4 nanoparticles were adsorbed on to the magnetic stirring bar when the stirring was stopped. After cooling to room temperature, the reaction solution was filtered through Celite in a pipette eluting with ethyl acetate. The volatile liquid was removed in vacuo and the residue was purified by flash column chromatography on silica gel (eluent: hexane–ethyl acetate = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the corresponding product. Fe3O4 nanoparticles were washed with ethyl acetate, air-dried and used directly for the next round of reactions without further purification.
1) to give the corresponding product. Fe3O4 nanoparticles were washed with ethyl acetate, air-dried and used directly for the next round of reactions without further purification.
Acknowledgements
We are grateful to the Canada Research Chair foundation (to CJL and AM), the CFI, NSERC and McGill University for support of our research. TQZ thanks the China Scholarship Council for a Visiting Scholarship. We thank Prof. Eric D. Salin, David A. Duford and Yongqing Xi of the Chemistry Department at McGill University for the ICP-AES and AAS analysis.Notes and references
- P. T. Anastas, J. C. Warner, Green Chemistry: Theory and Practice, Oxford University, Oxford, 1998 Search PubMed.
- D. Guin, B. Baruwati and S. V. Manorama, Org. Lett., 2007, 9, 1419 CrossRef CAS.
- (a) S. Sun and H. Zeng, J. Am. Chem. Soc., 2002, 124, 8204 CrossRef CAS; (b) A. H. Latham and M. E. Williams, Acc. Chem. Res., 2008, 41, 411 CrossRef CAS; (c) S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064 CrossRef CAS.
- For examples, see: (a) V. Polshettiwar, B. Baruwati and R. S. Varma, Chem. Commun., 2009, 1837 RSC; (b) S. Luo, X. Zheng, H. Xu, X. Mi, L. Zhang and J.-P. Cheng, Adv. Synth. Catal., 2007, 349, 2431 CrossRef CAS; (c) V. Polshettiwar, B. Baruwati and R. S. Varma, Green Chem., 2009, 11, 127 RSC; (d) M. Kotani, T. Koike, K. Yamaguchi and N. Mizuno, Green Chem., 2006, 8, 735 RSC; (e) D.-H. Zhang, G.-D. Li, J.-X. Lia and J.-S. Chen, Chem. Commun., 2008, 3414 RSC; (f) M. Kawamura and K. Sato, Chem. Commun., 2006, 4718 RSC; (g) M. Kawamura and K. Sato, Chem. Commun., 2007, 3404 RSC; (h) A. Hu, G. T. Yee and W. Lin, J. Am. Chem. Soc., 2005, 127, 12486 CrossRef CAS; (i) G. Chouhan, D. Wang and H. Alper, Chem. Commun., 2007, 4809 RSC; (j) R. Abu-Reziq, D. Wang, M. Post and H. Alper, Adv. Synth. Catal., 2007, 349, 2145 CrossRef CAS; (k) V. Polshettiwar and R. S. Varma, Chem.–Eur. J., 2009, 15, 1582 CrossRef CAS; (l) J. Ge, Q. Zhang, T. Zhang and Y. Yin, Angew. Chem., Int. Ed., 2008, 47, 8924 CrossRef CAS.
- For using superparamagnetic iron oxide as catalyst for the synthesis of α-aminonitriles, see: M. M. Mojtahedi, M. S. Abaee and T. Alishiri, Tetrahedron Lett., 2009, 50, 2322 Search PubMed.
- (a) M. A. Huffman, N. Yasuda, A. E. DeCamp and E. J. J. Grabowski, J. Org. Chem., 1995, 60, 1590 CrossRef CAS; (b) M. Konishi, H. Ohkuma, T. Tsuno, T. Oki, G. D. VanDuyne and J. Clardy, J. Am. Chem. Soc., 1990, 112, 3715 CrossRef CAS; (c) M. Miura, M. Enna, K. Okuro and M. Nomura, J. Org. Chem., 1995, 60, 4999 CrossRef CAS; (d) A. Jenmalm, W. Berts, Y. L. Li, K. Luthman, I. Csoregh and U. Hacksell, J. Org. Chem., 1994, 59, 1139 CrossRef CAS.
- For representative reviews on multi-component reactions, see: (a) Multicomponent Reaction (ed. J. Zhu and H. Bienaymé), Wiley-VCH, Weinheim, 2005 Search PubMed; (b) G. H. Posner, Chem. Rev., 1986, 86, 831 CrossRef CAS; (c) R. W. Armstrong, A. P. Combs, P. A. Tempest, S. D. Brown and T. A. Keating, Acc. Chem. Res., 1996, 29, 123 CrossRef CAS; (d) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef; (e) D. M. D'Souza and T. J. J. Müller, Chem. Soc. Rev., 2007, 36, 1095 RSC; (f) D. Tejedor and F. Garcia-Tellado, Chem. Soc. Rev., 2007, 36, 484 RSC; (g) B. Ganem, Acc. Chem. Res., 2009, 42, 463 CrossRef CAS.
- (a) C.-J. Li, Acc. Chem. Res., 2002, 35, 533 CrossRef CAS; (b) C. Wei and C.-J. Li, Green Chem., 2002, 4, 39 RSC; (c) C.-J. Li and C. Wei, Chem. Commun., 2002, 268 RSC; (d) J. H. Zhang, C. Wei and C.-J. Li, Tetrahedron Lett., 2002, 43, 5731 CrossRef CAS; (e) C. Wei and C.-J. Li, J. Am. Chem. Soc., 2002, 124, 5638 CrossRef CAS; (f) C. Wei and C.-J. Li, J. Am. Chem. Soc., 2003, 125, 9584 CrossRef CAS; (g) C. Wei, Z. Li and C.-J. Li, Org. Lett., 2003, 5, 4473 CrossRef CAS; (h) Z. Li, C. Wei, L. Chen, R. S. Varma and C.-J. Li, Tetrahedron Lett., 2004, 45, 2443 CrossRef CAS; (i) B. Huang, X. Yao and C.-J. Li, Adv. Synth. Catal., 2006, 348, 1528 CrossRef CAS . For a review on A3-coupling and AA3-coupling, see: C. Wei, Z. Li and C.-J. Li, Synlett, 2004, 1472 Search PubMed.
- For selected examples of A3-coupling reported by others, see: (a) Fischer and E. M. Carreira, Org. Lett., 2001, 3, 4319 CrossRef CAS; (b) S. Satoshi, K. Takashi and Y. Ishii, Angew. Chem. Int. Ed., 2001, 40, 2534 CrossRef CAS; (c) R. Fassler, D. E. Frantz, J. Oetiker and E. M. Carreira, Angew. Chem. Int. Ed., 2002, 41, 3054 CrossRef CAS; (d) C. Koradin, K. Polborn and P. Knochel, Angew. Chem. Int. Ed., 2002, 41, 2535 CrossRef CAS; (e) N. Gommermann, C. Koradin, K. Polborn and P. Knochel, Angew. Chem. Int. Ed., 2003, 42, 5763 CrossRef CAS; (f) N. Gommermann and P. Knochel, Chem. Commun., 2003, 2324 RSC; (g) C. Koradin, N. Gommermann, K. Polborn and P. Knochel, Chem. Eur. J., 2003, 9, 2797 CrossRef CAS; (h) N. Gommermann, A. Gehrig and P. Knochel, Synlett, 2005, 2796 CAS; (i) N. Gommermann and P. Knochel, Synlett, 2005, 2799 CAS; (j) N. Gommermann and P. Knochel, Chem. Commun., 2005, 4175 RSC; (k) S. Yasuike, C. C. Kofink, R. J. Kloetzing, N. Gommermann, K. Tappe, A. Gavryushin and P. Knochel, Tetrahedron: Asymmetry, 2005, 16, 3385 CrossRef CAS; (l) N. Gommermann and P. Knochel, Tetrahedron, 2005, 61, 11418 CrossRef CAS; (m) N. Gommermann and P. Knochel, Chem. Eur. J., 2006, 12, 4380 CrossRef CAS; (n) T. F. Knoepfel, P. Aschwanden, T. Ichikawa, T. Watanabe and E. M. Carreira, Angew. Chem. Int. Ed., 2004, 43, 5971 CrossRef; (o) P. Aschwanden, C. R. J. Stephenson and E. M. Carreira, Org. Lett., 2006, 8, 2437 CrossRef CAS; (p) V. K. Y. Lo, Y. Liu, M. K. Wong and C. M. Che, Org. Lett., 2006, 8, 1529 CrossRef CAS.
- W. W. Chen, R. V. Nguyen and C.-J. Li, TetrahedronLett., 2009, 50, 2895 CrossRef CAS.
- P. Li, Y. Zhang and L. Wang, Chem. Eur. J., 2009, 15, 2045 CrossRef CAS.
- (a) S. L. Buchwald and C. Bolm, Angew. Chem. Int. Ed., 2009, 48, 5586 CrossRef CAS; (b) P.-F. Larsson, A. Correa, M. Carril, P.-O. Norrby and C. Bolm, Angew. Chem. Int. Ed., 2009, 48, 5691 CrossRef CAS.
- Fe3O4 powder (99.99%), cyclohexanecarbaldehyde, piperidine, and phenylacetylene were purchased from Sigma-Aldrich. Cyclohexanecarbaldehyde and piperidine were distilled before use. Analysis by using Perkin-Elmer 603 atomic absorption spectrophotometer showed that 99.99% Fe3O4 powder and phenylacetylene were Cu-free (below detecting limit).
| This journal is © The Royal Society of Chemistry 2010 |