Facile palladium catalyzed Suzuki–Miyaura coupling in air and water at ambient temperature†
Alexander N. Marzialea, Stefan H. Faula, Thomas Reinerb, Sven Schneiderb and Jörg Eppinger*a
aBio- & Organometallic Catalysis Laboratories, KAUST Catalysis Center, 4700 King Abdullah University of Science and Technology, Thuwal, 23955-6900, Kingdom of Saudi Arabia. E-mail: jorg.eppinger@kaust.edu.sa
bTechnische Universität München, Department Chemie, Lichtenbergstr. 4, D-85747, Garching, Germany
First published on 7th October 2009
Abstract
A new palladacyclic catalyst yields high activities in aqueous Suzuki–Miyaura coupling at room temperature. Using an optimized protocol, a broad range of products can be isolated in good to excellent yields and high purity by simple filtration.
The application of water as an environmentally benign and economically favourable alternative to organic solvents has developed into an highly active field of research addressing current requirements in synthetic chemistry and catalysis.1–4 In contrast to common organic reaction media it is non-toxic, non-flammable and cheap.5 Particularly, catalytic cross-coupling reactions have been successfully applied in the aqueous phase.5,7 Regarding functional group tolerance and scope of application, Suzuki–Miyaura reactions have become one of the most powerful tools to selectively generate biaryls.8 During the last decade several groups described biphasic protocols for Suzuki–Miyaura couplings commonly based on water-soluble catalysts, including contributions by Buchwald,9 Beller,10 Miyaura,11 Plenio,12,13 Shaughnessy,14 Genet,15 and Leadbeater.6 Lipshutz and co-workers reported room temperature couplings in water utilizing nonionic ambiphiles or micelle forming additives.16 Alternatively, phase-transfer catalysis17 or mixtures of water and organic co-solvents have been employed.17,18 Further approaches include heating by microwave irradiation6,19–21 or application of supported catalysts.22–24 Yet, despite a variety of advantageous features, aqueous cross-coupling protocols typically necessitate co-solvents, high catalyst loading,25–27 elevated temperatures28 and/or tedious product work-up by column chromatography.6,13–28
Arguably the greatest advantage of performing cross-coupling reactions in neat aqueous buffer represents the possibility of facile separation of the phase, which is formed by solid lipophilic biaryl coupling products, yet such examples remain scarce.29,12 In this communication we present an ambient temperature protocol for aqueous Suzuki–Miyaura couplings based on a newly developed Bedford-type30 catalyst 4, facilitating product isolation by simple filtration. Such a protocol meets the criteria of “click-reactions”, which were defined by Sharpless to tolerate a broad scope of substrates, exhibit high yields, no or only inoffensive byproducts, insensitivity against air and water, no or only benign solvents and – most importantly – ease of product isolation.31,32 Hence, we systematically tested a variety of palladium catalysts known to be active in aqueous cross-coupling reactions.30,29,33 Based on the pH-dependence for catalyst activities in Suzuki reactions, which was established by Ogo et al.,33 conversion of bromophenol with phenylboronic acid at pH = 11 (carbonate buffer) was chosen as test reaction (Table 1). Reaction temperatures had to be kept slightly above room temperature (30 °C) to guarantee reproducible results. Under the desired conditions (aqueous buffer without any additives, room temperature, vessel open to air, palladium loading <0.05 mol%, product isolation by simple filtration), water soluble catalyst precursors including typical palladium salts with (9, 10) or without (8) stabilizing water-soluble phosphine ligand and cationic palladacycles (6) delivered only poor to moderate yields after 16 hrs of reaction time. Non-ionic palladium complexes proved to be more efficient catalysts under these reaction conditions. Improved yields were found for the asymmetric pincer complex 7, which has recently been applied by Domínguez and co-workers under similar conditions,29 yet working at high catalyst loadings (2 mol%). Best cross-coupling results were achieved using Bedford-type palladacyclic catalysts30 after optimizing the substitution pattern. In particular, the phosphine substituents proved to be of high influence with iso-propyl groups being most beneficial. Correspondingly, the new palladacycle 4 achieved 91% yield.
 | ||
| Fig. 1 Catalysts tested in aqueous Suzuki–Miyaura cross-coupling. | ||
| ||
|---|---|---|
| Entry | Catalyst | Yield (%)a |
| Reaction conditions: 2 mmol (1 eq.) of 4-bromophenol, 1.0 eq. phenylboronic acid, 20 ml buffer (1.0 M, NaOH/NaHCO3, pH = 11), r.t., 16 h, air, cat. ([Pd] = 0.02 mol%).a Yields were determined by GC.b [Pd]:HP(O)iPr2 = 1:1. | ||
| 1 | 1 | 42 |
| 2 | 2 | 73 |
| 3 | 3 | 33 |
| 4 | 4 | 91 |
| 5 | 5 | 30 |
| 6 | 6 | 32 |
| 7 | 7 | 64 |
| 8 | (NBu4)2[Pd2Br6] (8) | 35 |
| 9 | (NBu4)2[Pd2Br6]/P(OH)iPr2 (9)b | 39 |
| 10 | Pd(OAc)2/P(OH)iPr2 (10)b | 56 |
Having identified this catalyst as a possible candidate for a cross-coupling “click-chemistry” protocol, we investigated the influence of various reaction parameters on product yields (Table 2). A catalyst loading of [Pd] = 4 × 10−2 mol L−1 proved to be necessary to achieve quantitative conversion, while at [Pd] = 2 × 10−2 mol L−1 the best TON (4550) was achieved. Examination of the dependence of isolated yields on substrate concentration revealed an optimal concentration of bromophenol of 0.1 mol L−1. While a decrease in yield for lower substrate concentrations seems to reflect the typical reaction profile of cross-coupling catalysis (1st order in aryl halide), reduced yields at higher substrate concentrations can be attributed to the formation of a solid product phase at low conversions. After this phase has been formed, the lipophilic compounds (aryl halide and catalyst) are trapped since mixing is hampered. Thus, the substrate concentration has to be chosen such that formation of the product layer, which facilitates work-up by filtration, occurs at close to quantitative conversion only. This interpretation is supported by the marked decrease in reaction rate after the solid layer has started to form. Using catalyst 4, 76% yield can be isolated after 30 min, surpassing the 16 hrs value of all other catalysts tested under the same conditions (Table 1). Interestingly, an excess of boronic acid also leads to decreased yields due to enhanced formation of the undesired homocoupling by-product. Among the different boron nucleophiles tested, quantitative conversion could only be achieved for phenylboronic acid. Potassium phenyltrifluoroborate can also well be used under these conditions yielding 90% of the coupling product, while no conversion is observed for sodium tetraphenylborate.
| |||
|---|---|---|---|
| Entry | Parameters | Yield (%) | |
| Reaction conditions: 20 ml buffer (1.0 M, NaOH/NaHCO3, pH = 11), r.t., air.a 1.0 eq. of borane nucleophile.b [4] = 0.04 mol%.c Reaction time 16 h.d Reaction time 2 h.e Ar-BR3− = phenylboronic acid.f [Ar-Br] = 0.1 mol L−1. | |||
| 1 | [4]/mol%a,c,e,f | 0.005 | 48 |
| 2 | 0.01 | 91 | |
| 3 | 0.02 | ≥99 | |
| 4 | [Ar-Br]/mol L−1a,b,c,e | 0.05 | 84 |
| 5 | 0.1 | ≥99 | |
| 6 | 0.25 | 74 | |
| 7 | reaction time/ha,b,e,f | 0.5 | 76 |
| 8 | 2 | 83 | |
| 9 | 16 | ≥99 | |
| 10 | BR3−a,b,c,f | B(OH)3− | ≥99 |
| 11 | BF3− | 90 | |
| 12 | BPh3− | 0 | |
| 13 | [Ar-BR3−]/[Ar-Br]b,d,e,f | 1 | 83 |
| 14 | 1.5 | 81 | |
The substrate range of catalyst 4 in the Suzuki–Miyaura cross-coupling reaction was tested under the optimized reaction conditions using a combination of four different aryl bromides and nine boronic acids. Among all possible combinations 16 biphenylic coupling products precipitated during the reaction and were isolated in good to excellent yields and selectivities by filtration (Table 3, entries 1–16). Formation of boronic acid homocoupling by-products has been attributed to palladium peroxo complexes, which are generated in the presence of oxygen during catalysis.34 Although cross-coupling reactions were carried out in an open vessel under an atmosphere of air, less than 1% of homocoupling was observed. Correspondingly, washing the residue twice with desalinated water was sufficient to obtain pure products,35 as determined by 1H NMR spectroscopy and elemental analysis. Various functionalities are tolerated for the aryl bromide, including aldehyde, ketone, carboxylic and hydroxylic groups. Furthermore, a broad variety of arylboronic acids bearing methyl, chlorine, fluorine, carboxylic, methoxy, and 3,4-methylendioxy residues were coupled successfully. Hence, the optimized protocol is applicable to electron-rich and electron-poor aryl bromides and arylboronic acids. The heteroaromatic boron substrates 2-furanyl and 2-thiophenyl boronic acid (Table 3, entries 17 and 18) could only be converted in moderate yields using aryl iodides. Hence, in these cases, isolation of products by filtration was not practical. Aryl chlorides in general and non-polar aryl bromides, such as bromobenzene or bromoanisol, could not be converted at 30 °C. Notably, the reactivity difference established for aryl bromides and chlorides can be used for selective couplings as demonstrated for the reaction of 4-Cl-phenylboronic acid with 4-bromophenol (Table 3, entry 5–7).
| ||
|---|---|---|
| Entry | Producta | Isolated yield (%) |
| Reaction conditions: 2 mmol (1 eq.) 4-bromophenol, 1.0 equiv. boronic acid, 20 ml buffer (1.0 M, NaOH/NaHCO3, pH = 11), air, cat. (0.02 mol% palladacycle 4). Reaction times not optimized.a Carboxylic acids isolated as corresponding sodium salts. Note on product representation: arene on right side originates from aryl bromide, arene on left side from boronic acid.b Purification by column chromatography necessary.c The corresponding aryl iodide has been used. | ||
| 1 |  | ≥99 |
| 2 |  | 83 |
| 3 |  | ≥99 |
| 4 |  | 65 |
| 5 |  | ≥99 |
| 6 |  | 83 |
| 7 |  | 72 |
| 8 | 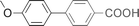 | 90 |
| 9 |  | ≥99 |
| 10 | 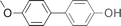 | 93 |
| 11 | 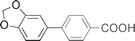 | 83 |
| 12 |  | 81 |
| 13 |  | ≥99 |
| 14 |  | ≥99 |
| 15 |  | 93 |
| 16 |  | 88 |
| 17 |  | 49b,c |
| 18 |  | 31b,c |
Conclusions
In conclusion, we have developed a simple protocol for Suzuki–Miyaura cross-coupling catalysis at room temperature in pure aqueous buffer, facilitating isolation of a broad variety of cross-coupling products by simple filtration in excellent yields and purity. Hence, this protocol meets all the criteria associated with “click-reactions”. In particular, avoiding the addition of organic additives, which are usually difficult to separate from the desired product offers the ability to beneficially exploit solubility differences. We are currently investigating possibilities to broaden this concept to other catalytic reactions.Experimental
General procedure for the Suzuki cross-coupling: 0.2 ml of a catalyst stock solution (0.002 mol L−1) containing palladacycle 4 (4.0 mg, 4.7 μmol) in 2.34 ml of dichloromethane were placed in a Schlenk tube. The solvent was removed in vacuo and 2 mmol (1 eq.) of the according aryl bromide in 20 ml of a NaHCO3/NaOH buffer (pH = 11, c = 1.0 M) were added. The reaction was vigorously stirred for 10 min at r.t. Subsequently 2 mmol (1 eq.) of the respective boronic acid were added. The reaction mixture was stirred for 16 h at 30 °C before the product was filtered. After washing the residue twice with 5 ml of deionized water, the analytically pure product was obtained.Acknowledgements
We are grateful to the Stifterverband für die deutsche Wissenschaft (Projekt-Nr. 11047 (ForschungsDozentur Molekulare Katalyse), J.E.), the DFG (Emmy-Noether Programm (SCHN950/2-1), S.S.), the Elitenetzwerk Bayern (graduate fellowship for A.N.M. and T.R.), and the IDK NanoCat for funding of this project.Notes and references
- I. T. Horváth, Green Chem., 2008, 10, 1024–1028 RSC; C.-J. Li, Acc. Chem. Res., 2002, 35, 533–538 CrossRef CAS.
- B. H. Lipshutz and S. Ghorai, Aldrichim. Acta, 2008, 41, 59–72 CAS; F. Alonso, I. P. Beletskaya and M. Yus, Tetrahedron, 2008, 64, 3047–3101 CrossRef CAS.
- A. R. Sheldon, Green Chem., 2005, 7, 267–278 RSC.
- S. Liu and J. Xiao, J. Mol. Catal. A: Chem., 2007, 270, 1–43 CrossRef CAS.
- Organic Reactions in Aqueous Media, ed. C.-J. Li and T.-H. Chan, Wiley, New York, 1997 Search PubMed; Organic Synthesis in Water ed. P. A. Grieco, Academic Press, Dordrecht, The Netherlands, 1997 Search PubMed; Aqueous-Phase Organometallic Catalysis, ed. B. Cornils and W. A. Herrmann, Wiley-VCH, Weinheim, 2nd edn, 2004 Search PubMed; K. H. Shaughnessy and R. B. DeVasher, Curr. Org. Chem., 2005, 9, 585 Search PubMed.
- N. E. Leadbeater, Chem. Commun., 2005, 2881 RSC.
- C. J. Li, Chem. Rev., 2005, 105, 3095–3165 CrossRef CAS; I. P. Beletskaya, A. V. Cheprakov, in Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E. Negishi, Wiley, New York, 2002, vol. 2, pp. 2957-3006 Search PubMed.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- K. W. Anderson and L. Buchwald, Angew. Chem., Int. Ed., 2005, 44, 6173–6177 CrossRef CAS.
- M. Beller, J. G. E. Krauter, A. Zapf and S. Bogdanovic, Catal. Today, 1999, 48, 279–290 CrossRef CAS; M. Beller, J. G. E. Krauter and A. Zapf, Angew. Chem., Int. Ed. Engl., 1997, 36, 772–774 CrossRef CAS.
- M. Ueda, M. Nishimura and N. Miyaura, Synlett, 2000, 6, 856–858.
- C. A. Fleckenstein and H. Plenio, Green Chem., 2007, 9, 1287–1291 RSC.
- C. A. Fleckenstein, S. Roy, S. Leuthäußer and H. Plenio, Chem. Commun., 2007, 2870–2872 RSC; C. A. Fleckenstein and H. Plenio, Chem.–Eur. J., 2007, 13, 2701–2716 CrossRef CAS; C. A. Fleckenstein and H. Plenio, J. Org. Chem., 2008, 73, 3236–3244 CrossRef CAS; C. A. Fleckenstein and H. Plenio, Chem.–Eur. J., 2008, 14, 4267–4279 CrossRef CAS.
- L. R. Moore and K. H. Shaughnessy, Org. Lett., 2001, 3, 2757–2759 CrossRef CAS; L. R. Moore and K. H. Shaughnessy, Org. Lett., 2004, 6, 225–228 CrossRef CAS; R. Huang and K. H. Shaughnessy, Organometallics, 2006, 25, 4105–4112 CrossRef; L. r. Moore, E. C. Western, R. Craciun, J. M. Spruell, D. A. Dixon, K. P. O'Halloran and K. H. Shaughnessy, Organometallics, 2008, 27, 576–593 CrossRef CAS.
- J. P. Genêt, A. Linquist, E. Blart, V. Mouriès and M. Savignac, Tetrahedron Lett., 1995, 36, 1443–1446 CrossRef CAS; C. Dupuis, K. Adiey, L. Charruault, V. Michelet, M. Savignac and J. P. Genêt, Tetrahedron Lett., 2001, 42, 6523–6526 CrossRef CAS.
- B. H. Lipshutz, T. B. Petersen and A. R. Abela, Org. Lett., 2008, 10, 1333–1336 CrossRef CAS; B. H. Lipshutz and A. R. Abela, Org. Lett., 2008, 10, 5329–5332 CrossRef CAS.
- C. Nájera, J. Gil-Moltó, S. Karlström and L. R. Falvello, Org. Lett., 2003, 5, 1451–1454 CrossRef CAS; C. Nájera, J. Gil-Moltó and S. Karlström, Adv. Synth. Catal., 2004, 346, 1798–1811 CrossRef CAS; L. Botella and C. Nájera, Angew. Chem., Int. Ed., 2002, 41, 179–181 CrossRef CAS; K. Botella and C. Nájera, Angew. Chem., 2002, 114, 187–189 CrossRef; D. A. Alonso, C. Nájera and M. C. Pacheco, J. Org. Chem., 2002, 67, 5588–5594 CrossRef CAS; L. Botella and C. Nájera, J. Organomet. Chem., 2002, 663, 46–57 CrossRef CAS; D. A. Alonso, L. Botella, C. Nájera and M. C. Pacheco, Synthesis, 2004, 10, 1713–1718.
- R. B. DeVasher, L. R. Moore and K. H. Shaughnessy, J. Org. Chem., 2004, 69, 7919–7927 CrossRef CAS; R. B. DeVasher, J. M. Spruell, D. A. Dixon, G. A. Broker, R. D. Rogers and K. H. Shaughnessy, Organometallics, 2005, 24, 962–971 CrossRef CAS.
- R. K. Arvela and N. E. Leadbeater, Org. Lett., 2005, 7, 2101–2104 CrossRef.
- K. M. Dawood, Tetrahedron, 2007, 63, 9642–9651 CrossRef CAS.
- V. Polackova and S. Toma, Chem. Pap., 2007, 61, 41–45 Search PubMed; V. Polackova, M. Hutka and S. Toma, Ultrason. Sonochem., 2005, 12, 99–102 CrossRef CAS.
- K. Worm-Leonhard and M. Meldal, Eur. J. Org. Chem., 2008, 5244–5253 CrossRef CAS.
- M. Mora, C. Jiménez-Sanchidrián and J. R. Ruiz, J. Mol. Catal. A: Chem., 2008, 285, 79–83 CrossRef CAS.
- M. Meise and R. Haag, ChemSusChem, 2008, 1, 637–642 CrossRef CAS.
- L. Liu, Y. Zhang and B. Xin, J. Org. Chem., 2006, 71, 3994–3997 CrossRef CAS.
- Y. Han, H. V. Huynh and G. K. Tan, Organometallics, 2007, 26, 6581–6585 CrossRef CAS.
- T. Brendgen, M. Frank and J. Schatz, Eur. J. Org. Chem., 2006, 2378–2383 CrossRef CAS.
- A. M. Sheloumov, P. Tundo, F. M. Dolgushin and A. A. Koridze, Eur. J. Inorg. Chem., 2008, 572–576 CrossRef CAS.
- B. Inés, R. SanMartin, F. Churruca, E. Domínguez, M. K. Urtiaga and M. I. Arriortua, Organometallics, 2008, 27, 2833–2839 CrossRef CAS; B. Inés, I. Moreno, R. SanMartin and E. Domínguez, J. Org. Chem., 2008, 73, 8448–8451 CrossRef CAS.
- R. B. Bedford and S. L. Welch, Chem. Commun., 2001, 129–130 RSC; R. B. Bedford, S. L. Hazelwood (née Welch) and M. E. Limmert, Chem. Commun., 2002, 2610–2612 RSC; R. B. Bedford, C. S. J. Cazin and S. L. Hazelwood (née Welch), Angew. Chem., Int. Ed., 2002, 41, 4120–4122 CrossRef CAS; R. B. Bedford, S. L. Hazelwood (née Welch), P. N. Horton and M. B. Hursthouse, Dalton Trans., 2003, 4164–4174 RSC; R. B. Bedford and M. E. Blake, Adv. Synth. Catal., 2003, 345, 1107–1110 CrossRef CAS; R. B. Bedford, Chem. Commun., 2003, 1787–1796 RSC.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS; S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275–3279 CrossRef CAS.
- E. J. Moses and A. D. Moorhouse, Chem. Soc. Rev., 2007, 36, 1249–1262 RSC.
- S. Ogo, Y. Takebe, K. Uehara, T. Yamazaki, H. Nakai, Y. Watanabe and S. Fukuzumi, Organometallics, 2006, 25, 331–338 CrossRef CAS.
- C. Adamo, C. Amatore, I. Ciofini, A. Jutand and H. Lakmini, J. Am. Chem. Soc., 2006, 128, 6829–6836 CrossRef CAS.
- While the products received by our procedure are pure enough for general synthesis, they are likely to contain trace amounts of Pd, which would need to be removed for medicinal applications to meet the allowable level in drug substances of 5 ppm. See: C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889–900 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and analytical data. See DOI: 10.1039/b915436a |
| This journal is © The Royal Society of Chemistry 2010 |



