Assessing the greenness of some typical laboratory ionic liquid preparations
Maggel Deetlefs* and Kenneth R. Seddon
The QUILL Research Centre, David Keir Building, Stranmillis Road, Belfast, BT9 5AG, UK. E-mail: quill@qub.ac.uk
First published on 8th December 2009
Abstract
The greenness, or lack thereof, of various ionic liquid syntheses and purification methodologies are assessed using a common tool used in strategic planning viz. strengths weaknesses opportunities threats (SWOT) analysis, including their adherence to the twelve principles of green chemistry, % atom economies and E-factors.
 Maggel Deetlefs | Maggel joined the QUILL Research Centre as a post-doctoral researcher in 2001 and has since advanced to the position of Assistant Director, with the dual-purpose of managing the Centre and maintaining her own research portfolio. Her research interests focus on the green synthesis and applications of ionic liquids, including purpose-specific design and developing simple methods to predict their physical properties. |
 Ken Seddon | Ken Seddon was appointed as chair of Inorganic Chemistry at the Queen's University of Belfast in 1993, where in 1999, he also co-founded the QUILL Centre with Prof. W. J. Swindall OBE. The QUILL Centre currently has a complement of 89 staff, including 64 research staff, students and technicians, as well as 14 associated academics. For more details about Ken, Maggel, and QUILL see http://quill.qub.ac.uk |
When are ionic liquid syntheses green?
Although ionic liquids still uphold their baptism as ‘green’, the use of the term, and indeed what constitutes a green ionic liquid synthesis, continues to be misinterpreted. At the heart of the misinterpretation, and many spurious claims, lies the assumption that ionic liquids are green because of their negligible vapour pressure.1,2 However, there are many other factors that determine whether an ionic liquid is, or is not, green, particularly the ionic liquid preparation itself (which is generally not considered or reported in the literature). It is also often overlooked that the very designer classification3 of ionic liquids means that they can be engineered to possess distinctly ‘non-green’ attributes (viz. toxicity, explosivity,4 non-biodegradability, etc.), and they can be (and have been) obtained by distinctly dirty procedures (e.g. using harmful organic solvents). In other words, the term ‘green’ should only be applied to an ionic liquid if both the ionic liquid, and the process used to produce it are green, i.e. if all twelve principles of green chemistry apply (Fig. 1).5 | ||
| Fig. 1 The twelve principles of green chemistry.5 | ||
The twelve principles of green chemistry have been formalised and extensively promoted since the 1990s by their progenitor, Prof. Paul Anastas,5,6 and, recently, were elegantly condensed by Prof. Martyn Poliakoff and co-workers into a mnemonic for easy communication, viz. PRODUCTIVELY.7 Undeniably, it is challenging for a chemical process to incorporate all twelve principles of green chemistry, but the sine qua non is that it must always be crystal-clear whether a reported process is truly green, or whether only parts of it are green. Needless to say, the same overarching green approach should apply to ionic liquid syntheses too, and it must thus be spelled out, especially in the open literature, what exactly is green about the ionic liquid and/or its preparation or use, if anything at all.
Since many excellent reviews8–11 already exist that describe various ionic liquid syntheses and purifications, here we have confined ourselves to assessing the ‘greenness’ of some common, laboratory-scale ionic liquid preparative methods i.e. purification procedures (Fig. 2)12 and synthetic routes (Fig. 3).9,12,13 Although it would be desirable to assess ionic liquid preparations conducted at the industrial scale, the information is not available for a credible study. Moreover, we have only assessed the preparative routes themselves and have not considered the fate14 of the ionic liquid starting materials or products.
 | ||
| Fig. 2 Typical ionic liquid purification routes.12 | ||
 | ||
| Fig. 3 Typical ionic liquid synthetic routes. | ||
In addition, we have also gauged the relative pros and cons of using differing energy sources to promote ionic liquid syntheses, viz. conductive (conventional) heating, microwave irradiation, ultrasonic irradiation, and simultaneous microwave and ultrasonic irradiation. For convenience, we have concentrated on ionic liquids containing 1-alkyl-3-methylimidazolium cations, [Cnmim]+.
Although many other synthetic routes are also available to prepare ionic liquids, e.g. carbene15 and alkylsulfate9,16 routes, the general principles outlined here apply only to the hydrophobic and hydrophilic synthetic procedures discussed, but the method of analysis could be applied.
The scales of justice
The assessment of the greenness of ionic liquid syntheses is a question of scale, and size really matters! This is because the greenness factors associated with laboratory-scale ionic liquid preparations are quite different than those that are relevant to industrial-scale syntheses. For example, in industrial-scale processes, excess 1-haloalkanes are typically recycled, whereas in the laboratory they are usually discarded. Another example is that on an industrial scale, the use of conductive heating (e.g. superheated steam) is more convenient, energy efficient and economical than employing microwave irradiation, while the opposite is true for laboratory-scale preparations.17 Therefore, according to the 6th principle of green chemistry, industrial-scale ionic liquid preparations are greener than their laboratory-scale cousins.It is important to re-emphasise that in this paper, all greenness arguments are focussed solely on the preparative steps of laboratory-scale ionic liquid preparations (<2 kg). Although our approach will be expanded in the future to include a cradle-to-grave approach for both small and large scales preparations, our primary aim was to first develop a user-friendly greenness assessment protocol. As more information becomes available for large scale ionic liquid syntheses, our evaluation protocol will be extended to gauge the greenness of industrial scale ionic liquid preparations by including, for example, the twelve principles of green engineering6 in our assessments, as well as quantitative considerations of energy efficiency using different heating sources. Ultimately, we envisage that our greenness analyses will also incorporate life cycle assessments, which take into account the greenness of ionic liquid starting materials plus the ionic liquid product itself.
What principles of green chemistry are relevant to typical ionic liquid preparations?
Of the twelve principles of green chemistry, the 4th, 7th, 9th and 10th principles5 are irrelevant to the laboratory-scale ionic liquid preparations discussed here. This is because the 4th, 7th and 10th principles of green chemistry apply to the ionic liquid product itself rather than the procedure, while the 9th principle is associated with catalytic methodologies and hence is also not relevant to the typical ionic liquid preparations summarised in Fig. 2 and 3. In contrast, the 8th, 11th and 12th principles of green chemistry apply to all the ionic liquid syntheses discussed here since (i) derivatisation (8th principle) is not used in any of the ionic liquid syntheses, (ii) in-process monitoring (11th principle), although currently under development (e.g. lab-on-a-chip),18 is not yet ready for implementation, and (iii) ionic liquids, by virtue of their negligible vapour pressure, minimise the potential for chemical accidents such as fires and explosions (12th principle). To summarise, only eight of the twelve principles of green chemistry are relevant to the synthetic procedures discussed here, viz. 1st, 2nd, 3rd, 5th, 6th, 8th, 11th and 12th.Although, strictly speaking, the twelve principles of green chemistry only apply to synthetic procedures, the 1st, 5th, 6th and 12th principles are relevant to ionic liquid purification procedures and, therefore, we have assessed the purification of the salts according to these four principles too.
Atom economy and the E-factor
In addition to applying the twelve principles of green chemistry to evaluate the green credentials of ionic liquid syntheses and purifications, these methods can also be assessed using two additional measures, viz. atom economy19 and the E-factor.20Atom economy (also known as atom efficiency) provides, for a given reaction, the ratio between the mass of the atoms making up the final product(s) and the mass of the atoms that are incorporated in all the reactants, eqn (1). It should be noted that the atom economy is a theoretical construct based solely on the stoicheiometry of a given reaction, and assumes 100% yield. Stated more simply, atom economy measures how many of the atoms present in the starting materials form part of the final product, and is reported as a percentage value with those values closest to 100% reflecting superior atom economies. For example, the preparation of 1-alkyl-3-methylimidazolium halide salts using quaternisation (Routes 1(a)–(d), Fig. 2) are 100% atom efficient, regardless of the yield obtained, since no by-products are formed. On the other hand, ionic liquids prepared using one-pot (Routes 2(a)–(c), Fig. 2) or metathesis reactions (Route 3, Fig. 2) will be <100% atom efficient since a stoicheiometric amount of MCl waste is generated.
 | (1) |
The problem with employing atom economy to evaluate the green credentials of a reaction is that it does not take into account that some reactions with favourable stoicheiometries require large excesses of reagents, give poor yields, and often generate large amounts of unwanted by-products. This, of course, highlights the basic flavour in the atom economy concept, viz. it is necessary but not sufficient, since a reaction giving 0.5% yield can still be described as 100% atom efficient. As a result, the E-factor concept was introduced, where all compounds that are not product are classified as waste. The nature of the E-factor equation, eqn (2), dictates that the greenest chemical reactions have E-factor values close to zero. In brief, the E-factor gives a much truer reflection of the greenness of a chemical reaction than atom economy, since all generated waste is accounted for.
 | (2) |
The ‘ideal’E-factor is reflected in the 2nd principle of green chemistry that states: ‘Synthetic methods should be designed to maximise the incorporation of all materials used in the process into the final product’.5 In other words, for a chemical process to have an E-factor of zero, all materials used in a process (and not just the reagents) should be contained in the final product if the process is truly green.
If we necessarily assume the process of producing an ionic liquid is the process of producing a pure, isolated ionic liquid, then the E-factor for the process, which combines Fig. 2 and Fig. 3, must be considered. Although every chemical reaction will have its own unique % atom economy and E-factor, to simplify discussion, the atom economies and E-factors associated with ionic liquid preparations and purifications (Fig. 2 and 3) have respectively been designated as low/medium/high and poor/good/excellent, since the literature has rarely given enough detail to allow definitive values to be assigned.
Strengths, weaknesses, opportunities and threats (SWOT) analyses
In order to simultaneously assess the green credentials of the selected synthetic and purification procedures of ionic liquids in terms of the twelve principles of green chemistry, atom economy, and the E-factor, we have applied a common tool used in strategic planning, viz. strengths, weaknesses, opportunities, threats (SWOT) analysis.21 For ionic liquid preparation and purification methods, SWOT analyses involve specifying the objective(s) of a given procedure (e.g. obtaining an ionic liquid that is >99% pure) and identifying the internal and external factors that are favourable and unfavourable to achieving that objective. Thus, SWOT analyses give an overview of the relative advantages or disadvantages of ionic liquid syntheses and purifications. In brief, the SWOT analyses provided here (represented skeletally in Fig. 4) give a measure of the balance between good and bad for some common ionic liquid preparation and purification procedures, and indicate potential directions for improving these methods. | ||
| Fig. 4 Skeletal strengths weaknesses opportunities threats (SWOT) analysis. | ||
Greenness assessment: traditional synthesis of 1-alkyl-3-methylimidazolium halide salts
It is safe to say that the methods used to prepare 1-alkyl-3-methylimidazolium halide salts11 have changed very little since they were first reported in 1982;22 most reported syntheses still involve reaction of 1-methylimidazole with an excess of 1-haloalkane and are promoted using conductive heating, Route 1(a), Fig. 2. However, from a green chemistry perspective, much room for improvement exists in the syntheses of 1-alkyl-3-methylimidazolium halide salts, particularly regarding the use of more efficient energy sources and reducing/eliminating the need for organic solvents during both synthesis and purification; all these issues are discussed below.The literature shows that the vast majority of 1-alkyl-3-methylimidazolium halide salt preparations are executed using traditional heating under reflux, although nowadays an atmosphere of dry dinitrogen is sometimes used since it has been found to promote the production of colourless ionic liquids11 and also prevents the formation of hydrated halide salts from adventitious water.23
Almost without exception, reported preparations of 1-alkyl-3-methylimidazolium halide salts use an excess of 1-haloalkane,24 which means that the reactions are not in line with the 2nd principle of green chemistry that states: ‘Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product’.5 It also means that although the preparations of the halide salts are 100% atom efficient, with every atom of the starting materials being incorporated into the final product, their E-factors are very poor, since excess 1-haloalkane is required to promote completion of the reactions at a reasonable rate. It also follows that the smaller the excess of 1-haloalkane, the lower the E-factor value will be, provided no organic solvent is used during the preparation. For example, the E-factors for the preparation of [C4mim]Cl (molar mass = 174.1 g mol−1) are 0.106 and 0.005 when 20% and 1% mol excesses of 1-chlorobutane (molar mass = 92.56 g mol−1) are employed, respectively.
Some reported syntheses of 1-alkyl-3-methylimidazolium halide salts also make use of an organic solvent;25 to reduce the viscosity of the reaction mixture and thus improve mass transfer, but also to control the reaction temperature and prevent product scrambling.26 However, the employed molecular solvent, as well as the employed excess of 1-haloalkane, require removal and subsequent disposal, which also do not comply with the 1st principle of green chemistry that states: ‘It is better to prevent waste than to treat or clean up waste after it has formed’.5 Therefore, the practice of employing an organic solvent during the preparation of 1-alkyl-3-methylimidazolium halide salts further increases the E-factor and is highly undesirable. For example, if 100 g of toluene is used during the synthesis of [C4mim]Cl (and using a 20 mol% excess of 1-chlorobutane), the E-factor increases from 0.106 to 0.681.
The 5th principle of green chemistry states that: ‘The use of auxiliary substances (e.g. solvents, separation agents, etc.) should be made unnecessary wherever possible and innocuous when used,’5 which means that, ideally, no solvent should be used during preparation, but if a solvent is to be used, it must be green and recycled. In order to align with the 1st, 2nd and 5th principles of green chemistry, and have low E-factor values, neither an excess of 1-haloalkane nor organic solvent should be used during the synthesis of 1-alkyl-3-methylimidazolium halide salts.
The use of conductive heating (usually an oil bath or heating mantle) to prepare 1-alkyl-3-methylimidazolium halide salts is also in opposition with the 6th principle of green chemistry that states: ‘Energy requirements should be recognized for their environmental and economic impacts and should be minimised. Synthetic methods should be conducted at ambient temperature and pressure.’5 Therefore, since conductive heating is slow, it is also energy inefficient because the transfer of heat from the heat source to the reaction mixture depends on the thermal conductivities of all the materials that must be penetrated such as the flask and solvent. In contrast, microwave-promoted ionic liquid preparations require lower (heating) energy inputs since microwaves penetrate the reaction mixture directly and are not limited by the thermal conductivities of the reaction flask, for example. Therefore, microwave-promoted preparations align better with the 6th principle of green chemistry than conventionally-heated ionic liquid syntheses.
When all the above-mentioned negative factors associated with the traditional syntheses of 1-alkyl-3-methylimidazolium halide ionic liquids are considered, it is safe to say that their preparations are not green. Indeed, of the eight relevant principles of green chemistry, they only comply with three principles (viz. the 8th, 11th and 12th) which apply to all the ionic liquid syntheses discussed here anyway. On the other hand, the E-factors for the preparations fall between good and excellent, provided excess 1-haloalkane is kept to a minimum and no harmful organic solvent is used during the preparation, and any solvent that is used is recycled. As a whole, however, the conductively-heated preparations of 1-alkyl-3-methylimidazolium halide salts possess a lot of room for improvement, as indicated by their SWOT analysis (vide infra).
Greenness assessment: purification of 1-alkyl-3-methylimidazolium halide salts
The purifications of 1-alkyl-3-methylimidazolium halide salts (Scheme 1) are generally dirty, with poor E-factors, since the excess 1-haloalkane, unconverted 1-methylimidazole, as well as any solvent used during their preparation, require removal and are thus out of line with the 5th principle of green chemistry.5![Schematic for the purification of [C4mim]X ionic liquids.](/image/article/2010/GC/b915049h/b915049h-s1.gif) | ||
| Scheme 1 Schematic for the purification of [C4mim]X ionic liquids. | ||
In our laboratories, removal of the excess 1-haloalkane and unconverted 1-methylimidazole is typically achieved by first adding a solvent such as ethanenitrile to the crude ionic liquid mixture and then repeatedly washing this phase with ethyl ethanoate. Once the washing process is complete, the two organic solvents are removed under reduced pressure (using a rotary evaporator). Since the amount of organic solvent used for the purification, as well as the excess of reagents, are classified as waste in the E-factor equation, the more of each that is used, the higher the E-factor will be for a given purification procedure.
In less experienced groups, the purification of 1-alkyl-3-methylimidazolium halide salts is often performed on the bench, and thus the salts absorb water from the atmosphere, that also requires removal. It must be noted, however, that this adventitious water cannot be completely removed from ionic liquids containing halide anions (especially chloride), as they form, typically, very stable hydrates (see, for example, Fig. 5).23
 | ||
| Fig. 5 Structures of 1,3-dimethylimidazolium chloride hemihydrate (left) and 1,2-dimethyl-3-ethylimidazolium chloride hemihydrate (right) showing hydrogen bonding (dashed lines), as determined by single crystal X-ray diffraction.23 | ||
Therefore, if water-free ionic liquids containing halide ions are desired, their preparation and purification must be conducted under strictly anhydrous conditions from start to finish, e.g. using Schlenk techniques, with rigorously predried reagents. Nevertheless, some adventitious water can be removed by heating 1-alkyl-3-methylimidazolium halide salts under reduced pressure at ca. 70 °C for several hours. Almost needless to say, removing water in this way requires a large energy input and thus contributes further to the green inefficiency (via non-compliance with the 6th principle of green chemistry)5 of the purification of 1-alkyl-3-methylimidazolium halide salts. Indeed, the purification of 1-alkyl-3-methylimidazolium halide salts, as summarised in Scheme 1, complies with none of the relevant principles of green chemistry viz. the 1st, 5th, 6th and 12th.
SWOT analysis: conductively-heated preparation of 1-alkyl-3-methylimidazolium halide salts and their subsequent purification
Examination of the SWOT analysis for the preparation of 1-alkyl-3-methylimidazolium halide salts using conductive heating and their subsequent purification (Fig. 6) shows that the overall methodology is dirty. Nevertheless, the procedure possesses some strengths, viz. the processes are well-established, simple, can be performed in even the most basic of laboratories to produce the salts on a small to medium scale (<1 kg), and possess 100% atom economies. On the other hand, both the preparations and purifications share the weakness of poor E-factors, since extensive purification is necessary to remove the excess of 1-haloalkane employed, which generates large volumes of solvent waste. Further weaknesses are that large-scale preparations (>1 kg) and purifications are laborious, and the use of conductive heating to promote the reactions, as well as to remove some residual water and organic solvents, is slow and energy inefficient. All the aforementioned weaknesses show that opportunities exist to develop a greener methodology to prepare pure 1-alkyl-3-methylimidazolium halide salts using conductive heating, and thus reduce the costs of their manufacture, especially on large scales. | ||
| Fig. 6 SWOT analysis: conductively-heated preparation of 1-alkyl-3-methylimidazolium halide salts and their subsequent purification. | ||
At present, the implementation of REACH (registration, evaluation, authorisation and restriction of chemicals)27 legislation is a threat to the continued low-cost manufacturer of many chemicals, but especially for new products, since they require a large initial financial investment. Therefore, research scientists aiming to develop any new industrial-scale ionic liquid synthesis need to be extremely familiar with REACH legislation in order to achieve their aim. A further threat that exists for the synthesis of 1-alkyl-3-methylimidazolium halide salts using conductive heating is that starting material costs will undoubtedly rise and, therefore, the challenge facing chemists is to render preparations greener and concomitantly cheaper.
Greenness assessment: ionic liquid syntheses promoted by microwave irradiation
It has long been recognised that much more efficient energy sources exist to promote chemical reactions than conductive heating. In particular, microwave irradiation,13,28–31 has started to attract attention for the preparation of ionic liquids (Routes 1(b) and 2(a), Fig. 3), and a wide variety of ionic liquids have been prepared to date using microwave reactors.32The promotion of 1-alkyl-3-methylimidazolium halide syntheses using microwave irradiation is favoured by an ionic conduction heating mechanism.30 This is because ionic liquids absorb microwave irradiation not only by a dipole rotation mechanism, but they also oscillate back and forth under the influence of the electric field of the microwave.13,30 These ‘ionic oscillations’ result in collisions with neighbouring ions and molecules, which generate heat, and thus speed up the reaction. In other words, the more ionic liquid product that has formed during a given preparation, the faster the reaction will proceed. In brief, microwave irradiation represents a far more efficient mode of heating to prepare ionic liquids than conductively-heated syntheses. In other words, ionic liquids represent a superlative method for microwave heating.
At present, the implementation of microwave irradiation to prepare ionic liquids continues to evolve, but a question that remains unanswered is: how much greener are microwave-promoted preparations than traditional methods? From a very simplistic standpoint, tremendous energy savings have already been demonstrated for laboratory-scale ionic liquid syntheses,13,29 since microwave-promoted reactions occur far more rapidly than the same preparations performed using conductive heating. This strongly aligns with the 6th principle of green chemistry5 by keeping the energy input of the syntheses to a minimum.
The first papers describing microwave syntheses of ionic liquids employed domestic microwave ovens that offered no temperature and pressure control and thus gave irreproducible results.31–33 Since then, more reliable results have been obtained using commercial microwave reactors (Fig. 7), which have allowed the temperature and pressure of reactions to be moderated, rendering the procedures far safer and more reproducible than their domestic predecessors.34
 | ||
| Fig. 7 Commercially available microwave reactors for batch synthesis (left) and for continuous flow synthesis (right). Photographs courtesy of Milestone s.r.l., Sorisole, BG, Italy. | ||
It is worth noting that while the first reports describing microwave-assisted ionic liquid syntheses focussed on their preparation via quaternisation (Route 1(b), Fig. 3), later reports33,35,36 showed that a one-pot approach (Route 2(a), Fig. 3) could also be used. Since the microwave-assisted procedures require much smaller 1-haloalkane excesses (∼1 mol%) compared to conventional preparations (up to 400 mol%),24 they align well with the 1st principle of green chemistry and their E-factors are far superior to traditional synthetic routes, especially conducted in the absence of additional solvent. In other words, if no organic solvent is used during the synthesis, the preparations also align with the 2nd principle of green chemistry and the best E-factors for the preparations are obtained. In brief, the microwave-assisted syntheses of 1-alkyl-3-methylimidazolium halide salts comply with seven of the eight relevant principles of green chemistry (viz. the 1st, 2nd, 5th, 6th, 8th, 11th and 12th) while the analogous one-pot microwave-assisted syntheses align with six (viz. the 1st, 5th, 6th, 8th, 11th and 12th). It must be noted that although the one-pot microwave-assisted ionic liquid syntheses generate a stoicheiometric amount of MX waste (e.g. NaCl), the waste salt is far less harmful/toxic and straightforward to dispose of compared to 1-haloalkane waste and thus the methodology aligns reasonably well with the 1st principle of green chemistry too.
Microwave-assisted vs. traditional ionic liquid preparations
The superior E-factors and energy efficiencies of microwave-assisted syntheses of ionic liquids show that they are far greener than conductively-heated preparations. However, the ‘degree of greenness’ depends on (i) the type of quaternisation being performed, since the kinetics of the reaction typically follow the order: CnH2n+1Cl < CnH2n+1Br < CnH2n+1I, (ii) the 1-haloalkane excess required to achieve complete conversion to product, (iii) the scale of the reaction, and (iv) whether any solvent is necessary to reduce the viscosity and/or reduce product scrambling.26 The proviso for the use of solvent in terms of green chemistry is that the solvent itself will have to be green (e.g. ethanol) and/or recycled to uphold the overall green credentials of a given preparation.The preparation of ionic liquids on scales >2 kg also requires special consideration, as this is moving from bench scale to semi-pilot plant scales. However, at semi-pilot plant scale, the twelve principles of green engineering6 must also be considered, but this falls outside the scope of this review. Nevertheless, current indications are that continuous flow reactors (see Fig. 7) represent the way forward in producing ionic liquids on large scales.32,37
SWOT analysis: microwave-promoted syntheses of ionic liquids
The SWOT analysis for the preparation of ionic liquids using microwave irradiation is shown in Fig. 8. | ||
| Fig. 8 SWOT analysis for the preparation of ionic liquids using microwave irradiation. | ||
The greatest green strengths of performing microwave-assisted ionic liquid preparations on a laboratory scale are the energy reduction by virtue of reduced reaction times and low 1-haloalkane excesses. Further strengths are that (i) different microwave reactors30 can be employed to execute the preparations on different scales,32 (ii) syntheses can be performed solvent-free, (iii) the reactions are 100% atom efficient and (iv) have excellent (low) E-factor values compared to traditional preparations. All the these strengths render the microwave-promoted syntheses of ionic liquids very green indeed.
Weaknesses associated with microwave-assisted preparation of ionic liquids are:
• Currently, there is a lack of energy efficiency data for syntheses vs. other methodologies
• Microwave reactors are expensive compared to traditional synthetic apparatus
• Discoloured ionic liquids are sometimes obtained at temperatures >75 °C38
• Ionic liquids decompose if overheated13,26,39
• The high viscosity of ionic liquids can produce mass transport problems during synthesis
• The scale-up of the syntheses is very expensive.
Despite the weaknesses mentioned above, opportunities exist for the development of green microwave reactor technology to produce ionic liquids. One major opportunity is green process development by establishing quantitative energy input data to definitively assess the greenness of microwave-assisted preparations vs. other methodologies. Another opportunity is the design and manufacture of custom-made reactors to produce ionic liquids on large scales.37 Both these opportunities may generate valuable intellectual property and could also reduce the cost of producing ionic liquids on large scales.
The largest threat to the development of microwave technology to produce ionic liquids on large scales by continuous flow is that it proves too expensive. Further threats include the inability, via design constraints, to incorporate in situ reaction monitoring (which aligns with the 11th principle of green chemistry) and the inability (albeit unlikely) to incorporate safety controls in industrial reactors to produce ionic liquids on large scales (which aligns with the 12th principle of green chemistry). Also, the low penetration of microwaves limits the size of the reactor; syntheses of over 2 kg will require a flow system.
Greenness assessment: syntheses of ionic liquids promoted by ultrasonic irradiation
At about the same time that the first reports describing the microwave-assisted preparations of ionic liquids were published, reports also began to appear describing ultrasound-promoted preparations of ionic liquids.40,41The promotion of chemical reactions with ultrasound is due to a physical phenomenon known as cavitation, which is the formation, growth, and implosive collapse of bubbles in a liquid.42 The collapse or implosion of such bubbles results in some fascinating physical effects, which include the formation of localised ‘hotspots’ in an elastic liquid and reduction of bubble size.43 In terms of ionic liquid synthesis, the formation of hotspots favours quaternisations (Route 1(c), Fig. 3), while the reduction of bubble size favours both quaternisations and metathesis (Route 3, Fig. 3)40 since improved mass transport overcomes the viscosity issues generally associated with ionic liquid syntheses. Hotspot formation, bubble size reduction and the reduced preparation times compared to traditional methods all combine to speed up ionic liquid syntheses, and thus represent a significant green advantage, especially if the preparations are performed solvent-free. Indeed, it is worth noting that at present, the majority of papers describing ultrasound-promoted syntheses of ionic liquids have focussed on ‘one-pot’ reactions (Route 2(b), Fig. 3).
Despite the apparent green advantages of ultrasound-assisted ionic liquid preparations, a phenomenon which renders these preparations inadequate is that, almost without exception, ionic liquids discolour and decompose when exposed to ultrasonic irradiation for the time required to obtain acceptable conversions.44,45 Needless to say, this decomposition of ionic liquids is a severe disadvantage to the successful and widespread implementation of the technology and, from a green chemistry perspective, does not align with the 1st principle of green chemistry, since very dirty purification and decolourisation of the salts is required, which also gives very poor E-factors. In addition, in a recent review, the highest reported tabulated yields to produce 1-alkyl-3-methylimidazolium halides and similar salts without halide anions are 95 and 90%, respectively.46 This contrasts with yields of >99% obtained by thermally-induced and microwave-assisted preparations, rendering ultrasound-assisted ionic liquid syntheses far less green.
Although ultrasound-assisted syntheses of 1-alkyl-3-methylimidazolium ionic liquids (Route 1(c) and 2(b), Fig. 3) comply with five of the eight relevant principles of green chemistry (viz. the 5th, 6th, 8th, 11th and 12th), they do not follow the 1st and 2nd principles, since they give poor yields (compared to thermal and microwave routes), and the ionic liquid products require extensive purification. This is especially true for ionic liquids prepared by anion exchange under the influence of ultrasonic irradiation (Route 3, Fig. 3), which only comply with three of the eight (viz. 8th, 11th and 12th) principles of green chemistry, and these are met by all ionic liquid preparations anyway. All the ultrasound-assisted ionic liquid syntheses considered here have poor E-factors too and thus are not as green as their microwave-assisted analogues.
SWOT analysis: ultrasound-promoted syntheses of ionic liquids
The SWOT analysis for the preparation of ionic liquids promoted by ultrasonic irradiation (Fig. 9) reveals that its unique strength (compared to traditional and microwave-assisted syntheses) is improved mass transport. However, almost without exception, the preparations have very poor E-factors due to the discoloured ionic liquid products, which require extensive purification and decolourisation efforts that produce large volumes of organic solvent and solid waste (vide supra). In addition to the major weakness of a poor E-factor, other weaknesses of ultrasound-assisted ionic liquid syntheses include the lack of quantitative energy efficiency data, the cost of the apparatus, and the lack of demonstrated large-scale production. It must be pointed out that ionic liquid discolouration is far more severe under ultrasonic conditions than under microwave irradiation and, furthermore, the salts decompose under even mild ultrasonic conditions.44 | ||
| Fig. 9 SWOT analysis for the preparation of ionic liquids using ultrasonic irradiation. | ||
The greatest opportunities that exist for ultrasound-promoted ionic liquid syntheses are (i) to develop green procedures that do not lead to the discolouration and decomposition of ionic liquids, and (ii) to determine the true energy efficiency of the preparations compared to other methodologies by collecting quantitative energy input data, thus creating the opportunity for (iii) reactor design and (iv) generating valuable intellectual property.
It must be reiterated that ultrasound-assisted ionic liquid preparation will probably only find commercial application if its severe shortcomings (or threats in terms of the SWOT analysis) are overcome by future research and development efforts. Such research and development efforts will, similarly to microwave-assisted preparations, have to include safety controls and in situ reaction monitoring to align with the 11th and 12th principles of green chemistry.
Greenness assessment: simultaneous use of microwave and ultrasonic irradiation to prepare ionic liquids
Recent studies have shown that the simultaneous use of microwave and ultrasonic irradiation can be used to promote ionic liquid syntheses by one-step (Route 1(d), Fig. 3), two-step (Routes 1(a) then Route 3, Fig. 3), as well as one-pot (Route 2(c), Fig. 3) procedures.36,46,47The simultaneous use of microwave and ultrasonic irradiation to prepare ionic liquids should offer the cumulative benefits of the individual irradiations, viz. excellent coupling of microwaves with ionic liquids plus improved mass transport. Therefore, the time and energy saved using microwave and ultrasonic irradiation simultaneously would represent a significant green advantage, especially if the syntheses are solvent-free. However, using this technology to prepare 1-alkyl-3-methylimidazolium halide salts (Route 1(d), Fig. 3) gives very poor yields (<5%) and can also take significantly longer than using microwave or ultrasound irradiation alone.36 Therefore, the combined use of microwave and ultrasonic irradiation to prepare 1-alkyl-3-methylimidazolium halide salts has a very poor E-factor, and uses significantly more energy than using the individual types of irradiation alone, rendering the syntheses dirty. In addition, a major problem that is anticipated for all ionic liquid syntheses using microwave and ultrasonic irradiation simultaneously is that discoloured ionic liquid products will be obtained, requiring extensive decolourisation, which will have a further negative effect on the E-factors.
Although the simultaneous microwave/ultrasound-assisted preparations of 1-alkyl-3-methylimidazolium halide (Route 1(d), Fig. 3) and non-halide (Route 2(c), Fig. 3) salts comply with five of the eight relevant principles of green chemistry (viz. the 5th, 6th, 8th, 11th and 12th), they do not follow the 1st and 2nd principles, since they give poor yields (compared to thermal and microwave routes) and the ionic liquid products require extensive purification. In addition, the E-factors of the preparations are poor due to the need to decolourise the ionic liquid products.
SWOT analysis: simultaneous use of microwave and ultrasonic irradiation to prepare ionic liquids
The simultaneous use of microwave and ultrasonic irradiation to prepare ionic liquids offers both the best and worst of the individual technologies (A Tale of Two Technologies?), and the SWOT analysis shows that this is indeed true (Fig. 10). | ||
| Fig. 10 SWOT analysis for the preparation of ionic liquids using simultaneous ultrasound and microwave irradiation. | ||
The expected strengths of the technology include (i) rapid preparations with (ii) good energy efficiencies compared to traditional methods, (iii) improved mass transport (vs. conductive heating and microwave-assisted preparations), (iv) the potential to perform the transformations solvent-free, and (v) high atom economies. At present, however, the limited number of studies describing syntheses have shown that the technology works best for one-pot preparations (Route 2(c), Fig. 3), and is less successful for preparing ionic liquids containing halide anions.36 The SWOT analysis clearly shows, without labouring the point, that at present, the combined microwave/ultrasound irradiation technology has both obvious advantages and severe disadvantages.
Greenness assessment: purification of ionic liquids with non-halide anions
Ionic liquid purification procedures can be divided into two main categories, viz. purification of hydrophobic ionic liquids and purification of hydrophilic ionic liquids (Fig. 2). To simplify discussion for the former and latter categories, we have selected two stereotypical salts as representatives, viz. 1-butyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}-amide, [C4mim][NTf2], and 1-butyl-3-methylimidazolium trifluoromethanesulfonate, [C4mim][OTf]. It must be noted that 1-alkyl-3-methylimidzaolium halide salts fall in the above-mentioned hydrophilic category, but their purification and associated green performance have already been discussed (vide supra).On examination of the twelve principles of green chemistry, it is clear that only the 1st, 5th, 6th and 12th principles are relevant to ionic liquid purification. This is because the remaining principles of green chemistry only address synthetic methodologies.
Regardless of whether a one- or two-step methodology is employed to prepare [C4mim][NTf2] or [C4mim][OTf] (Fig. 3), a stoicheiometric amount of MX waste (usually NaCl) is generated. If not removed from the ionic liquid product, the presence of the metal halide waste will severely affect the physical properties of the ionic liquid.10,12 The removal of MX from both [C4mim][NTf2] and [C4mim][OTf] is achieved by washing the crude ionic liquid product with water, although the ease with which this is achieved, and the respective green performances, vary considerably.
Although a stoicheiometric amount of MX waste is generated during two-step ionic liquid syntheses, it is not toxic waste, and thus does not require incineration. Furthermore, the metal halide waste is not contaminated with organics, which means that both one- and two-step syntheses to produce [C4mim][NTf2] and [C4mim][OTf] closely align with the 1st principle of green chemistry.5
Greenness assessment: purification of hydrophobic vs. hydrophilic ionic liquids
In order to remove MX from hydrophilic [C4mim][OTf], dichloromethane, a dense solvent, is usually added to the crude ionic liquid mixture. The dichloromethane is added since the ionic liquid preferentially dissolves therein, allowing repeated washing of the ionic liquid-containing phase with cold water to remove the MX waste more easily. The residual dichloromethane, as well as the water that remains in the ionic liquid as a result of the washing procedure, require removal that is achieved by first using a rotary evaporator and then drying the salt in vacuo at ∼70 °C for many hours, which is extremely energy inefficient. Almost needless to say, the use of dichloromethane to purify [C4mim][OTf] is an extremely dirty practice, since it is a toxic solvent that is detrimental to both humans and the environment.48 Furthermore, the larger the volume of dichloromethane used, the poorer the E-factor of the procedure.The removal of MX from hydrophobic [C4mim][NTf2] is far easier than its removal from [C4mim][OTf] because it does not readily mix with water and, therefore, washing the salt with water to extract MX is both faster and more efficient. This purification is also greener, since no organic solvent is required, as is the case for hydrophilic salts, to aid with the removal of MX. In principle, the water phase can also be recycled by distillation and reused, although some contaminated ionic liquid, now severely contaminated with MX, will remain, since even hydrophobic ionic liquids exhibit mutual solubility with water.2 Moreover, the water must be removed in order to obtain the pure salt; this is achieved by drying the salt in vacuo at ∼70 °C for many hours, which is extremely energy inefficient.
In brief, the purification procedure of [C4mim][OTf] is far less efficient than that of its [C4mim][NTf2] cousin, since a significant amount of the ionic liquid is ‘lost’ to the organic phase and higher levels of MX also remain in the purified ionic liquid. This is evidenced by the lower chloride content levels achievable for [C4mim][NTf2]49 than for [C4mim][OTf].12,50 In addition, less energy is required to remove residual water from [C4mim][NTf2] than for [C4mim][OTf] by heating the salts in vacuo, as the former should, by definition, hold less water than the latter. Although the purification of these ionic liquids does not comply with the relevant principles of green chemistry (viz. the 1st, 5th, and 6th), the hydrophobic ionic liquid processes are much greener than the hydrophilic, both in total isolated yield and avoidance of organic solvents.
SWOT analyses: purification of hydrophobic and hydrophilic ionic liquids
The SWOT analyses for the purification of [C4mim][NTf2] and [C4mim][OTf] show that the former procedure is the greener (Fig. 11). This is mainly due to the purification of [C4mim][OTf] requiring the use of dichloromethane, which is an extremely harmful compound. Another major reason why the purification of [C4mim][OTf] is dirty, is because much greater losses of the ionic liquid to the water phase occur compared to the purification of [C4mim][NTf2] and it also requires longer heating in vacuo to remove residual water.![SWOT analyses for the purification of [C4mim][NTf2] (left) and [C4mim][OTf] (right).](/image/article/2010/GC/b915049h/b915049h-f11.gif) | ||
| Fig. 11 SWOT analyses for the purification of [C4mim][NTf2] (left) and [C4mim][OTf] (right). | ||
Decolourisation of ionic liquids
All practicing ionic liquid synthetic chemists know that the salts are sometimes obtained as pale yellow to black products. Ionic liquids containing halide anions are particularly susceptible to discolouration, and the cause of the colour can be transferred to derived ionic liquids prepared by metathesis (Route 3, Fig. 3). Although intense chromophores (extinction coefficients > 106 mol l−1 cm−1) are the suspected discolouration culprits,51 the true cause (and indeed the types of chromophores responsible) remains a mystery. To date, attempts to isolate the chromophores by column chromatography have failed to provide enough material for identification. This failure indicates the extremely low levels of the chromophores in ionic liquids (probably ppb levels). It is worth noting that even some of the most intensely coloured ionic liquids are usually analytically pure to NMR spectroscopy and mass spectrometric techniques.The colour of an ionic liquid is usually not detrimental when using the salts as solvents (provided that they contain minimal levels of other impurities such as chloride and/or water), but colourless ionic liquids are essential for spectroscopic studies, in order to eliminate interference from the suspected chromophoric impurity resonances that usually appear in the UV-Vis spectra; the impurity is also often strongly luminescent.
Conclusions
In conclusion, we believe this is the first critical assessment of the greenness of the synthetic procedures and purification methodologies commonly used for the synthesis of ionic liquids, which are summarised in Table 1.| Methodology | E-factor | Atom economy |
| |
|---|---|---|---|---|
| a The 4th, 7th, 9th and 10th principles of green chemistry do not apply. | ||||
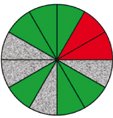
| Route 1 (a) | Good–excellent | High | 3 (8th, 11th, 12th) | 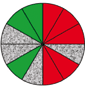 |
| Route 1 (b) | Excellent | High | 7 (1st, 2nd, 5th, 6th, 8th, 11th, 12th) |  |
| Route 1 (c) | Poor | Low–high | 5 (5th, 6th, 8th, 11th, 12th) | 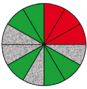 |
| Route 1 (d) | Poor | High | 5 (5th, 6th, 8th, 11th, 12th) | 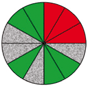 |
| Route 2 (a) | Poor–good | Low–medium | 7 (1st, 2nd, 5th, 6th, 8th, 11th, 12th) |  |
| Route 2 (b) | Poor | Low–medium | 5 (5th, 6th, 8th, 11th, 12th) |  |
| Route 2 (c) | Very poor | Low–medium | 5 (5th, 6th, 8th, 11th, 12th) |  |
| Route 3 | Poor | Low–medium | 3 (8th, 11th, 12th) |  |
To maintain green credibility, ionic liquids must be both green in application and in their synthesis. The above discussions clearly illustrate that laboratory-scale ionic liquid synthesis and purification can certainly be considered as green if microwave-assisted synthesis is employed. It is also clear that the purification of hydrophobic ionic liquids is intrinsically greener than hydrophilic ionic liquids. However, it is important to emphasise that for industrial scale ionic liquid preparations, we will have to readdress how to determine greenness.
With current trends towards the design of non-toxic, biodegradable ionic liquids, the new challenges become to develop improved purification procedures for hydrophilic ionic liquids alongside a universal requirement for development of in situ on-line analytical monitoring for industrial scale syntheses. Overall, the judgement provided here for the synthesis and purification of ionic liquids is: ‘Green, but not green enough’.
Acknowledgements
M.D. and K.R.S. gratefully acknowledge the EPSRC for funding under their Portfolio Partnership Scheme (Grant no. EP/D029538/1) and also thank Milestone, particularly Dr. Mauro Iannelli, for the supply of their microwave units to QUILL. M.D. thanks Drs J. D. Holbrey and M. Nieuwenhuyzen for help in generating the images for Fig. 5 and Prof. Chris Strauss for helpful discussions. K.R.S. also wishes to thank the Institute of Chemical Sciences and Engineering at the EPFL (Switzerland) for providing the opportunity for tranquil meditation.Notes and references
- M. J. Earle, J. M. S. S. Esperança, M. A. Gilea, J. N. C. Lopes, L. P. N. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS; L. P. N. Rebelo, J. N. C. Lopes, J. M. S. S. Esperança and E. Filipe, J. Phys. Chem. B, 2005, 109, 6040–6043 CrossRef CAS.
- M. Deetlefs and K. R. Seddon, “Ionic liquids: fact and fiction”, Chim. Oggi – Chem. Today, 2006, 24, 16–17 Search PubMed , 20–23.
- M. Freemantle, “Designer solvents - Ionic liquids may boost clean technology development”, Chem. Eng. News, 1998, 76, 32–37.
- A. R. Katritzky, S. Singh, K. Kirichenko, J. D. Holbrey, M. Smiglak, W. M. Reichert and R. D. Rogers, Chem. Commun., 2005, 868–870 RSC.
- P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686–694 CrossRef CAS; P. T. Anastas, and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- P. T. Anastas and J. B. Zimmerman, Environ. Sci. Technol., 2003, 37, 94A CrossRef.
- S. Tang, R. Bourne, R. Smith and M. Poliakoff, Green Chem., 2008, 10, 268–269 RSC.
- J. H. Davis Jr., C. M. Gordon, C. Hilgers, and P. Wasserscheid, in Ionic Liquids in Synthesis, eds. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2003, pp. 7-21 Search PubMed; P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 Search PubMed.
- A. J. Carmichael, M. Deetlefs, M. J. Earle, U. Fröhlich and K. R. Seddon, in Ionic Liquids as Green Solvents: Progress and Prospects, eds. R. D. Rogers and K. R. Seddon, American Chemical Society, Washington D.C., 2003, pp. 14–31 Search PubMed.
- A. Stark, and K. R. Seddon, in Kirk-Othmer Encyclopaedia of Chemical Technology, ed. A. Seidel, John Wiley & Sons, Inc., Hoboken, New Jersey, 2007, pp. 836–920 Search PubMed.
- C. M. Gordon, and M. J. Muldoon, in Ionic Liquids in Synthesis, eds. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2008, pp. 7–25 Search PubMed.
- K. R. Seddon, A. Stark and M.-J. Torres, Pure Appl. Chem., 2000, 72, 2275–2287 CrossRef CAS.
- M. Deetlefs and K. R. Seddon, Green Chem., 2003, 5, 181–186 RSC.
- D. Kralisch, A. Stark, S. Koersten, G. Kreisel and B. Ondruschka, Green Chem., 2005, 7, 301–309 RSC; D. Reinhardt, F. Ilgen, D. Kralisch, B. Koenig and G. Kreisel, Green Chem., 2008, 10, 1170–1181 RSC.
- M. J. Earle and K. R. Seddon, Preparation of imidazole carbenes and the use thereof for the synthesis of ionic liquids, WO Pat. 2001077081 ( 2001).
- P. Wasserscheid, R. van Hal and A. Boesmann, Green Chem., 2002, 4, 400–404 RSC; P. Wasserscheid, R. van Hal, A. Bosmann, J. Esser, and A. Jess, in Ionic Liquids as Green Solvents: Progress and Prospects, eds. R. D. Rogers, and K. R. Seddon, American Chemical Society, Washington D.C., 2003, pp. 57–69 Search PubMed.
- C. R. Strauss, Org. Process Res. Dev., 2009, 13, 915–923 Search PubMed.
- G. McHale, C. Hardacre, R. Ge, N. Doy, R. W. K. Allen, J. M. MacInnes, M. R. Bown and M. I. Newton, Anal. Chem., 2008, 80, 5806–5811 CrossRef CAS.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS.
- R. A. Sheldon, Chem. Ind., 1992, 23, 903–906 Search PubMed; R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- R. C. Appleby, Modern Business Administration, Pearson Education Ltd., 1994 Search PubMed.
- J. S. Wilkes, J. A. Levisky, R. A. Wilson and C. L. Hussey, Inorg. Chem., 1982, 21, 1263–1264 CrossRef CAS; A. A. Fannin, D. A. Floreani, L. A. King, J. S. Landers, B. J. Piersma, D. J. Stech, R. L. Vaughn, J. S. Wilkes and J. L. Williams, J. Phys. Chem., 1984, 88, 2614–2621 CrossRef.
- A. E. Elaiwi, Mass Spectrometry of Organic and Chlorometallated Salts, D. Phil. Thesis, University of Sussex, Sussex ( 1994).
- G. P. Smith, A. S. Dworkin, R. M. Pagni and S. P. Zingg, J. Am. Chem. Soc., 1989, 111, 525–530 CrossRef CAS.
- P. Bonhôte, A.-P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef CAS.
- A. J. Jeapes, R. C. Thied, K. R. Seddon, W. R. Pitner, D. W. Rooney, J. E. Hatter and T. Welton, Process for recycling ionic liquids, WO Pat. 2001015175 ( 2001).
- Health and Safety Executive, REACH (Registration, Evaluation, Authorisation & restriction of CHemicals), http://www.hse.gov.uk/reach/index.htm, 2008.
- R. S. Varma and V. V. Namboodiri, Pure Appl. Chem., 2001, 73, 1309–1331 CrossRef CAS.
- B. M. Khadilkar and G. L. Rebeiro, Org. Process Res. Dev., 2002, 6, 826–828 Search PubMed.
- N. E. Leadbeater, and H. M. Torenius, in Microwaves in Organic Synthesis, ed. A. Loupy, Wiley-VCH, Weinheim, 2006, pp. 327-361 Search PubMed.
- R. S. Varma and V. V. Namboodiri, Chem. Commun., 2001, 643–644 RSC.
- È. Boros, K. R. Seddon and C. R. Strauss, Chemical processing with microwaves and ionic liquids, Chim. Oggi – Chem. Today, 2008, 26, 28–30 Search PubMed.
- V. V. Namboodiri and R. S. Varma, Tetrahedron Lett., 2002, 43, 5381–5383 CrossRef.
- B. A. Roberts and C. R. Strauss, Acc. Chem. Res., 2005, 38, 653–661 CrossRef CAS.
- D. Q. Xu, B. Y. Liu, S. P. Luo, Z. Y. Xu and Y. C. Shen, Synthesis, 2003, 2626–2629 CrossRef CAS.
- G. Cravotto, C. Gaudino Emanuela, L. Boffa, J.-M. Lévêque, J. Estager and W. Bonrath, Molecules, 2008, 13, 149–156 Search PubMed.
- T. Erdmenger, R. M. Paulus, R. Hoogenboom and U. S. Schubert, Aust. J. Chem., 2008, 61, 197–203 CrossRef CAS.
- C. G. Begg, M. R. Grimmett and P. D. Wethey, Aust. J. Chem., 1973, 26, 2435–2438 CAS.
- A. K. Abdul-Sada, P. W. Ambler, P. K. G. Hodgson, K. R. Seddon and N. J. Stewart, Ionic liquids of imidazolium halide for oligomerization or polymerization of olefins, World Pat. WO 95 21871 ( 1995).
- J.-M. Lévêque, J.-L. Luche, C. Pétrier, R. Roux and W. Bonrath, Green Chem., 2002, 4, 357–360 RSC.
- V. V. Namboodiri and R. S. Varma, Org. Lett., 2002, 4, 3161–3163 CrossRef CAS.
- E. A. Nepparis, Phys. Rep., 1980, 61, 159–251 CrossRef.
- Synthetic Organic Sonochemistry, ed. J.-L. Luche, Plenum Press, New York, 1998 Search PubMed.
- J. D. Oxley, T. Prozorov and K. S. Suslick, J. Am. Chem. Soc., 2003, 125, 11138–11139 CrossRef CAS.
- X. Li, J. Zhao, Q. Li, L. Wanga and S. C. Tsang, Dalton Trans., 2007, 1875–1880 RSC.
- J.-M. Lévêque, J. Estager, M. Draye, G. Cravotto, L. Boffa and W. Bonrath, Monatsh. Chem., 2007, 138, 1103–1113 CrossRef CAS.
- G. Cravotto and P. Cintas, Chem.–Eur. J., 2007, 13, 1902–1909 CrossRef CAS.
- Scorecard – The Pollution Information Site, Dichloromethane, http://www.scorecard.org/chemical-profiles/summary.tcl?edf_substance_id=75-09-2, 2005.
- G. W. Driver, Physical Chemistry and Thermodynamics of Ionic Liquids and Ionic Liquid Solutions, PhD Thesis, The Queen's University of Belfast, Belfast ( 2006).
- C. Villagrán, M. Deetlefs, W. R. Pitner and C. Hardacre, Anal. Chem., 2004, 76, 2118–2123 CrossRef CAS.
- M. J. Earle, C. M. Gordon, N. V. Plechkova, K. R. Seddon and T. Welton, Anal. Chem., 2007, 79, 758 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |