The fate of metals in wastewater treated by the activated sludge process and membrane bioreactors: A brief review†
Ana
Santos
and
Simon
Judd
Centre for Water Science, Cranfield University, Bedford, Bedfordshire, UK MK43 0AL
First published on 24th November 2009
Abstract
The fate of metals in wastewater treatment by the conventional activated sludge process (ASP) and membrane bioreactors (MBRs) is reviewed. The review outlines the environmental and health impacts of metals, but focuses primarily on data reported for removal of toxic metals, and some other high-profile inorganic micropollutants such as aluminium and arsenic, by wastewater treatment processes. Information from pilot and full scale plants is included, with corroboratory reports from bench-scale tests. General trends in removal across different metals are considered, along with the impact of the key process operating determinant of solids retention time. It is concluded that the only consistent trend in metals removal is that it is most effectively achieved through efficient solids separation, and that this represents the primary advantage offered by the MBR. As such, MBRs achieve averaged metals removals which are consistently but not dramatically higher than the ranges reported by the ASP: 64–92% vs. 51–87%, with no more than a 55% decrease on average in effluent concentration. The slightly greater removal attained is attributable to the additional suspended solids retention attained by the membrane process. In either case, further removal of metals would demand a tertiary process for removal of the dissolved material.
 Ana Santos | Ana Santos has a degree in Biotechnology Engineering at Algarve University, Portugal. She is a PhD student at the Centre for Water Science at Cranfield University focusing her research on heavy metal removal by conventional activated sludge treatment and MBRs. |
 Simon Judd | Simon Judd has been at the Centre for Water Science at Cranfield University since 1992, and has managed most of the membrane bioreactor programmes conducted within the Centre. He has authored/co-authored over 50 papers and three reference books on membrane technology, and contributed keynotes, conducted consultancy and delivered short courses in MBR technology globally. |
Environmental impactThe paper examines published data on attainable removal for toxic metal micropollutants, summarising and appraising the wealth of information in this area. Metals form a key group of micropollutants to be subject to environmental legislation limiting their concentration in discharged effluents; an understanding of their fate in wastewater treatment processes is essential in informing decisions to be made regarding process modification or replacement. The key conclusion reached is that arguably the most advanced biotreatment process available, the membrane bioreactor which provides complete solids removal combined with enhanced biotreatment, does not perform significantly better than the conventional activated sludge process. This has significant implications regarding the setting of realistic effluent quality standards and the benefit of any proposed tertiary treatment process. |
1 Metals in the environment
The arising impacts of metals on humans and aquatic environments has been of major concern since the early 1970s. Various metal compounds are natural components of the Earth's crust and thus provide a background concentration. However, it is the anthropogenic sources that are of primary concern, since they elevate the naturally low concentrations to potentially harmful levels. Since they cannot be degraded, metals persist in the environment and tend to accumulate throughout the food chain as they are absorbed by living organisms. In small quantities, some of these elements (e.g.chromium, copper, iron, manganese and zinc) perform important physiological functions. However, if ingested beyond threshold concentrations they can cause acute or chronic toxicity in higher organisms, microorganisms and plants,1 and it is this which has led to increasingly stringent legislation. Under the EU Water Framework Directive (2000/60/EC), environmental quality standards for metals are set according to the risk imposed and impacts on the whole ecosystem and on human health. Metals classified as potentially hazardous to the aquatic environment include cadmium, lead, mercury and nickel as priority substances (Annex X of the WFD), while copper, chromium and zinc are classified as potential main pollutants (Annex VIII).2 Standards are provided as threshold values below which no adverse impact is expected on either human health or the environment (Table 1).| Substance | Environmental Quality Standards (EQS) | ||||||
|---|---|---|---|---|---|---|---|
| a These standards are related to the water hardness to take into account bioavailability of metals. | |||||||
| Freshwater, µg l −1 | Marine, µg l −1 | ||||||
| List I | |||||||
| Cadmium (total; dissolved) | 5 | 2.5 | |||||
| Mercury (total; dissolved) | 1 | 0.3 | |||||
| List II | |||||||
| Hardness, µg l −1 CaCO 3 | |||||||
| 0–50 | >50–100 | >100–150 | >150–200 | >200–250 | >250 | ||
| Chromium (dissolved) | 2 | 10 | 10 | 20 | 20 | 20 | 5 |
| Copper (dissolved) | 0.5 | 3 | 3 | 3 | 8 | 12 | 5 |
| Lead (dissolved) | 4 | 10 | 10 | 20 | 20 | 20 | 10 |
| Nickel (dissolved) | 8 | 20 | 20 | 40 | 40 | 40 | 15 |
| Zinc (dissolved) | 8 | 15 | 15 | 50 | 50 | 50 | 10 |
Much of the recent aquatic toxicology in particular, which has driven legislation in this area, has been concerned with the prediction of effects of pollutants with respect to effect of interaction between the different metals,4–9 the significance and impact of environmental factors such as temperature, incoming solar radiation or other seasonal factors,10–14 and factors influencing the bioavailability of metals.15–20 Recorded toxic effects include damaged or reduced mental and central nervous function, lower energy levels, and damage to blood composition, lungs, kidneys, liver, and other vital organs. Long-term exposure may result in slowly progressing physical, muscular, and neurological degenerative processes that mimic Alzheimer's disease, Parkinson's disease, muscular dystrophy, and multiple sclerosis. Allergic reactions are not uncommon, and repeated long-term contact with some metals or their compounds may even cause cancer.21
2 Metals in wastewater treatment
Toxic metals are almost ubiquitous in wastewater, arising from a diverse range of sources. In the past, industrial activities have always been a significant source of heavy metals.22–27 However, increasingly stringent trade effluent legislation have led to cleaner manufacturing technology and improved effluent treatment,28 as well as a general reduction in manufacturing in western countries. The reduced industrial emissions has proportionally increased the contribution from more dilute diffuse sources such as traffic related emissions (e.g. vehicle exhaust, brake linings, tyres, asphalt wear, gasoline/oil leakage), effluents from small businesses (e.g. car washes, dental uses), domestic effluents (e.g. metals leaching from household pipes, especially copper, and derived from household goods such as detergents), buildings (e.g. copper roofing material, galvanized steel as a zinc source, drainage water) and several other chemical treatment processes.28,29 Metal concentrations in wastewater thus vary widely between locations (Table 2).| Location | Cd | Cr | Cu | Pb | Hg | Ni | Zn | Reference |
|---|---|---|---|---|---|---|---|---|
| Whitlingham, UK, 1987 | 2.2 | 25.4 | 289 | 50 | 1.6 | 26 | 346 | 31 |
| Oxford, UK, 1979 | 6 | 63 | 161 | 158 | — | 40 | 1650 | 22, 23 |
| Thessaloniki, Greece, 2003 | 3.3 | 40 | 79 | 39 | — | 770 | 470 | 28 |
| Mean, highly polluted waters | 3.8 | 42.8 | 176.3 | 82.3 | 1.6 | 278.7 | 822 | |
| %SD, highly polluted waters | 2 | 19 | 105.8 | 65.8 | — | 425.6 | 719.7 | |
| Takatori, Japan, 1991 | 0.9 | 5.1 | 64 | 18 | — | 6.7 | 224 | 32 |
| Hendriksdal, Stockholm, 2002 | 0.23 | 4 | 78 | 3.6 | 0.1 | 6.2 | 150 | 29 |
| Survey of 30 different WwTW, UK, 2006 | 0.8 | 12 | 78 | 25 | 0.5 | 14 | 155 | 34 |
| Ribeirao Preto, Brazil, 2007 | 0.2 | 6.9 | 17 | 37 | 0.1 | — | 79 | 33 |
| Mean, moderately polluted waters | 0.6 | 8 | 53.0 | 26.7 | 0.3 | 10.4 | 152.7 | |
| %SD, moderately polluted waters | 0.4 | 3.6 | 32.0 | 9.6 | 0.3 | 5.2 | 72.5 | |
| Overall mean | 1.9 | 22.3 | 109.4 | 47.2 | 0.6 | 143.8 | 439.1 | |
| Overall % Standard Deviation (SD) | 108 | 99 | 82 | 108 | 123 | 213 | 125 |
Metals fate in conventional activated sludge processes (ASPs, Fig. 1a) has been extensively studied over the past 30 years. Publications within the past 3–4 years alone include studies of nitrification inhibition35,36 and biosorption,37–40 as well as examination of their fate in pilot or full scale plants.41,42 Metals in wastewater may occur as attached to suspended solids via surface bound organic ligands or adsorbed on to a major insoluble matrix component (e.g. iron(III) oxide, aluminium hydroxide etc.); insoluble salts; inorganic complex solids, or as free or organically bound soluble forms; their speciation may depend on the influent metal concentration, influent chemical oxygen demand (COD), hardness, alkalinity and pH of the wastewater.43 Their relative distribution can be estimated from the volatile suspended solid (VSS) fraction, roughly equating to the surface-bonded organic ligands, the non-volatile suspended solids (NVSS), which represents the insoluble matter, and soluble COD which includes the soluble organic ligands.44,45 Since metals are not biodegradable, their removal by a biological process is dependent on physicochemical processes, and ultimately partitioning between soluble and insoluble species according to the above fractions. Their removal during biological treatment can be by adsorption of soluble metal by the bioreactor activated sludge flocs and/or by settlement of the insoluble metal species with the sludge in the secondary settlement tank,46 and several factors thereby impact on their removal:
• plant operation parameters such as sludge age, hydraulic residence time (HRT), dissolved oxygen concentration and suspended solids removal which determine bulk sludge quality such as mixed liquor suspended solids concentration (MLSS) and stirred sludge volume index (SSVI),45,47–50
• physicochemical parameters such as metal type, species and concentration, metal salt solubility temperature, pH, concentration of chelating agents and particle size51–70 and
• biochemical species, and specifically the extracellular polymeric substances (EPS) concentration.54,59,61,67–76
It follows that maximizing metals removal demands minimisation of soluble species and the improvement of solids removable by clarification.
3 Membrane bioreactors and metals in wastewater treatment
The advantages offered by membrane bioreactors (MBRs, Fig. 1b) for the treatment of wastewaters are well documented. These relate primarily to the high quality effluent produced, with effluents ostensibly free of suspended solids and pathogens, and the process intensity, provided by its small footprint compared to the classical ASP.78 However, their hydraulic performance is limited by fouling of the membrane surface, an extensively studied phenomenon and recently comprehensively reviewed,77 and clogging or blocking of the channels between the membranes,78 which is almost entirely unexplored.The study of metals in wastewater, as they relate to MBRs, largely falls into three groups:
a) use of metal-based coagulants for anti-fouling,79
b) treatment of metals-laden industrial effluents,80–82 and
c) fate of metal micropollutants in municipal and industrial wastewater treatment.83,84
An advantage of an MBR is its ability to operate with uncoupled hydraulic and solids retention times (HRT and SRT respectively). Operation at long SRTs allows a smaller footprint plant with lower sludge production, and also high MLSS concentrations which tend to provide better nitrification.78 The impact of MLSS on metals removal is thus a key consideration for MBRs, as well as the more significant aspect of the retention of all suspended solids.
4 Metals removal by conventional ASPs
Metals removal by classical biotreatment is, for the reasons outlined in Section 2, vagarious, and values have been found to vary widely (Table 3). Of key importance is the source of the wastewater, municipal, industrial or a combination of both, which impacts primarily on metal type, speciation (and specifically solids![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid partitioning) and concentration. The performance of a conventional ASP thus depends to some extent on that of the upstream primary settler and the downstream secondary clarifier, since the former reduces the solids loading and the latter reintroduces solids in the return activated sludge. Whilst primary settlement removes metals associated with settleable particles, a significant colloidal or dissolved fraction passes through to the biological secondary treatment.43
liquid partitioning) and concentration. The performance of a conventional ASP thus depends to some extent on that of the upstream primary settler and the downstream secondary clarifier, since the former reduces the solids loading and the latter reintroduces solids in the return activated sludge. Whilst primary settlement removes metals associated with settleable particles, a significant colloidal or dissolved fraction passes through to the biological secondary treatment.43
| Cd | Cr | Cu | Pb | Hg | Ni | Zn | Reference | |
|---|---|---|---|---|---|---|---|---|
| a Average of the initial concentration and percentage removal from different wastewater types: municipal and combined and municipal, respectively. | ||||||||
| Concentration | 3.3 | 40.0 | 79.0 | 39.0 | — | 770.0 | 470.0 | 28 |
| % removal | 55 | 50 | 58 | 31 | — | 44 | 43 | |
| Concentration | 20.0 | — | 90.0 | 50.0 | — | — | 460.0 | 1,a |
| % removal | 14 | — | 52 | 34 | — | — | 88 | |
| Concentration | 0.6 | 9.0 | 65.0 | 18.0 | — | 11.0 | — | 85 |
| % removal | 50 | 67 | 73 | 78 | — | 27 | — | |
| Concentration | 9.6 | 37.5 | 35.3 | 6.8 | 2.4 | 26.0 | 810.0 | 42 |
| % removal | 98 | 61 | 83 | 70 | 68 | 80 | 63 | |
| Concentration | 6.0 | — | 161.0 | 158.0 | — | — | — | 22 |
| % removal | 88 | — | 96 | 93 | — | — | — | |
| Concentration | — | 63.0 | — | — | — | 40.0 | 1650.0 | 23 |
| % removal | — | 68 | — | — | — | 75 | 95 | |
| Concentration | 21.0 | 177.5 | 193.5 | 524.5 | — | 104 | 809 | 24,a |
| % removal | 67 | 74 | 65 | 79 | — | 12.5 | 69 | |
| Concentration | 6.0 | 290.0 | 310.0 | 230.0 | 7.0 | 330.0 | 2400.0 | 51 |
| % removal | 83 | 79 | 74 | 94 | 86 | 18 | 77 | |
| Concentration | 6.0 | 80.0 | 90.0 | 270.0 | — | 70.0 | 600.0 | 87 |
| % removal | 50 | 63 | 33 | 81 | — | 29 | 58 | |
| Concentration | 18.0 | 59.0 | 170.0 | 160.0 | — | — | 353.0 | 49 |
| % removal | 11 | 78 | 61 | 43 | — | — | 48 | |
| Concentration | 1.8 | 38.0 | 56.0 | 62.0 | 2.8 | 28.0 | — | 86 |
| % removal | 90 | 60 | 55 | 69 | >95 | 25 | — | |
| Concentration | 0.3 | 18.8 | 43.4 | 9.6 | 8.0 | 9.7 | 427.3 | 83 |
| % removal | 12 | 66 | 79 | 62 | 98 | 79 | 66 | |
Iron (Fe) and aluminium (Al) are used as coagulants, though mainly in potable water treatment and can reach concentrations of several mg l−1 both in municipal and industrial wastewaters.42 Aluminium, a basic metal, has been measured at concentrations between 400 and 2500 µg l−1 and removed at efficiencies of between 44 and 95%.42,83 A similar trend is evident with Fe, which also forms a sparingly soluble trivalent hydroxide, where concentrations have ranged from 480 to 2400 µg l−1 and removal efficiencies from 79 to 90%,42,51,83,85 with only one exception where removal was low at 21%28 which also coincided with the lowest influent concentration.
Other transitional metals have been found in a wide range of concentrations (Tables 2–3), but generally lower than Fe. Cadmium (Cd) is present at low levels, with some peak concentrations (0.3 to 21 µg l−1). Relatively poor removals (12–14%) have been recorded by a number of authors,1,49,83 whilst other authors observed much removal (50–98%) at similarly low influent levels of 0.6 to 9.6 µg l−1.22,24,28,42,51,85–87 Cobalt (Co) has been also measured at low levels (2 µg l−1) and removed at efficiencies of 36–50%.83,85 For manganese (Mn), the influent concentration tends to be higher 60–120 µg l−1 but not significantly better removed (33–72%). 24,28,51 Copper (Cu), nickel (Ni) and chromium (Cr) were measured at variable influent levels of 35–310 µg l−1,1,22,24,28,42,49,51,83,85–87 9.7–770 µg l−1.23,24,28,42,51,83,85–87 and 9–290 µg l−1,23,24,28,42,49,51,83,85–87 respectively, with the corresponding removal efficiency ranges being 33–96%, 18–80% and 50–79%. Mercury (Hg) has been found at much lower levels (2.4–8 µg l−1) but is removed by up to 68–98%83,86,42,51 with an exception of 17% removal at influent concentration of 1 µg l−1.49 Lead (Pb) also has variable influent concentration but is also generally removed at efficiencies ranging from 31 to 94%.1,22,24,28,42,49,51,83,85–87 Finally, zinc (Zn), the most abundant transitional metal in wastewater, is generally removed at efficiencies between 43 and 95%.1,23,24,28,42,49,51,83,87 The metalloid Arsenic (As) is often present at low levels, average influent concentrations of 4.3 µg l−1, and removed at efficiencies between 9 and 60%.42,83,86
5 Metals removal by MBRs
Since MBR membranes are able to reject, by size exclusion, all particulate matter below 0.1µm in size77,78 removal of metals associated with suspended solids would be expected to be quantitative. According to the metals removal data listed in Table 4, removals of over 95% total metal have been recorded for five of the seven metals (the exceptions being Ni and Zn). This would appear to indicate that metals arise primarily in the suspended form or else substantially precipitate in the treatment process. Even metals present at low concentrations tend to be significantly removed by MBRs: for example Co, measured at a influent concentration of 2.6 µg l−1, has been shown to be 77–85% removed.89| Cd | Cr | Cu | Pb | Hg | Ni | Zn | Conditions | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | 0.3 | 18.8 | 43.4 | 9.6 | 7.95 | 9.71 | 427.3 | 4.8–9 g l−1 MLSS | 83 | |
| % removal | >27 | 75 | 95 | 74 | 94 | 86 | 94 | 40–60 days SRT | ||
| Concentration | 1.8 | 38.0 | 56.0 | 61.7 | 2.8 | 27.8 | — | 11 days SRT | 86 | |
| % removal | >90 | >95 | 85 | 58 | >95 | 40 | — | 4 g l−1 MLSS | ||
| Concentration | 1.8 | 38 | 56 | 61.7 | 2.8 | 27.8 | — | 200 days SRT | ||
| % removal | >90 | >95 | 89 | 63 | >95 | 46 | — | 9 g l−1 MLSS | ||
| Concentration | 1.8 | 38.0 | 56.0 | 61.7 | 2.8 | 27.8 | — | >1000 days SRT | ||
| % removal | >90 | >95 | 72 | 50 | >95 | 66 | — | 18 g l−1 MLSS | ||
| Concentration | 1.8 | 38.0 | 56.0 | 61.7 | 2.8 | 27.8 | — | >1000 days SRT | ||
| % removal | >90 | >95 | 90 | 70 | >95 | 64 | — | 16 g l−1 MLSS | ||
| Concentration | 1.0 | - | 53.0 | 50.0 | 1.2 | 74.0 | 274.0 | 190 days SRT | 89 | |
| % removal | >50 | - | 90 | 88 | 58 | 50 | 51 | 14 hrs HRT | ||
| Concentration | 1.0 | - | 53.0 | 50.0 | 1.2 | 74.0 | 274.0 | >300 days SRT | ||
| % removal | >50 | - | 79 | >98 | >92 | 89 | 94 | 14 hrs HRT | ||
| Concentration | 0.5 | 18.5 | 53.9 | 9.6 | 8.0 | 8.7 | 461.0 | 55–67 days SRT | 90 | |
| % removal | >50 | 75 | 96 | 74 | 94 | 79 | >90 | 8 hrs HRT | ||
| Concentration | — | - | — | — | — | — | — | 190 days | 88 | |
| % removal | >99 | - | 89 | 65 | — | 45 | 54 | 14 hrs HRT | ||
| Concentration | — | - | — | — | — | — | — | >200 days | ||
| % removal | >99 | - | 72 | 50 | >99 | 65 | 80 | 14 hrs HRT | ||
| Concentration | — | - | — | — | — | — | — | 10 days SRT | 41 | |
| % removal | — | 56 | 75 | >68 | 97 | 32 | 59 | |||
| Concentration | — | - | — | — | — | — | — | 20 days SRT | ||
| % removal | — | - | 81 | — | 95 | — | — | |||
| Concentration | — | - | — | — | — | — | — | 30 days SRT | ||
| % removal | — | 85 | 89 | — | 98 | — | 66 | |||
| Concentration | — | 56.6 | 9.8 | 8.6 | 0.7 | 16.6 | 1233.0 | — | 42 | |
| % removal | — | 72 | 90 | 74 | 92 | 72 | 83 | |||
| Concentration | — | - | — | — | — | — | — | 18.4 days SRT | 84 | |
| % removal | 80 | - | — | 90 | 95 | — | — | 7.9 hrs HRT | ||
| Concentration | — | 746.0 | 1345.0 | 16.0 | — | 33.7 | — | — | 95 | |
| % removal | — | 89 | 49 | 100 | — | 100 | — | — | ||
Impacts of SRT (or, by implication, MLSS concentration) appear to vary. For example, for the ubiquitous Al and Fe—widely used coagulants in potable water treatment—removals range from 89 to above 99% and 77 to 98% respectively. The removal efficiency increases with increasing SRT by 8–11% and around 10% for Al and Fe respectively over a SRT range of 190 up to >300 days in both cases.41,42,83,88–90 Other metals where improved retention at longer SRTs has been demonstrated include Co, Cr, Cu, Ni, Pb and Zn. Increasing MLSS concentration from 3 to 10 g l−1 has been shown to increase Cr(III) removal from 45 to 90%, since Cr(III) ions have high affinity for the biomass flocs and are subsequently effectively retained by the membranes93 (Fig. 2); an increase in SRT from 10 to 30 days was shown to increase Cr removal from 56 to 85%;41 the removal efficiency of Ni and Pb increases from 40 to 89% and 50 to >98% respectively on increasing the SRT from 11 to >1000 days.86,89,90 The removal efficiency for Zn, one of the most abundant of the transitional metals and measured at concentrations up to 1.2 mg l−1,42 has been shown to increase from 51 to 94% on increasing the SRT from 10 to >300 days.41,88,89 Overall, evidence suggests increasing SRT and/or MLSS tends to increase removal efficiency of most metals by between 12 and 66%, but it also appears that a threshold concentration is reached at very high SRTs (>1000 days).86
 | ||
| Fig. 2 Cr removal vs. MLSS and pH93 | ||
Metals showing no impact of SRT tend to be present at low concentrations or else are substantially retained by the biomass. Cadmium, which is thought to have a high affinity for biomass or biomass-bound ligands,72,92 is almost completely removed either by membrane separation processes or conventional activated sludge processes with subsequently little increase with SRT.86,89 Silver (Ag), removed by 90–99%,41,88,89 is similarly unaffected by SRT, as are other non-abundant metals such as vanadium (V).88,89 Likewise, little or no increase in retention with SRT has been demonstrated for As, whose average influent concentration is below 4 µg l−1.41,83,84,86,89,90 and for which considerable variation in removal (3–69%) has been reported. Increasing the SRT from a range of 11 to >1000 days, increased removal only marginally, from 3 to 37%, according to two studies.86,89
Conflicting data has been published for some species. Retention of Hg, which has been recorded at concentrations of 1.2–8 µg l−1,42,83,86,89,90 has been shown to be unaffected by SRT in some cases41,94 but to increase with SRT in others.86,88,89 For Cu, which is relatively abundant and ubiquitous in wastewaters, removal has generally been shown to increase with SRT.41,86,94 However, decreased removal with increasing SRT reported by some authors88,89 has been attributed by them to a concomitant increase in organic matter. Lead has also been shown to decrease removal with increasing SRT,86,88 other than for a single anomaly which was attributed to inconsistent influent concentrations.91
The impact of pH adjustment and coagulant dosing has been studied by some authors. Copper removal has been shown to be substantially increased by alum addition along with an extended SRT, with removals increased from 64 to 80% through dosing with alum and further increased to 94% on increasing the SRT from 30 to 50 days. pH adjustment had comparatively little impact on removal efficiency over a narrow range (7.4 to 8.3).94 On the other hand, Cr, which occurs in environment in oxidation states of III and VI with some pH dependency, has shown to be more effectively removed on increasing the pH from 3 to 9 due to formation of the Cr(III) hydroxide precipitate above pH ∼ 6.93 Adsorption onto the biomass flocs is also increased, and solids retention by the membranes subsequently yields higher removals.93 However, the pH of wastewater treatment works biomass in practice rarely rises above a value of 8.
6 MBR vs. ASP data
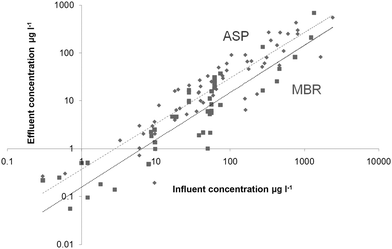
There have been a large number of bench-scale studies devoted to elucidating the mechanism for metals removal by ASP or MBR biomass, many of which have been based on the premise that metals complexation by natural organic ligands in the wastewater significantly impact on their removal.37–40,51–54,56–75 These studies have ranged from the identification and study of the protagonist organic functional groups37,40,59,61,63,65,72–75 and the impact on the physicochemical interactions of factors such as temperature,39 the identity and concentration of ions in solution competing for the binding sites,39,54,66,67,69,70–75 pH, bulk dissolved organic matter (DOM) concentration,38,56–70 and process operational determinants such as HRT and SRT.51,55 MBRs may be expected to provide enhanced removal by adsorption than ASPs due to the higher MLSS concentration at which they operate combined with the smaller floc size.77Notwithstanding the extensive discussion and progress made in determining speciation and mechanisms generally, data trends from pilot and full-scale plant operation across different studies remain vagarious, as indicated in Tables 3–4. No patterns in % removal with either metals type or chemical behavior can be discerned. The only consistent overall trend relates to effluent vs influent concentration, which follows a simple power relationship for both the ASP (Fig. 3) and the MBR (Fig. 4) regardless of the metal, albeit with significant data scatter below the line of parity (effluent concentration = influent concentration). Thus for the aggregated data (Fig. 5):
| Cout = m Cinn |
 | ||
| Fig. 3 Effluent vs. influent metals concentration, ASP data. | ||
 | ||
| Fig. 4 Effluent vs. influent metals concentration, MBR data. | ||
| Cd | Cr | Cu | Pb | Hg | Ni | Zn | |
|---|---|---|---|---|---|---|---|
| ASP | |||||||
| Mean Concentration | 8.5 | 81.3 | 117.5 | 139.0 | 5.0 | 154.3 | 886.6 |
| % SD | 91 | 107 | 71 | 113 | 59 | 163 | 78 |
| Mean % Removal | 62 | 69 | 68 | 74 | 87 | 46 | 68 |
| % SD | 52 | 13 | 25 | 30 | 16 | 60 | 25 |
| MBR | |||||||
| Mean Concentration | 1.3 | 124.0 | 178.2 | 39.1 | 3.4 | 32.8 | 533.9 |
| % SD | 50 | 203 | 230 | 63 | 81 | 71 | 75 |
| Mean % Removal | 74 | 83 | 83 | 73 | 92 | 64 | 75 |
| % SD | 34 | 16 | 15 | 22 | 11 | 32 | 23 |
| Difference in % removal, MBR vs. ASP | 12 | 14 | 15 | −1 | 5 | 18 | 7 |
| Mean % reduction in residual, MBR vs. ASP | 32% | 45% | 47% | −4% | 38% | 27% | 22% |
All evidence supports the premise that metals removal is predominantly by solids rejection, rather than adsorption of the dissolved species. In most cases at moderate concentrations (>1 µg l−1 in the influent), where data are available, the effluent dissolved concentration from ASP data is either the same or indeed higher than the influent concentration unless influenced by chemical changes directly impacting on salt solubility (such as pH, counter ion concentration or a change in oxidation state). This is reflected in published data for a number of the transitional metals, including Co, Cr, Cu, Fe, Ni, Pb and Zn.28,51,85 Dissolved Mn on the other hand, which is subjected to change in oxidation state from soluble MnII to insoluble MnIV during aerobic treatment, has been shown to be significantly removed by an ASP.28 Whilst the MBR might be expected to provide a higher capacity for the dissolved metals, due to the very significant increase in solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid interfacial area of MBR sludge, this does not appear to be the case in practice. Whilst the MBR might be expected to provide a higher capacity for the dissolved metals, due to the very significant increase in solid
liquid interfacial area of MBR sludge, this does not appear to be the case in practice. Whilst the MBR might be expected to provide a higher capacity for the dissolved metals, due to the very significant increase in solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid interfacial area of MBR sludge, this does not appear to be the case in practice.
liquid interfacial area of MBR sludge, this does not appear to be the case in practice.
7 Conclusions
Metals as micropollutants have been less extensively studied than the higher profile endocrine-disrupting organic substances,97 yet data for their removal extends back almost 40 years; they have recently attracted increased interest due to legislation such as WFD. A number of conclusions can be drawn from the body of work on metals removal by the conventional activated sludge process (ASP) and that from membrane bioreactors (MBRs):1. General trends suggest that MBRs offer slightly greater metals removal than ASPs, presumably through enhanced clarification provided by the membranes which are able to reject, by size exclusion, all particulate matter below 0.1µm in size. The decrease in residual effluent concentration (Table 5) provided by the MBR compared with the ASP, based on the entire body of information provided in Tables 3 and 4, is no more than 50%, Cu and Cr showing the most significantly improved removal. Aggregated graphical data (Fig. 5) suggests an overall reduction of 56% on average.
2. Removals by conventional ASPs followed the order Ni<Cd<Cu<Cr = Pb<Zn<Hg, cf. Ni<Pb<Cd<Zn<Cr<Cu<Hg for MBRs. Given the very broad scatter in the data, the small differences in the order of removal reported cannot be determined with any certainty. Moreover, and notwithstanding expectations based on equilibrium thermodynamics, there is no apparent trend in removal with increasing influent concentration either for ASPs or MBRs. On the other hand, some authors have claimed hydraulic retention time to have a significant impact on removal for conventional ASPs,96 indicating that there may be a kinetic element in metal retention.
3. Whilst some authors report an increase in removal with solids retention time, the increase is generally small and varies between studies. For the ASP, the metal removal efficiency depends on factors such as MLSS, suspended solids removal and COD, factors which are linked to SRT such that the impact of the latter is not easily predicted and is dependent not only on metal species but also on wastewater quality. Notionally, a higher SRT would be expected to provide increased capacity through a higher solids surface area for metals adsorption. This effect would then be expected to be enhanced in an MBR since flocs are generally smaller in size78 and the membrane retains macromolecular materials in addition to particulates. It has been postulated that increased concentrations of dissolved organic at longer SRTs may actually promote dissolution of metals and lead to slightly elevated concentrations.93
4. Given the above findings, removal of metals down to very low levels is likely to demand tertiary polishing processes based either on chemical precipitation adsorption or membrane nanofiltration to target the dissolved species, which evidence suggests will be substantially in complexed form. Whereas for other organic and ostensibly biodegradable micropollutants the installation of an MBR may be justified on the basis of their improved removal over that attained by a conventional ASP,97 the improvement in metals removal offered by an MBR is marginal in comparison. Given the high energy and voluminous waste generated by the MBR, it is questionable that the selection of this process technology for improved metals removal alone is justifiable either on a cost or carbon footprint basis.
References
- K. B. Chipasa, Waste Manage., 2003, 23, 135–143 CrossRef CAS.
- European Communities, Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Water Policy, Official Journal of the European Union, vol. L, 2000, 327–371 Search PubMed.
- SEPA (Scottish Environmental Protection Agency), Environmental Quality Standards (EQS) List, Issue No. 1, Annex G, 2004 Search PubMed.
- C. Barata, S. J. Markich, D. J. Baird, G. Taylor and A. Scares, Aquat. Toxicol., 2002, 60, 85–99 CrossRef CAS.
- P. Chandra and A. R. Khuda-Bukhsh, Ecotoxicol. Environ. Saf., 2004, 58, 194–201 CrossRef CAS.
- J.A. Hansen, P. G. Welsh, J. Upton, D. Cacela and A. D. Dailey, Environ. Toxicol. Chem., 2002, 21, 67–75 CrossRef CAS.
- D. Montvydiene and D. Marciulioniene, Environ. Toxicol., 2004, 19, 351–358 CrossRef CAS.
- W. P. Norwood, U. Borgmann, D. G. Dixon and A. Wallace, Hum. Ecol. Risk Assess., 2003, 9, 795–811 CrossRef CAS.
- V. R. Sindhe and R. S. Kulkami, J. Environ. Biol., 2004, 25, 365–368 Search PubMed.
- T. S. Babu, T. A. Akhtar, M. A. Lampi, S. Tripuranthakam, D. G. Dixon and B. M. Greenberg, Plant Cell Physiol., 2003, 44, 1320–1329 CrossRef CAS.
- T. K. Ghosal and A. Kaviraj, Chemosphere, 2002, 46, 1099–1105 CrossRef CAS.
- E. H. W. Heugens, T. Jager, R. Creyghton, M. H. S. Kraak, A. J. Hendriks, N. M. van Straalen and W. Adrrdraal, Environ. Sci. Technol., 2003, 37, 2145–2151 CrossRef CAS.
- K. Maraldo and I. Dahllof, Aquat. Toxicol., 2004, 69, 189–198 CrossRef CAS.
- R. S. Rathore and B. S. Khangarot, Ecotoxicol. Environ. Saf., 2002, 53, 27–36 CrossRef CAS.
- K. A. C. de Schamphelaere, P. M. Vasconcelos, F. M. G. Tack, H. E. Alien and C. R. Janssen, Environ. Toxicol. Chem., 2004, 23, 1248–1255 CrossRef CAS.
- J. Eggleton and K. V. Thomas, Environ. Int., 2004, 30, 973–980 CrossRef CAS.
- C. R. Janssen, D. G. Heijerick, K. A. C. de Schamphelaere and H. E. Alien, Environ. Int., 2003, 28, 793–800 CrossRef CAS.
- C. D. Luider, J. Crusius, R. C. Playle and P. J. Curris, Environ. Sci. Technol., 2004, 38, 2865–2872 CAS.
- S. Meylan, R. Behra and L. Sigg, Environ. Sci. Technol., 2004, 38, 3104–3111 CrossRef.
- M. Ravichandran, Chemosphere, 2004, 55, 319–331 CrossRef CAS.
- International Occupational Safety and Health Information Centre: Metals. In Basics of Chemical Safety, Chapter 7, Sep. Geneva: International Labour Organization, 1999 Search PubMed.
- J. N. Lester, R. M. Harrison and R. Perry, Sci. Total Environ., 1979, 12, 13–23 CrossRef CAS.
- S. Stoveland, M. Astruc, J. N. Lester and R. Perry, Sci. Total Environ., 1979, 12, 25–34 CrossRef CAS.
- J. A. Davis and J. Jacknow, J. Water Pollut. Control Fed., 1975, 47, 2292–2297 Search PubMed.
- L. A. Klein, M. Lang, M. Nosh and S. L. J. Kirschner, Water Pollut. Control Fed., 1974, 46, 2653–2662 Search PubMed.
- H. Stonerook, J. Kerr, G. Newell and L. Ferell, Water Pollut. Control Fed., 1984, 56, 1093–1098 Search PubMed.
- K. J. Yost and R. F. Wukasch, Environ. Monit. Assess., 1983, 3, 61–76 CrossRef CAS.
- M. Karvelas, A. Katsoyiannis and C. Samara, Chemosphere, 2003, 53, 1201–1210 CrossRef CAS.
- L. Sorme and R. Lagerkvist, Sci. Total Environ., 2002, 298, 131–145 CrossRef CAS.
- D. Ziolko, O. V. Martin, M. D. Scrimshaw, and J. N. Lester, 2009, priv. comm.
- J. N. Lester, in Heavy Metals in Wastewater and Sludge Treatment Processes. Sources, Analysis and Legislation, CRC Press Inc., Boca Raton, USA, 1987, vol. I Search PubMed.
- M. Chino, K. Moriyama, H. Saito and T. Morn, Water, Air, Soil Pollut., 1991, 57–58, 829–837 CrossRef CAS.
- A. S. Oliveira, A. Bocio, T. M. B. Trevilato, A. M. M. Takayanagui, J. L. Domingo and S. I. Segura-Muñoz, Environ. Sci. Pollut. Res., 2007, 14, 483–489 CrossRef CAS.
- K. L. Rule, S. D. W. Comber, D. Ross, A. Thornton, C. K. Makropoulos and R. Rautiu, Water Environ. J., 2006, 20, 177–184 Search PubMed.
- S. J. You, Y. P. Tsai and R. Y. Huang, J. Hazard. Mater., 2009, 165, 987–994 CrossRef CAS.
- D. Dionisi, C. Levantesi, M. Majone, L. Bornoroni and M. de Sanctis, Ind. Eng. Chem. Res., 2007, 46, 6762–6769 CrossRef CAS.
- F. Pagnanelli, S. Mainelli, L. Bornoroni, D. Dionisi and L. Toro, Chemosphere, 2009, 75, 1028–1034 CrossRef CAS.
- A. Hammaini, F. Gonzalez, A. Ballester, M. L. Blazquez and J. A. Munoz, J. Environ. Manage., 2007, 84, 419–426 Search PubMed.
- Z. Al-Qodah, Desalination, 2006, 196, 164–176 CrossRef CAS.
- B. Yuncu, F. D. Sanin and U. Yetis, J. Hazard. Mater., 2006, 137, 990–997 CrossRef CAS.
- A. Conklin, C. Eaton, K. Bourgeous, L. Holmes, K. Smith and J. Beatty, Proc. Water Environment Federation, 2007, 3338–3359 Search PubMed.
- G. Carletti, F. Fatone, D. Bolzonella and F. Cecchi, Water Sci. Technol., 2008, 57, 1329–1336 Search PubMed.
- J. N. Lester, in Heavy Metals in Wastewater and Sludge Treatment Processes. Treatment and Disposal, CRC Press Inc., Boca Raton, USA, 1987, vol. II Search PubMed.
- S. Kempton, R. Sterritt and J. N. Lester, Sci. Total Environ., 1987, 63, 247–258 CrossRef CAS.
- A. C. Rossin, R. M. Sterritt and J. N. Lester, Water, Air, Soil Pollut., 1982, 17, 185–198 CAS.
- R. M. Sterritt and J. N. Lester, Water Res., 1981, 15, 59–65 CrossRef CAS.
- K. Y. Chen, C. S. Young, T. K. Jan and N. Rohatgi, J. Water Pollut. Control Fed., 1974, 46, 2663–2675 Search PubMed.
- S. Stoveland, Ph.D Thesis, Imperial College, London, 1978.
- H. G. Brown, C. P. Hensley, G. L. McKinney and J. L. Robinson, Environ. Lett., 1973, 5, 103–114 CrossRef CAS.
- E. F. Barth, M. B. Ettinger, B. V. Salotto and G. N. McDermott, J. Water Pollut. Control Fed., 1965, 37, 86–96 Search PubMed.
- B. G. Oliver and E. G. Cosgrove, Water Res., 1974, 8, 869–874 CrossRef CAS.
- W. Stumm and H. Bilinski, Water Pollut. Res. Proc. of 6th Int. Conf., 1973, 39–49 Search PubMed.
- M. H. Cheng, J. W. Patterson and R. A. Minear, J. Water Pollut. Control Fed., 1975, 47, 362–376 Search PubMed.
- T. Rudd, R. M. Sterritt and J. N. Lester, Water Res., 1984, 18, 379–384 CrossRef CAS.
- S. Stoveland and J. N. Lester, Sci. Total Environ., 1980, 16, 37–54 CrossRef CAS.
- P. O. Nelson, A. K. Chung and M. C. Hudson, J. Water Pollut. Control Fed., 1981, 53, 1323–1333 Search PubMed.
- B. R. Fristoe and P. O. Nelson, Water Res., 1983, 17, 771–778 CrossRef CAS.
- R. M. Sterritt and J. N. Lester, Conference on Heavy Metals Environment, Heidelberg, Germany, 1983 Search PubMed.
- C. T. Tien and C. P. Huang, J. Environ. Eng., 1987, 113, 285–299 CrossRef CAS.
- C. T. Tien and C. P. Huang, International Conference on Heavy Metals Environment, Amsterdam, Netherlands, 1991 Search PubMed.
- K. Fukushi, D. Chang and S. Ghosh, Water. Sci. Technol., 1996, 34, 267–272 CrossRef CAS.
- J. Wang, C. P. Huang, H. E. Allen, I. Poesponegoro, H. Poesponegoro and L. R. Takiyama, Water Environ. Res., 1999, 71, 139–147 CrossRef CAS.
- M. Ledin, Earth-Sci. Rev., 2000, 51, 1–31 CrossRef CAS.
- T. Lebeau, D. Bagot, K. Jezequel and B. Fabre, Sci. Total Environ., 2002, 291, 73–83 CrossRef CAS.
- M. Esparza-Soto and P. Westerhoff, Water Res., 2003, 37, 2301–2310 CrossRef CAS.
- J. Wang, C. P. Huang and H. E. Allen, Water Res., 2003, 37, 4835–4842 CrossRef CAS.
- G. Ozdemir, T. Ozturk, N. Ceyhan, R. Isler and T. Cosar, Bioresour. Technol., 2003, 90, 71–74 CrossRef CAS.
- R. Pardo, M. Herguedas, E. Barrado and M. Vega, Anal. Bioanal. Chem., 2003, 376, 26–32 CAS.
- I. Savvaidis, M. Hugues and R. Poole, Antonie van Leeuwenhoek, 2003, 84, 99–107 CrossRef CAS.
- S. Comte, G. Guibaud and M. Baudu, J. Hazard. Mater., 2008, 151, 185–193 CrossRef CAS.
- M. J. Brown and J. N. Lester, Water Res., 1979, 13, 817–837 CrossRef CAS.
- M. J. Brown and J. N. Lester, Water Res., 1982, 16, 1539–1548 CrossRef CAS.
- M. J. Brown and J. N. Lester, Water Res., 1982, 16, 1549–1560 CrossRef CAS.
- G. Guibaud, N. Tixier, A. Bouju and M. Baudu, Chemosphere, 2003, 52, 1701–1710 CrossRef CAS.
- Y. Liu, M. C. Lam and H. H. P. Fang, Water Sci. Technol., 2001, 43, 59–66 Search PubMed.
- S. Comte, G. Guibaud and M. Baudu, Process Biochem., 2006, 41, 815–823 CrossRef CAS.
- F. Meng, S. R. Chae, A. Drews, M. Kraume, H. S. Shin and F. Yang, Water Res., 2009, 43, 1489–1512 CrossRef CAS.
- S. J. Judd, Trends Biotechnol., 2008, 26, 109–116 CrossRef CAS.
- H. F. Zhang, B. S. Sun, X. H. Zhao and Z. H. Gao, Sep. Purif. Technol., 2008, 63, 341–347 CrossRef CAS.
- R. Zaloum, S. Lessard, D. Mourato and J. Carriere, Water Sci. Technol., 1994, 30, 21–27 CAS.
- S. Chuichulcherm, S. Nagpal, L. Peeva and A. Livingston, J. Chem. Technol. Biotechnol., 2001, 76, 61–68 CrossRef CAS.
- N. Canter, Tribology and Lubrication Technology, 2006, 62, 30–39 Search PubMed.
- F. Fatone, P. Battistoni, P. Pavan and F. Cecchi, Water Sci. Technol., 2006, 53, 111–121 Search PubMed.
- S. Cattaneo, F. Marciano, L. Masotti, G. Vecchiato, P. Verlicchi and C. Zaffaroni, Water Sci. Technol., 2008, 58, 1789–1796 Search PubMed.
- R. Buzier, M. H. Tusseau-Vuillemin, C. M. dit Meriadec, O. Rousselot and J. M. Mouchel, Chemosphere, 2006, 65, 2419–2426 CrossRef CAS.
- F. Fatone, A. L. Eusebi, P. Pavan and P. Battistoni, Water Sci. Technol., 2008, 57, 505–511 Search PubMed.
- P. Roberts, H. R. Hegi, A. Weber and H. R. Krahenbuhl, Prog. Wat. Technol., 1977, 8, 301–306 Search PubMed.
- L. Innocenti, D. Bolzonella, P. Pavan and F. Cecchi, Desalination, 2002, 146, 467–474 CrossRef CAS.
- F. Cecchi, L. Innocenti, D. Bolzonella and P. Pavan, Annali di Chimica, 2003, 93, 381–388 CAS.
- F. Fatone, D. Bolzonella, P. Battistoni and F. Cecchi, Desalination, 2005, 183, 395–405 CrossRef CAS.
- J. Q. Jiang, Water Sci. Technol., 2001, 44, 89–98 Search PubMed.
- P. Battistoni, G. Fava and M. L. Ruello, Water Res., 1993, 27, 821–827 CrossRef CAS.
- S. Malamis, E. Katsou, D. Chazilias and M. Loizidou, J. Membr. Sci., 2009, 333, 12–19 CrossRef CAS.
- A. G. El-Din, R. W. Holden II, G. J. Borgnini, S. Truchan Jr, R. Shamskhorzani, Proc. of the 78th Annual Water Environment Federation Technical Exposition and Conference, Washington, DC, Water Environmental Federation, Alexandria, Virginia, 2004 Search PubMed.
- E. Dialynas and E. Diamadopoulos, Desalination, 2009, 238, 302–311 CrossRef CAS.
- T. A. Ozbelge, H. O. Ozbelge and M. Tursun, Chem. Eng. Process., 2005, 44, 23–32 CrossRef CAS.
- J. Sipma, B. Osuna, N. Collado, H. Monclús, G. Ferrero, J. Comas and I. Rodriguez-Roda, Desalination, in press Search PubMed.
Footnote |
| † Part of a themed issue dealing with water and water related issues. |
| This journal is © The Royal Society of Chemistry 2010 |