Effects of eggs on plasma lipoproteins in healthy populations†
Maria Luz
Fernandez
*
Department of Nutritional Sciences, The University of Connecticut, 3624 Horsebarn Road Extension, Storrs, CT 06269. E-mail: maria-luz.fernandez@uconn.edu; Fax: +1 (860) 486-3674; Tel: +1 (860) 486-5547
First published on 19th October 2010
Abstract
Extensive research has not clearly established a link between egg consumption and risk for coronary heart disease. This lack of connection can be explained by two major reasons: First, eggs are a good source of numerous nutrients including lutein and zeaxanthin, potent antioxidants, which may exert a protective effect against lipoprotein oxidation. Second, it has been well established that dietary cholesterol increases the concentrations of both circulating LDL and HDL cholesterol in those individuals who experience a higher increase in plasma cholesterol following egg consumption (hyper-responders). It is also important to note that 75% of the population experiences a mild increase or no alterations in plasma cholesterol concentrations when challenged with high amounts of dietary cholesterol (normal responders and hypo-responders). Egg intake has been shown to promote the formation of large LDL and HDL subclasses in addition to shifting individuals from the LDL pattern B to pattern A, which is less atherogenic. For these reasons, dietary recommendations aimed at restricting egg consumption should be taken with caution and not include all individuals. We need to acknowledge that diverse healthy populations experience no risk in developing coronary heart disease by increasing their intake of cholesterol but in contrast, they may have multiple beneficial effects by the inclusion of eggs in their regular diet.
Introduction
Dietary guidelines aimed at reducing the risk for coronary heart disease (CHD) recommend no more than 300 mg of dietary cholesterol per day, a recommendation that bears on egg consumption.1 Although the American Heart Association has modified its previous policy of eating only two eggs per week to allowing the consumption of one yolk per day while restricting the rest of the dietary cholesterol,2 eggs continue to be regarded with caution by the majority of the population. It is important to note that guidelines from other countries such as Canada,3 New Zealand4 and the European countries5 do not support the idea of recommending an upper limit for dietary cholesterol and their policies are restricted to control the intake of saturated fat and trans fat as major determinants of blood cholesterol concentrations.Extensive research does not support a relationship between egg intake and CHD incidence.6,7 A review of multiple case-controlled studies measuring intake of cholesterol and disease incidence, reported that a relationship could not be clearly established between this dietary component and increase in CHD risk. Furthermore, data gathered from the Lipid Research Clinics Prevalence Follow-up Study,8 which examined both men and women (n = 4546) found no significant relationships between deaths attributable to CHD and dietary cholesterol intake. Analyses of several studies,9–11 including the elderly population,12 have also failed to find an association between the incidence of CHD and egg consumption. More recent studies also indicate the lack of correlation between egg intake and risk for coronary heart disease, or stroke.13,14 It is noteworthy to mention that recent studies have also shown that diabetic individuals may not benefit from egg consumption14 and that this may increase their risk for all cause mortality.15 However, the intent of this review is to focus on healthy populations.
The lack of association between egg intake and CHD reported in these epidemiological studies4–10,12–14 could partly be explained by the fluctuations in response to dietary cholesterol among all individuals, which varies from no changes, slight increase or higher increases in plasma cholesterol. However, it is important to note that for those individuals (25% of the population) who have a higher response to dietary cholesterol, there is a consistent increase in both plasma LDL cholesterol (LDL-C) and HDL cholesterol (HDL-C) concentrations with no alterations in the LDL-C/HDL-C ratio, a major determinant of CHD risk. This inter-individual variation in response to dietary cholesterol can be attributed in part to differences in absorption rates,16 the body's ability to down regulate cholesterol synthesis17 or to increase biliary excretion.18,19 These variations could have a significant genetic component, mediated in part by candidate genes involved in lipoprotein metabolism. The presence of a variant allele may influence the metabolism of cholesterol in a way that deviates from classical understanding.
Gene polymorphisms and response to dietary cholesterol
There are very limited studies addressing the effects of different genotypes on the response to dietary cholesterol. One study reported that individuals identified as homozygous for the variant adenosine binding cassette transporter (ABC)G5 allele (G/G) have greater plasma total cholesterol response to dietary cholesterol intake.20 In contrast to this report, we have found that individuals possessing the C/C genotype experienced a greater increase in both LDL cholesterol (P < 0.05) and a trend for lutein (P = 0.08) after consuming 3 eggs for one month compared to those individuals with the C/G (heterozygote) or G/G genotypes (homozygotes).21 These results, although obtained from a small number of subjects, suggest that the ABCG5 polymorphism may play a role in the plasma response to dietary cholesterol and carotenoids.21The effect of the A278-C promoter polymorphism on the rate limiting enzyme of conversion of cholesterol to bile acids, cholesterol 7α-hydroxylase (CYP7A1) was studied in 496 normolipidemic individuals.22 Subjects were challenged with a mean intake of 742 mg per day of dietary cholesterol for 3–4 weeks. All subjects had a significant increase in HDL-C following the dietary cholesterol challenge. The CYP7A1 polymorphism was found to have a significant effect on the increases in HDL cholesterol.22 However, the APOC3 and APOC4 polymorphisms although associated to plasma lipid parameters, have not been found to be related to the responses to dietary cholesterol.23
Eggs and plasma cholesterol responses in different populations
The current dietary recommendations of no more than 300 mg of dietary cholesterol per day pose a controversial issue for those individuals who might derive health benefits by including eggs in their diets while simultaneously, they are not increasing their risk for CHD. An analysis of various cholesterol-feeding studies, conducted over a 50 year period, has produced evidence that a modest increase in total cholesterol of 0.056–0.061 mmol L−1 (95% CI 0.051–0.069 mmol L−1) can be predicted in response to a 100 mg per day increase in dietary cholesterol.7 If this moderate increase is used as a reference, those who experience elevations in TC higher than 0.061 mmol L−1 (95% CI 0.064–0.14 mmol L−1) would be classified as hyper-responders to dietary cholesterol. The hypo-responders would be those who experience an increase of <0.036 mmol L−1 (95% CI 0.025–0.049 mmol L−1).7Clinical trials conducted in children,24 younger adults25,26 and the elderly27,28 have clearly demonstrated that while hyper-responders, which correspond to 25% of the population exhibit increases in both LDL-C and HDL-C with the result of no changes in the LDL-C/HDL-C ratio. These responses to dietary cholesterol are independent of fat intake.24–28 Recent studies in my laboratory have also shown that during a weight loss intervention, participants consuming 3 eggs per day had no increases in LDL-C while a significant increase in HDL-C was observed.29 In contrast, subjects consuming egg substitutes had no changes in HDL-C following the intervention.29 Further, when lipoprotein particle size and subclasses were analyzed by nuclear magnetic resonance (NMR), significant increases in both LDL and HDL particles were observed as well as an increase in lecithin cholesterol acyl transferase (LCAT) and cholesterol ester transfer protein (CETP) activities.30 These data suggest that dietary cholesterol provided by eggs enhances reverse cholesterol transport. Fig. 1 presents comparisons between subjects consuming 3 whole eggs or the equivalent amount of egg substitutes on different parameters of HDL metabolism.30
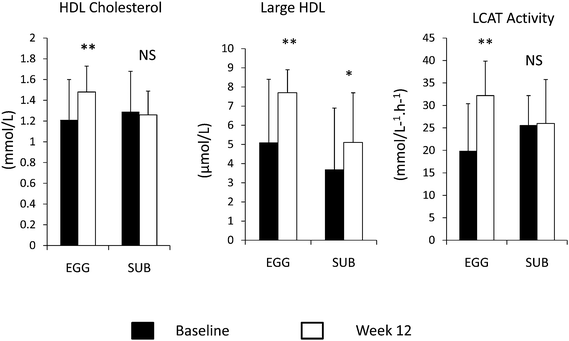 | ||
| Fig. 1 Comparisons in different parameters of HDL metabolism in subjects who consumed 3 eggs (EGG) or the equivalent of 3 egg substitutes (SUB) at baseline (black bar) and at week 12 (white bar). NS = non significant; * P < 0.05; ** P < 0.001. | ||
Responses to dietary cholesterol in children
Eggs are a central food item in Mexico, which spans all socio-economic classes. Because the northern part of Mexico is characterized by dyslipidemias conducive to the metabolic syndrome and CHD,31,32 we evaluated the effects of consuming two whole eggs per day compared to egg whites only, on plasma lipids and the atherogenicity of the LDL particle in Mexican children aged 10–12 y.24 We reported that the increases in plasma cholesterol due to dietary cholesterol was present in 1/3 of the children and was associated with increases in both LDL and HDL with no alterations in the LDL-C/HDL-C ratio. However, when we adjusted the body weight to 70 kg, the response to dietary cholesterol was not present in any of the children. In addition, egg consumption resulted in the formation of buoyant LDL particles associated with pattern A. The significance of this finding derives from the high prevalence of pattern B LDL present in this population.33 A predominance of LDL particles in this pattern B sub-class has been shown to be associated with a three-fold increase in CHD risk,34 which may be due to the easy entry of this particle into the arterial wall and its high susceptibility to oxidation. Thus, egg consumption did not alter the LDL-C/HDL-C ratio in these Mexican children and there was a shift of LDL size to a less atherogenic particle.The potential beneficial effects of eggs in children suffering from Smith-Lemli-Opitz syndrome, which is a condition of impaired cholesterol synthesis and birth defects related to mental retardation, was evaluated.35 Dietary cholesterol provided by eggs increased both LDL-C and HDL-C in these subjects suggesting that egg intake may be a potential therapeutic effect for this condition.
Responses to dietary cholesterol in young adults
Early reports from the Framingham study show a lack of association between dietary cholesterol and heart disease.36 Other studies conducted in the 1980s demonstrated that there were no differences in plasma cholesterol and triglyceride concentrations in young males consuming either 150 mg per 1000 kcal or 500 mg per 1000 kcal of dietary cholesterol.37 Similar results were reported in young adult males when they ate 400 versus 1400 mg of dietary cholesterol for 4 weeks.38More recent studies conducted in 40 men aged 20–50 years old25 and in 51 pre-menopausal women, 50% of which were of Hispanic origin26 reported that men and women classified as hypo-responders to dietary cholesterol (70% of the population), had no changes in LDL or HDL-C after consuming 3 eggs per day for 30 days. In contrast, those individuals who were classified as hyper-responders did experience an increase in both LDL-C and HDL-C. In addition, intake of eggs resulted in the production of larger LDL particles with no increased susceptibility to oxidation.39 These results indicate that pre-menopausal women and men with initial plasma cholesterol concentrations that place them at a low risk for CHD do not experience the development of an atherogenic lipoprotein profile following the consumption of additional dietary cholesterol, regardless of their response classification.
A study carried out in 18 healthy lacto-vegetarian Indians demonstrated an increase in blood cholesterol in subjects after 4 weeks of consuming one boiled egg per day.40 However, the mean LDL-C and HDL-C were not different after 8 weeks of egg consumption although some hyper-responders still presented elevations in total cholesterol after this period of time.40
Responses to dietary cholesterol in adults
By the year 2020, the number of people worldwide over the age of 60 is expected to reach one billion, which suggests that the incidence of age-related disease will continue to increase. The physiologic and economical changes, as well as the increased risk of chronic disease, associated with advancing age places the elderly at an interesting crossroads. Widely accepted risk factors that have been identified for CHD may not be applicable to elderly populations. Although elevated plasma cholesterol concentrations have been shown to predict CHD risk in middle-aged individuals, this parameter does not seem to be relevant for the elderly demographic.41 However, low-fat diets are commonly prescribed to many elderly individuals in an attempt to lower elevated cholesterol concentrations. Unfortunately, this can result in an increase in dietary carbohydrates. This change in diet composition can be detrimental, causing increases in triglycerides, which are generally accompanied by low HDL-C levels. Low HDL-C has been identified as the best indicator of CHD risk in elderly individuals.27Furthermore, the consumption of a diet high in simple sugars can cause changes in lipoprotein metabolism that result in the production of smaller, denser LDL particles.42 In a study conducted in post-menopausal women and men 60 y or older, similar results were observed compared with the younger populations,27,28 no changes in the LDL-C/HDL-C in those individuals classified as hyper-responders and a shift to the larger, more buoyant LDL particle. Furthermore, the increases in HDL were associated with larger HDL particles and the number of LDL particles and apo B concentrations did not change following egg consumption confirming that egg intake resulted in the formation of larger LDL.43 In this study,27 participants were challenged by eating 3 eggs per day. We decided to investigate the effects of consuming only one egg per day for 12 weeks in a population of subjects aged 40 to 65 y.44 Surprisingly, we observed no increases in LDL cholesterol, while an increase in HDL cholesterol was reported for all subjects43 implying once more the role of dietary cholesterol in promoting reverse cholesterol transport.
Similar results were reported in a study conducted in 110 elderly Japanese subjects who were fed additional 750 mg of dietary cholesterol for 4 weeks.28 Subjects experienced a significant increase in HDL-C with no changes in LDL-C at the end of the dietary treatment. However, there were significant increases in the larger LDL particle as well as the less dense HDL (HDL2).28
Different plasma lipid responses (total cholesterol, LDL-C, HDL-C and LDL-C/HDL-C ratio) to egg consumption between hyper and hypo-responders in various studies are summarized in Table 1.
| Population | Intervention | LDL-C | HDL-C | LDL-C/HDL-C Ratio | |||
|---|---|---|---|---|---|---|---|
| Hyper-R | Hypo-R | Hyper-R | Hypo-R | Hyper-R | Hypo-R | ||
| a Ballesteros et al., 2004 (ref. 24). b Herron et al., 2002 (ref. 26). c Mutungi et al., 2008 (ref. 29). d Ata et al., 2010 (ref. 44). | |||||||
| Childrena | 2 eggs/d for 4 weeks |

|

|

|

|

|

|
| Womenb | 3 eggs/d for 4 weeks |

|

|

|

|

|

|
| Menc | 3 eggs/d 12 weeks, weight- loss |

|

|

|

|

|

|
| Men/Womend | 1 egg/d for 12 weeks |

|

|

|

|

|

|
Responses to dietary cholesterol during insulin resistance
To evaluate whether insulin resistance with or without obesity influences the response to dietary cholesterol,45 197 healthy subjects participated in a randomized crossover design and were fed 0, 2 and 4 eggs per 4 weeks with one month washout between periods. The subjects were classified as insulin sensitive (IS n = 65), insulin resistant (IR n = 75) and obese insulin-resistant (OIR n = 58). IR and IS subjects had a significant increase in LDL-C of 7.8 and 3.3% only after consuming 4 eggs while OIR subjects had no changes in LDL-C when consuming 0, 2 or 4 eggs. In contrast HDL-C was significantly increased for all groups even after the consumption of 2 eggs. These studies suggest that dietary management of OIR individuals should focus more on restricting calories rather than dietary cholesterol.28Eggs and carotenoids
Eggs also are major sources of lutein and zeaxanthin, two potent antioxidants, which in addition to their protective effects against macular degeneration and cataract formation,44,46 they may also play a role in decreasing the susceptibility of the LDL particle to oxidation.47 Epidemiological studies indicate an inverse relationship between intake of these carotenoids and both cataract and age-related macular degeneration.47 These carotenoids circulate in plasma mostly carried in the HDL particle.Plasma lutein and zeaxanthin have been shown to increase significantly after egg supplementation in moderately hypercholesterolemic men and women.48 We have demonstrated that in pre-menopausal women and younger men classified as hyper-responders, there was a significant increase in lutein and zeaxanthin compared to those individuals classified as hypo-responders.49 We also found that female hyper-responders had significantly higher plasma lutein concentrations than male hyper-responders following egg consumption despite the finding that the dietary intake of lutein + zeaxanthin was similar between the groups. One explanation for why female hyper-responders had higher plasma lutein levels than men could be found in lipoprotein analysis. Female hyper-responders had significantly higher plasma HDL-C levels (76.0 ± 14.0 mg dL−1) than males (48.7 ± 7.8 mg dL−1) therefore they experienced the same carotenoid response possibly due to the fact that HDL is the major lipoprotein transporting lutein in plasma. When LDL and HDL subclasses were evaluated in an elderly population, a significant correlation was found between large HDL and lutein and zeaxanthin content following intake of 3 eggs for 4 weeks.43
Conclusion
This review of epidemiological data and results from recent clinical trials related to egg consumption present reliable evidence that in multiple populations including children, young adults and the elderly, there are consistent results on both plasma cholesterol distribution in lipoprotein subclasses and on the formation of larger LDL and HDL. The maintenance of the LDL-C/HDL-C ratio, the presence of more buoyant LDL without increases in the susceptibility of LDL to oxidation, the increased concentration of large HDL and the higher plasma lutein and zeaxanthin seen in these individuals clearly suggest that healthy populations benefit from egg consumption.Acknowledgements
The sole author has the responsibility for all the parts of the manuscript.References
- A. H. Lichenstein, L. J. Appel, M. Brands and M. Carnethon, et al. Diet and Lifestyle recommendations Revision 2006, A scientific statement from the American Heart Association Scientific Committee Circulation, 2006, 114, pp. 82–96 Search PubMed.
- American Heart Association, 2004, http://www.americanheart.org.
- J. Genest, R. McPherson, J. Frolhlich, T. Anderson and N. Campbell, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult- 2009 recommendations, Can. J. Cardiol., 2009, 25, 567–579 Search PubMed.
- Ministry of Health: Health and Nutrition guidelines for healthy adults, New Zealand: Ministry of Health, 2003, 18–21 Search PubMed.
- Task force members: I. Graham, D. Atar and K. Borsch-Johnsen, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Fourth Joint Task Force of the European Society of Cardiology and Other societies on cardiovascular disease prevention in clinical practice, Eur. Heart J., 2007, 28, 2375–2414 Search PubMed.
- F. B. Hu, M. J. Stampfer, E. B. Rimm and J. E. Manson, et al. A prospective study of egg consumption and risk of cardiovascular disease in men and women, JAMA, J. Am. Med. Assoc., 1999, 281, 1387–1394 Search PubMed.
- W. H. Howell, D. J. McNamara, M. A. Tosca and B. T. Smith, et al. Plasma lipid and lipoprotein responses to dietary fat and cholesterol, Am. J. Clin. Nutr., 1997, 65, 1747–1764 CAS.
- K. L. Esrey, L. Joseph and S. A. Grover, Relationship between dietary intake and coronary heart disease mortality: lipid research clinics prevalence follow-up study, J. Clin. Epidemiol., 1996, 49, 211–216 CrossRef CAS.
- D. J. McNamara, Dietary cholesterol and atherosclerosis, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2000, 1529, 310–320 CrossRef CAS.
- S. B. Kritchevsky, A review of scientific research and recommendations regarding eggs, J. Am. Coll. Nutr., 23, 596S–600S Search PubMed.
- R. Clarke, C. Frost, R. Collins, P. Appleby and R. Peto, Dietary lipids and blood cholesterol: quantitative meta-analysis of metabolic ward studies, Br. Med. J., 1997, 314, 112–117 CAS.
- H. M. Krumholz, T. E. Seeman and S. S. Merrill, et al. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years, JAMA, J. Am. Med. Assoc., 1994, 272, 1335–1340 Search PubMed.
- Y. Nakamura, et al. Egg consumption, serum total cholesterol concentrations and coronary heart disease incidence: Japan Public Health Center-based prospective study, Br. J. Nutr., 2006, 96, 921–8 CAS.
- A. I. Qureshi, F. K. Suri, S. Ahmed, A. Nasar, A. A. Divani and J. F. Kimani, Regular egg consumption does not increase the risk of stroke and cardiovascular diseases, Med. Sci. Monit., 2007, 13, CR1–8 Search PubMed.
- D. K. Houston, J. Ding, J. S. Lee, M. Garcia, A. M. Kanaya, F. A. Tylavsky, A. B. Newman, M. Visser and S. B. Kritchevsky, Dietary fat and cholesterol and risk of cardiovascular disease in older adults: The Health ABC Study, Nutr. Met. Card. Dis., 2010 DOI:10.1016/j.numecd.2009.11.007.
- D. J. McNamara, R. Kolb, T. S. Parker, H. Batwin, P. Samuel, C. D. Brown and E. H. AhrensJr, Heterogeneity of cholesterol homeostasis in man. Response to changes in dietary fat quality and cholesterol quantity, J. Clin. Invest., 1987, 79, 1729–39 CrossRef CAS.
- P. Mistry, N. E. Miller, M. Laker, W. R. Hazzard and B. Lewis, Individual variation in the effects of dietary cholesterol on plasma lipoproteins and cellular cholesterol homeostasis in man. Studies of low density lipoprotein receptor activity and 3-hydroxy-3methylglutaryl coenzyme A reductase activity in blood mononuclear cells, J. Clin. Invest., 1981, 67, 493–502 CrossRef CAS.
- P. J. Nestel and A. Poyster, Changes in cholesterol synthesis and excretion when cholesterol intake is increased, Metab. Clin. Exp., 1976, 25, 1591–1599 Search PubMed.
- E. C. R. Quintao and G. Sperotto, The role of dietary cholesterol in the regulation of human body cholesterol metabolism, Adv. Lipid Res., 1987, 22, 173–188 Search PubMed.
- R. M. Weggemans, P. L. Zoch, E. S. Tai and J. M. Ordovas, et al. ATP binding cassette G5 C1950G polymorphism may affect blood cholesterol concentrations in humans, Clin. Genet., 2002, 62, 266–299 CrossRef.
- K. L. Herron, M. M. McGrane, I. E. Lofgren, D. Waters, R. M. Clark, J. M. Ordovas and M. L. Fernandez, ABCG5 polymorphism contributes to the individual response to dietary cholesterol and to carotenoids present in eggs, J. Nutr., 2006, 136, 1161–1165 CAS.
- M. K. Hofman, R. M. Weggemans and P. L. Zock, et al. CyP7A1 A-278C polymorphism affects the response of plasma lipids after dietary cholesterol or cafestol intervention in humans, J. Nutr., 2004, 134, 2200–2204 CAS.
- K. L. Herron, I. E. Lofgren, X. Adiconis, J. Ordovas and M. L. Fernandez, Associations between plasma lipid parameters and APOC3 and APOA4 genotypes in a healthy population are independent of dietary cholesterol intake, Atherosclerosis, 2006, 184, 113–120 CrossRef CAS.
- M. N. Ballesteros, R. M. Cabrera, M. S. Saucedo and M. L. Fernandez, 2004. Dietary cholesterol does not increase biomarkers for chronic disease in a pediatric population at risk from Northern Mexico, Am. J. Clin. Nutr., 2004, 80, 855–861 CAS.
- K. L. Herron, S. Vega-Lopez, K. Conde, T. Ramjiganesh, S. Roy, N. Shachter and M. L. Fernandez, Men classified as hypo- or hyper-responders to dietary cholesterol feeding exhibit differences in lipoprotein metabolism, J. Nutr., 2003, 133, 1036–1042 CAS.
- K. J. Herron, S. Vega-Lopez, K. Conde, T. Ramjiganesh, N. Shachter and M. L. Fernandez, Pre-menopausal women classified as hypo-or hyper-responders, do not alter their LDL/HDL ratio following a high dietary cholesterol challenge, J. Am. Coll. Nutr., 2002, 21, 250–258 Search PubMed.
- C. M. Greene, T. L. Zern and R. Wood, et al. Dietary cholesterol provided by eggs does not result in an increased risk for coronary heart disease in an elderly population, J. Nutr., 135, 2793–2798 Search PubMed.
- Y. Homma, T. Kobayashi and H. Yamaguchi, et al. Apolipoprotein-E phenotype and basal activity of low-density lipoprotein receptor are independent of changes in plasma lipoprotein subfractions after cholesterol ingestion in Japanese subjects, Nutrition, 2001, 17, 310–314 CrossRef CAS.
- G. Mutungi, J. Ratliff, M. Puglisi, M. Torres-Gonzalez, U. Vaishnav, J. O. Leite, E. Quann, J. S. Volek and M. L. Fernandez, Dietary cholesterol from eggs increases HDL cholesterol in overweight men consuming a carbohydrate restricted diet, J. Nutr., 2008, 138, 272–276 CAS.
- G. Mutungi, D. Waters, J. Ratliff, M. J. Puglisi, R. M. Clark, J. S. Volek and M. L. Fernandez, Eggs distinctly modulate plasma carotenoid and lipoprotein subclasses in adult men following a carbohydrate restricted diet, J. Nutr. Biochem., 2010, 21, 261–267 CrossRef CAS.
- M. N. Ballesteros, R. M. Cabrera and M. S. Saucedo, et al. Identification of risk factors for chronic disease in a population of children from Northern Mexico, J. Nutr., 2005, 135, 70–73 CAS.
- R. L. Vidal-Quintanar, R. L. Mendivil, M. Peña and M. L. Fernandez, Lime-treated maize husks lower plasma LDL-cholesterol in normal and hypercholesterolemic adult men from northern Mexico, Br. J. Nutr., 1999, 81, 281–288 CAS.
- R. P. Mesink and M. B. Katan, Effect of dietary fatty acids on serum lipids and lipoproteins: a meta analysis of 27 trials, Arterioscler. Thromb., 1992, 12, 911–919 Search PubMed.
- R. M. Krauss, Atherogenic lipoprotein phenotype and diet-gene interactions, J. Nutr., 2001, 131, 340S–343S CAS.
- L. S. Merkens, W. E. Connor and L. M. Linck, et al. Effects of dietary cholesterol on plasma lipoproteins in Smith-Lemli-Opitz syndrome, Pediatr. Res., 2004, 56, 726–732 CrossRef CAS.
- T. R. Dawber, R. J. Nickerson, F. N. Brand and J. Pool, Eggs, serum cholesterol, and coronary heart disease, Am. J. Clin. Nutr., 1982, 36, 617–625 CAS.
- H. Ginsberg, N. A. Le, C. Mays, J. Gibson and W. V. Brown, Lipoprotein metabolism in nonresponders to increased dietary cholesterol, Arteriosclerosis, 1981, 1, 463–470 CAS.
- E. Flaim, L. F. Ferreri, F. W. Thye, J. E. Hill and S. J. Ritchey, Plasma lipid and lipoprotein cholesterol concentrations in adult males consuming normal and high cholesterol diets under controlled conditions, Am. J. Clin. Nutr., 1981, 34, 1103–1108 CAS.
- K. L. Herron, I. E. Lofgren, M. Sharma, J. S. Volek and M. L. Fernandez, A high intake of dietary cholesterol does not result in more atherogenic LDL particles in men and women independent of response classification, Metab. Clin. Exp., 2004, 53, 823–830 Search PubMed.
- G. Chakrabarty, R. L. Biljani and S. C. Mahapatra, et al. The effect of ingestion of egg on serum lipid profile in healthy young free-living subjects, Indian J. Physiol. Pharmacol., 2002, 46, 492–498 Search PubMed.
- K. L. Herron and M. L. Fernandez, Are the current dietary guidelines regarding egg consumption appropriate?, J. Nutr., 2004, 134, 187–190 CAS.
- C. D. Gardener, S. P. Fortmann and R. M. Krauss, Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women, JAMA, J. Am. Med. Assoc., 1996, 276, 875–881 Search PubMed.
- C. M. Greene, D. Waters, R. M. Clark, J. H. Contois and M. L. Fernandez, Plasma LDL and HDL characteristics and carotenoid content are positively influenced by egg consumption in an elderly population, Nutr. Metab., 2006, 3, 6 CrossRef.
- S. Ata, J. Barona, R. Kopec, J. Jones, M. Calle, S. Schwartz and M. L. Fernandez, Consumption of one regular egg or a lutein-enriched egg per day increases HDL cholesterol, reduces apolipoprotein B and the number of small LDL particles while increasing plasma carotenoids and macular pigment density in adult subjects, FASEB J., 24 Search PubMed.
- R. H. Knopp, B. Retzlaff and B. Fish, et al. Effects of insulin resistance and obesity on lipoproteins and sensitivity to egg feeding, Arterioscler., Thromb., Vasc. Biol., 2003, 23, 1437–1443 Search PubMed.
- R. Vishwanathan, E. F. Goodrow-Kotyla, B. R. Wooten, T. A. Wilson and R. J. Nicolosi, Consumption of 2 and 4 egg yolks/d for 5 weeks increases macular pigment concentrations in older adults with low macular pigment taking cholesterol-lowering statins, Am. J. Clin. Nutr., 2009, 90, 1272–9 CrossRef CAS.
- J. D. Ribaya-Mercado and J. B. Blumberg, Lutein and zeaxanthin and their potential roles in disease prevention, J. Am. Coll. Nutr., 2004, 23, 567S–587S Search PubMed.
- G. J. Handelman, Z. D. Nightingale and A. H. Lichtenstein, et al. Lutein and zeaxanthin concentrations in plasma after dietary supplementation with egg yolk, Am. J. Clin. Nutr., 1999, 70, 247–251 CAS.
- R. M. Clark, R. M. Clark, K. L. Herron, D. Waters and M. L. Fernandez, Hypo- and hyper-response to egg cholesterol predicts lutein and beta-carotene plasma concentrations in men and women, J. Nutr., 2006, 136, 601–607 CAS.
Footnote |
| † There was no financial support for this review; Maria Luz Fernandez has no conflicts of interest in any of the information presented in this manuscript. |
| This journal is © The Royal Society of Chemistry 2010 |
