On the challenge of quantifying man-made nanoparticles in the aquatic environment†
Alan G.
Howard
*
School of Chemistry, University of Southampton, Southampton, SO17 1BJ, UK. E-mail: agh@southampton.ac.uk
First published on 30th November 2009
Abstract
Technologies based on nanomaterials are developing daily, finding applications as diverse as new sensors for improved monitoring and detection, new medical imaging techniques, novel approaches to the treatment and remediation of contaminated land and green technologies for chemical production. An inevitable consequence of Man's exploitation of nanotechnology is both the deliberate and accidental release of manufactured nanomaterials into the environment. This presents the analytical science community with a challenge for which it is, at present, poorly prepared—the quantification of specific nanoparticles in the environment. The problem is the development of trace analysis methods targeted at solid phase species, rather than the dissolved species measured, for example, in a typical pesticide residue analysis. This will require the adoption of radically different approaches and techniques, many of which will be unfamiliar to the conventionally trained environmental analyst. This paper sets out to give a very brief overview of the techniques that are available, specifically questioning their suitability for the quantification of man-made nanoparticles in the aquatic environment. Suggestions are made as to how these techniques might be transferred from the characterization of synthetic products to the field of trace analysis. The analytical community is presented with a new frontier of environmental investigation that can only commence with the development of innovative approaches to the quantitative measurement of man-made nanomaterials in the environment.
 Alan G. Howard | Alan Howard was born in London, within the sound of Bow Bells. After obtaining a BSc in Chemistry (1972) and an MSc in Advanced Analytical Chemistry (1973) from the University of Bristol, he then carried out research into the mechanisms responsible for the accumulation of heavy metals in shellfish, receiving a PhD from the University of Bristol in 1976. Following a short period of postdoctoral work, he moved to the University of Southampton where he is currently a Senior Lecturer in analytical and environmental chemistry in the School of Chemistry. His main research interests are in the analytical applications of nanoparticles, trace analysis and the speciation and behavior of contaminants in the aquatic environment. |
Environmental impactNew man-made nanomaterials and their applications are appearing daily, but we still know little about the impact of these materials on the environment. The problem is the development of trace analysis methods targeted at solid phase species that are present at low concentrations in complex environmental matrices. With a long history of methods development in determining dissolved species, the switch to the measurement of trace concentrations of solids presents the analytical and environmental science communities with many novel challenges. If we are to be able to assess the environmental impact of the release of nanoparticles into the environment, we need to be able to quantify specific nanoparticles, and this will require innovative new analytical approaches. |
1. Colloids and nanoparticles
Within the nanoscale‡ size range, particles are made up of relatively small numbers of atoms and can exhibit novel properties that are not to be found in their larger relatives. Dramatic changes occur in the mechanical, optical, chemical, and electronic properties of materials on the nanoscale. Optical properties can change, surface effects can differ due the decreased radius of curvature of the particle and particles become of a size where they are able to move through cell membranes. Nanosized materials now in production range from small quantities of quantum dots, measuring 2–10 nm in diameter and made most commonly from cadmium and selenium, to bulk titania products used in sunscreens and paints. Technologies based on nanomaterials are developing daily, finding applications as diverse as new sensors for improved monitoring and detection, new medical imaging techniques, novel approaches to the treatment and remediation of contaminated land and green technologies for chemical production. However, an inevitable consequence of Man's exploitation of nanotechnology is both the deliberate and accidental release of manufactured nanomaterials into the environment.3As with any shift of materials production, it is necessary to understand the impact of these new additions to the environment. To allow us to assess the impacts and risks posed by nanomaterials, analytical methods need to be developed by which we can assess potential environmental exposure and understand their behavior in the environment. This will, however, require a step change in our ability to identify and quantify the man-made particles; particularly when they are present in an already complex environmental matrix.
This is not a problem that has a defined starting date, nor is it uniform in scale. At one extreme, the subject is dominated by natural processes such as soot formation during combustion. The release of man-made phase contrast materials from medical imaging may result in small quantities of nanoparticles being released locally, but nanosized titanium dioxide (TiO2) is already incorporated into many consumer products, including sunscreens and toothpastes, industrial products such as paints, lacquers and papers, and it is used as a photocatalyst in water treatment. World production of titania alone is believed to be over 40 thousand tons per year, a significant proportion of which is nanoparticulate. An indication of the range of small particles to be found in the environment is given in Fig. 1.
 | ||
| Fig. 1 Typical dimensions of common particles. | ||
For general overviews on the presence, chemistry and potential environmental consequences of natural and manufactured nanoparticles in the environment, the reader is directed to a number of excellent review articles that have been published on the subject.4–7
Recent scientific interest in man-made nanoparticles has revitalized interest in an area of aquatic environmental chemistry that previously came under the banner of colloid science. In the hydrosphere, there are biological nanoparticles (bacteria, viruses etc), nano-dimensioned humic materials, combustion products resulting from forest fires and nanoparticles emanating from black smokers at the Mid-Atlantic Ridge. Man-made nanoparticles arise from industrial processes such as combustion, mining, ore refining etc, but the deliberate manufacture of nanoparticles is not new. Many long-established commercial products are in fact of nanometric dimensions, including the multi-ton production of carbon black and fumed silica for applications in car tyres and plastic fillers. It is principally the range of nano-sized particles that are being deliberately designed to perform a specific function, produced and marketed that has increased environmental concern over recent years. In the same way that the total quantity of pesticide residues in a sample may be considered to be of less significance than a small quantity of a dioxin, it may eventually become clear that the absolute quantity of nanoparticles is of little significance if a minor nanoparticulate product is found to be environmentally damaging.
In general terms, there is little difference between the release of modern manufactured nanoparticles into the environment and the release of natural and ‘old-style’ nano-sized particles such as carbon black. That is not to say that they will have no distinct impact; their effects may arise from specific properties or from the scale of their release. It is therefore necessary to develop the means by which the behavior of such materials in the environment can be studied. In doing so, one particle type will have to be distinguished from another and quantitative methods will be required.
This article sets out to identify some of the problems that will have to be addressed if we are to be able to assess the impact of man-made/engineered nanoparticles in the aquatic environment. It does not attempt to review in great depth the approaches taken to characterize new nanoparticulate material, but should provide some indication of approaches that might be adaptable to the quantitative measurement of environmental nanoparticles. The article will focus on the difficulties the environmental scientist will encounter whilst trying to study the presence and behavior of man-made nanoparticles once they have dispersed within the aquatic environment. The concepts behind the study of man-made materials are similar to those involved in the study of natural nanoparticles, but the diversity of the natural material is expected to be greater than that of the manufactured materials. This may prove to be advantageous as the manufactured materials generally exhibit similarities of size, morphology and composition which should provide useful focal points for the development of quantitative methods. The task is challenging as it requires both a radical shift of thinking and the development of many new techniques and procedures.
1.1 The importance of small particles in the chemistry of water
A simple Stokes' law calculation of sedimentation velocity indicates that as particle size reduces, the sedimentation velocity rapidly decreases to a point where sedimentation is balanced against repulsive forces and Brownian motion. As particles become smaller, their specific surface area also increases, leading to such particles having large capacities to hold, by adsorption, complexation etc, secondary materials such as pollutants. Partitioning of contaminants occurs between the particulate and the dissolved phases.8 Whereas such partitioning occurring between larger suspended sediment particles and the water would be expected to result in the deposition of a contaminant associated with the particles to the (quasi-stationary) bottom sediments, where colloidal particles are concerned, the consequence is the maintenance of the contaminant suspended in the moving water. Association with colloids can therefore enhance the migration of surface-bound contaminants until such time as they are taken up by organisms or potentially filtered out as they permeate through rocks, aggregate or attach to other solid matter to give larger, sedimentable particles. Such aggregation-derived sedimentation is well established as occurring in estuaries as a result of ionic strength and acidity induced ‘flocculation’ during the mixing of freshwater and seawater.Many man-made nanoparticles are designed to have specific properties and such properties may not turn out to be environmentally benign. The most obvious example of such a material is titanium dioxide, a material that is known to enhance the degradation of organic material. Whilst the deliberate introduction of titania to enhance photo-degradation in the purification of water may be beneficial, the release of photo-active titania nanoparticles into the aquatic environment may result in unforeseen chemical and biological consequences.
1.2 The problem of distinguishing dissolved from particulate
Ever since the introduction of the 0.5 μm cellulose acetate membrane filter into oceanographic research by Goldberg9 in 1952, it has become a long-established practice for water scientists to employ a 0.45 μm filter to distinguish between the so-called ‘dissolved’ and ‘particulate’ phases. In the context of this article, this practice is clearly not compatible with an understanding of the role of natural colloids and man-made nanoparticles in the aquatic environment.10 Whilst, in theory, a filter cutoff at 450 nm might at first sight seem to be useful for the isolation of nanoparticles (having one dimension of 100 nm or less), this would only be suitable for quasi-spherical particles; nanorods, having hydrodynamic radii much greater than 450 nm would, in theory, be removed during filtration.Conventional filtration suffers from many limitations when used to filter particle suspensions, not least as a result of the differences between depth and barrier filtration. Depth filters trap particles both at the surface and throughout a bed of randomly-assembled fibers (glass or quartz microfiber, cellulose, and polypropylene). Whilst this results in depth filtration generally tolerating high flow rates and loading capacities, it cannot lead to a sharp particle size cutoff. Surface, or membrane filtration, on the other hand, primarily traps particles on a membrane surface. In theory, contaminants sufficiently smaller than the pores in the material pass through, while larger particles are trapped (Fig. 2). This is a complex area, compounded by problems arising from electrostatic interactions between the particle and the filter material, the topography of the filter surface, particle impaction onto the surface of the filter and other particles and clogging effects. Many filter types may offer the same nominal pore size, but in practice these are likely to yield significantly different performance characteristics when challenged with a range of different particle types and concentrations.
![Differing structures of common filter types influence filtration performance. [A. 1.6 μm Whatman GF/C filter (particles spiked into deionised water), B. 0.45 μm Millipore HA filter, C. 1 μm Nuclepore filter (particles spiked into estuarine water)].11](/image/article/2010/EM/b913681a/b913681a-f2.gif) | ||
| Fig. 2 Differing structures of common filter types influence filtration performance. [A. 1.6 μm Whatman GF/C filter (particles spiked into deionised water), B. 0.45 μm Millipore HA filter, C. 1 μm Nuclepore filter (particles spiked into estuarine water)].11 | ||
The presence of nanoparticulate contaminants in natural waters cannot therefore be adequately assessed by the application of an arbitrarily chosen filter. Whilst a 0.45 μm filter may well collect most particles larger than 450 nm, it is inevitable that it will also collect a large proportion of the smaller particles by electrostatic attraction, enforced particle aggregation on/within the filter as many particles attempt to simultaneously enter a filter pore and impaction of the particles on the surface of a track-etched filter. Anyone who has been actively involved in the filtration of a water sample will be well aware of how rapidly the initial water passes through the filter but how, often quite rapidly, the filter ‘clogs’ and filtration rate slows as the filter is used further. The mean effective filter particle size cutoff will inevitably be reducing as the filtration of the sample progresses. Whilst it is relatively easy to identify the many problems that arise from such a practice, concluding how to obtain meaningful analytical measurements of water contaminants by filtration is much more difficult.
The current interest in the industrial production of manufactured nanoparticles has focused attention on the use of filters to distinguish dissolved and particulate fractions in analysis. No longer is it possible to accept filtration based on a nominal cut-off of 0.45μm if this results in the unpredictable and variable assignment of the natural and man-made particles between the dissolved and particulate fractions. It is becoming more widely accepted that this filtration cannot be usefully employed and some modern procedures for the measurement of pesticide residues in water are now based on unfiltered samples.
For those requiring more detailed information, but often of a more qualitative nature, the reader is referred to a number of books that cover the study of nanoparticles in the environment.12–14
2. Measurement of environmental nanoparticles
Current interest in the development of all-things-nano is opening the eyes of environmental scientists to the need to develop ways by which nanoparticles can be quantified once they are released into the environment. The task is rather greater than that presented by the release of a new low to medium molecular weight organic herbicide into the environment. Nanoparticles range from the organic to the mineral, they are by definition not dissolved, and therefore are not compatible with most techniques that have been developed to study dissolvable species in complex matrices. Analysts are well aware of the large gulf between the characterisation of a single pure product of an organic synthesis and the additional workup and performance required of quantitative organic trace analysis. We are at a transition point at which mankind has developed new nanomaterials, has the means to characterize them as pure materials, but is at present rarely able to quantitatively track them once they have been released into complex environmental systems. The task of developing the necessary measurement capability will inevitably fall on trace analysts, many of whom will have little or no experience of measuring particulate species.2.1 What techniques are available?
In discussing how it might become possible to quantify man-made nanoparticles in the aquatic environment, an initial broad overview and evaluation will be made of the techniques and approaches that are already available. In most cases, it will become evident that the tools focus on the characterisation of synthetic products and are neither well suited to complex mixtures, nor to the measurement of low concentrations. A more detailed review of the characterization of engineered nanoparticles has recently been published.15Dealing with the complexity and sensitivity requirements of nanoparticle quantification may necessitate the linking of existing particle separation and measurement techniques with instrumental approaches drawn from other areas of analytical science, examples of which include hyphenation with element-specific mass spectrometric techniques, laser excited atomic emission from separated particles, electrochemical particle detection etc.
Arguably the simplest approach to the concentration of particulate material from an aqueous suspension is centrifugation. In its basic form, centrifugation can be employed to isolate particles from a water sample, but as the particles become increasingly small they require more powerful centrifuges to achieve satisfactory particle isolation. Simple centrifugation does not normally enrich the nanoparticles with respect to larger and unwanted solids, but principally isolates all solids from the water and dissolved species. Density gradient centrifugation is available to enhance the selectivity of the centrifugal isolation, but requires that the components for separation have densities within a specific gradient range. Aqueous density gradient systems commonly employed for biological systems usually have densities less than 1.4 g cm−3; less than the density of metal nanoparticles.
Solvent and small molecule/salt removal from the sample can also be successfully achieved by dialysis and ultra filtration. Larger volumes of water can be processed and clogging effects minimized by cross flow ultrafiltration.16
Small particles (<1 μm) can then be separated from larger particles using split-flow thin-cell fractionation (SPLITT). In this technique, the two fractions are separated by splitting a laminar flow of particle-containing liquid with a force (such as gravity) applied at right angles to the flow.17,18 No membrane is used in the separation and it might therefore be expected to be minimize (but not prevent) changes to fragile natural aquatic colloids and nanoparticle aggregates.
Little work has been carried out to investigate the all important quantitative aspects of nanoparticle recovery from natural waters.
Scanning electron microscopy (SEM) provides a view of particle morphology and allows the analyst to gain an impression of particle abundance, together with rough estimates of composition through energy-dispersive X-ray spectroscopy. Particle sizing can be carried out by microscopy, but is normally dependent on ‘manual’ measurements of individual particles, which can be extremely tedious and selective. Conventional electron microscopes are also high vacuum instruments, prone to sample changes arising from the need to dehydrate samples and coat them with gold prior to the microscopy. Environmental SEM instruments help with this problem somewhat as they allow the study of wet samples, but they do so at the expense of resolution. Even under optimal conditions, the best resolution obtainable from an SEM is ca 1 nm.
Transmission electron and helium ion microscopes offer the greatest resolution with quoted values as low as ca 50 pm. Whilst of great value in assessing particle structure and makeup, their application is generally limited to the characterization of new materials; they are high vacuum techniques that, with cellular and other wet particle structures, suffer from particle alteration during preparation and analysis. Their expense, limited availability and focus on single particle structures has meant that they have not been widely used for the study of diverse particle populations from environmental samples.
Atomic force microscopy obtains images through the measurement of very small forces resulting from the interaction between the atoms or molecules of the sample and a pointed tip that is scanned in two dimensions across the sample. Information about the tip movement provides three-dimensional images of the sample with a resolution of 10 nm. Unlike electron microscopes, the AFM can be used with samples in air and under liquid. Light can be delivered through the tip and when combined with a near-field optical microscope (SNOM) gives a spatial resolution of ca 30–50 nm.
Summary. Microscopy is a powerful means of visualising samples but it cannot be considered to be an ideal approach to quantification; the counting of single particles can be laborious and prone to poor sampling of the whole population. Particle counting for quantitative purposes will always be difficult due to the small field of view of the instrument when magnifications are sufficient to resolve nanoparticles.
The disc centrifuge separates particles by size using centrifugal sedimentation in a density gradient liquid that is held in an optically clear disc rotating at up to 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. When particles reach the outside edge of the rotating disc, they attenuate a light beam that passes through the disc. The light intensity is continuously recorded and a particle size distribution is calculated from this information. The working range depends on particle density, but is generally from ca. 75 μm to under 5 nm. Fixed speed runs have a limited dynamic range of ca. 70× but this can be expanded by gradually increasing the centrifugation speed during analysis.
000 rpm. When particles reach the outside edge of the rotating disc, they attenuate a light beam that passes through the disc. The light intensity is continuously recorded and a particle size distribution is calculated from this information. The working range depends on particle density, but is generally from ca. 75 μm to under 5 nm. Fixed speed runs have a limited dynamic range of ca. 70× but this can be expanded by gradually increasing the centrifugation speed during analysis.
Nanoparticle tracking analysis (NTA) is an alternative approach based on the relationship between the rate of Brownian motion and particle size through the Stokes–Einstein equation. The rate of particle movement is related to a sphere of equivalent hydrodynamic radius. The relationship depends on the viscosity of the liquid, the temperature and size of the particle, but it is not influenced by particle density or refractive index. The sample is injected into a viewing chamber and the individual particles are visualized and tracked by their scattering of laser light, viewed though an optical microscope equipped with a digital camera. The technique calculates particle size on a particle-by-particle basis. NTA is currently suitable for particles from 10 to 1000 nm. Extending the tracking into 3 dimensions potentially overcomes problems associated with the movement of the particle out of the focal plane of the microscope.21
Dynamic light scattering.22 When a laser beam is shone through a liquid containing suspended particles, the beam scatters off the particles in all directions, giving a scattering-angle-dependent intensity pattern. Brownian motion causes the intensity pattern to fluctuate randomly and measurement of the intensity fluctuations (at a given scattering angle) can give information about the particles, including their hydrodynamic radius. A knowledge of the particle and solution physical properties is however required, as these are components of the particle size calculation.
UV-vis spectrophotometry and fluorescence. Some classes of nanoparticles exhibit characteristic spectroscopic properties in the ultraviolet and visible regions of the electromagnetic spectrum that can be usefully exploited to distinguish, characterise and quantify the particles.
As the dimensions of semiconductor materials are reduced to the nanoscale, their dimensions approach the wavelength of the conduction electrons and “quantum confinement” occurs. Energy levels shift and the band gap becomes larger. CdSe or CdS semiconductor quantum dots, for example, absorb over a broad wavelength band and their fluorescence is related to the particle size; the larger the particle, the redder (lower energy) its fluorescence spectrum.
With some metal nanoparticles (e.g. Au, Ag) a different phenomenon, surface plasmon resonance, occurs as localized surface plasmon oscillations can give rise to intense coloration of the particle sols. Nanoparticle sols of noble metals exhibit strong absorption bands at ultraviolet-visible wavelengths that are not present in the bulk metal and the resulting characteristic absorption and fluorescence spectra can be exploited to determine particle size.23
Whilst reported studies have focused on the relationship between particle size and spectroscopic properties, spectroscopic properties may also prove to be valuable in distinguishing analytes from bulk material in complex mixtures and providing a basis for quantification.
Summary. Quite a number of techniques are available for determining the size of particles, but these have largely been developed for particle populations with a high level of monodispersity and are best suited to the characterization of ‘pure’ samples of man-made particles.24 The applicability of the common sizing techniques to the quantitative study of natural waters is not well established and results obtained by the various methods can, even for quite well defined systems, differ significantly. Such problems are compounded by particle size measurements often being highly dependent on a knowledge of the physical properties of the particles (such as density, refractive index etc) that are almost impossible to determine for natural samples. No one method can be recommended for all sample types.
Manipulation between immiscible solvent phases is the familiar basis of many conventional analytical procedures employed to extract and enrich molecular species. In solvent extraction techniques, such partition-based steps depend on the differential migration of dissolved analytes and matrix species between the two phases. Whilst dissolution of nanoparticles is not normally viable, affinities for different solvent types can be controlled by (temporary?) surface modification. Even a solvent interface can potentially be exploited to isolate man-made nanoparticles from water;25 gold nanoparticles (5 and 20 nm), with suitable modification, spread as a thin film at the liquid–liquid interface between water and an organic solvent.26
Flow cytometry, widely used in the biological sciences for the counting and sorting of cellular particles, has the power to distinguish particles through their light scattering and the use of multi-labeling fluorescence approaches. Future developments may include those based on the development of fluorescence staining of natural and engineered nanoparticles and the deliberate labeling of particles before release. Some extension of the normal working range (200 nm to 150 μm in diameter) of flow cytometry may first be required to cover single nanoparticles. Whilst conventionally based on detection by fluorescence and scattering, there may be potential in this area for the development of particle fractionation and counting linked to spectroscopic identification through, for example, fast response time ICP or laser excitation of the separated particles, with emission spectroscopy or mass spectrometric detection of the separated particle stream.
The disc centrifuge, whilst previously mentioned in the context of instruments marketed principally for particle sizing, is in fact a separation technique potentially well suited to the resolution of complex particle mixtures.
Field-flow fractionation (FFF) is similar to chromatography, but uses a thin flow channel through which a solvent transports the sample. As with liquid chromatography, the choice of detector (most normally ultraviolet absorption or fluorescence) can enhance the available physical and chemical information. It is a family of separation techniques, utilizing the same basic separation principle, but employing different force fields. Depending on the FFF variant that is used, different force fields (liquid flows, centrifugal forces, temperature gradients or gravity fields) are applied perpendicular to the separation channel. Under the influence of these force fields and the counteracting diffusion field, smaller particles are located in the faster flow and bigger particles are located in slower stream lines of the laminar flow inside the channel. This results in smaller particles eluting faster through the channel than the bigger ones.
FFF can provide high resolution separations of particles from 1 nm up to 100 μm in a liquid medium and gives information about diffusion coefficient and size directly. It can also be potentially linked (hyphenated) to a broad range of instruments offering more selective detection.
Hydrodynamic chromatography (HDC) is essentially FFF but with the secondary force arising from a thinning of the channel through which the particles flow. Larger molecules are more dominant in the centre of a narrow-bore capillary than smaller molecules, and thus move faster due to the parabolic flow profile. The interstices of a packed-bed column can act as the separation channel and HPLC-type columns packed with small nonporous particles (such as 2μm silica) have been used to separate 5–78 nm silica colloids. The silica colloids were monitored based on turbidity using a UV detector.27 HDC separations have been so far demonstrated on packed beds, open-tubular capillaries, and microchips.
Size exclusion chromatography (SEC) is normally applicable to particles smaller than 100 nm. Some successful nanoparticle separations have already been achieved28 but, requiring a porous column packing material, surface adsorption can cause unwanted column–analyte interactions necessitating the addition of additives to block active sites.
Electrophoretic techniques. The migration of particles in an electric field has been widely used in the biological sciences and charging of nanoparticles offers many potential approaches to the enrichment of nanoparticle extracts obtained from water samples. Several different approaches have been developed that have potential in the separation and measurement of nanoparticle populations. Capillary zone electrophoresis within fused silica capillaries and employing a UV-vis detector has, for example, been reported to separate differing sizes of polystyrene nanospheres and gold nanoparticles.29Isoelectric focusing is used extensively in biology to separate isoforms of proteins according to their isoelectric point (pI). With the right sample type, it can offer extremely high resolution of sample components using charge rather than the other most commonly employed discriminator, size. In a typical IEF gel electrophoresis experiment, the charged biomolecules migrate in a pH gradient formed by ampholytes within a polyacrylamide gel or column of density-stabilized solution. When an electric field is applied, molecules migrate to the position in the gel where they have zero charge. The particles themselves do not need to be charged if their surfaces can be altered by derivatisation; in the case of gold nanoparticles, by derivatization with a thiol terminated carboxylic acid.30
Summary. A number of different separation techniques have already proven suitable for the separation of particles based on their size, surface, density and charge characteristics. If enhanced by their linking (hyphenation) to more sensitive and selective detection systems, they should provide a sound foundation for the resolution of quite complex mixed particle systems.
3 Developing new strategies
The need to quantify manmade nanoparticles in water takes environmental analysis to an exciting new level. Having focused decades of attention on the measurement of dissolved pollutants, a major shift in approach will be needed to quantify particles in water. Taking a broad view of most analytical challenges involving the measurement of trace small molecule contaminants in the aquatic environment, the solutions can normally be broken down into a number of distinct stages. Measurements of dissolved molecular species are made possible by the application of powerful separation techniques to the problem after extensive sample extraction and pretreatment; when applied to particles in water, these steps would translate to:• identify the particles of interest (qualitative)
• isolate, if unavoidable, the particles from the water (quantitative extraction)
• if necessary, separate the particles from other matrix components (clean-up/enrichment)
• quantify the particles, discriminating between particle types (species) and/or properties such as size (quantification).
The concepts of qualitative and quantitative measurement of man-made nanoparticles in environmental samples are at a very early stage of development. The task of determining environmental nanoparticles normally lacks the convenience of being able to dissolve the analyte that commonly underpins procedures employed in the determination of solution phase species. In many ways, the problem is similar to that encountered in biology—identification and quantification of environmental particles such as viruses, bacteria and plankton.31 The sizes are similar and like microorganisms, man-made nanoparticles often have characteristic sizes, shapes, surface chemistries etc. What the nanoparticles lack (so-far) is the life force that permits them to be replicated in culture.
Our ability to characterize nanoparticles in environmental samples therefore depends on the nature of the particles themselves (whether, for example, they have similar distinctive shapes or distinctive chemical functionalities that would make them amenable to specific staining techniques), their concentration and the nature of the matrix. Studying systems to which distinctive man-made nanoparticles have been deliberately added is therefore often easier than studying low concentrations of difficult to identify particles in a complex environmental sample containing both man-made and natural nanoparticles.
In the early stages of the development of techniques with which to quantify environmental nanoparticles, it is likely that studies will be limited to systems involving nanoparticles that are easily recognizable (either by man or machine) amongst a complex mix of natural and man-made particles. This may be because they are:
• monodisperse; a common size can indicate a common origin
• have distinctive shapes; this may indicate an engineered synthesis not present in nature
• have characteristic chemical composition (including being radio-labeled); a composition that is 100% CdSe or Au may indicate man-made origins
• separable; they may possess distinct exploitable properties such as density, magnetism etc.
• able to be labeled; staining/fluorescence labeling exploiting surface chemistry to distinguish analyte particles from others.
The deliberate addition of identifiable particles to natural water can be a valuable approach to assist in the understanding of properties such as nanoparticle interaction with natural colloids or in particle migration studies. These are, however, only model systems and suffer from the limitations of any artificially devised system. There remains a critical need for methods to be developed by which man-made particles can be distinguished from natural particles and, just as importantly, quantified. Following the route of a classic trace analysis—extract, enrich, separate and measure—may provide a useful framework on which to base the exploration of potential means of developing quantitative nanoparticle trace analysis of natural waters. Taking the quantitative determination of gold nanoparticles as a potentially straightforward example with which to illustrate such a method, the scheme described in Fig. 3 might prove to be viable.
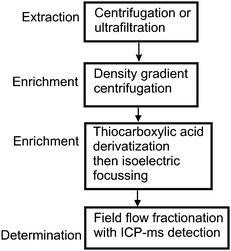 | ||
| Fig. 3 A hypothetical quantitative analysis scheme for gold nanoparticles in water. | ||
At each of the early stages of the procedure the objective is to remove as much of the water and potential interferands as possible. Extractions can be based on centrifugation, filtration, temporary aggregation etc. Initial choices are less refined and as we move through to the later stages, as the extract becomes both simpler and of lower volume, the specific properties of the analyte can be more fully exploited. In this case the well established modification of the gold surface with a thiocarboxylic acid results in a specific modification giving the gold surface a negative charge and in the process making it suitable for isoelectric focusing. This electrophoretic technique is not normally well suited to quantification and the choices for the final stage are based on the separation abilities of FFF and the detection of 197Au by ICP-MS32 (hyphenated instrumentation).
A less conventional approach, requiring some advanced instrument development, could be based on quantitative imaging. Gold nanoparticles are highly resistant to most chemical and thermal treatments and once the particles have been extracted from a water sample enrichment of the extract could be achieved by the application of ashing and acid digestion treatments. It should then prove possible to spread an aqueous suspension of the residue as a monolayer onto a suitable substrate such as glass; a process that can be facilitated by the modification of the substrate surface with thiol groups. With the development of modern image recognition techniques applied with automated electron microscopes, identification and quantitative counting of readily identified manmade nanoparticles should soon become possible.
These have been just two examples of the many approaches that might be taken. They are derived from current technologies and are meant to be purely illustrative of the vast potential of this new area of analysis.
4 Conclusions
At present, very little is known about the fate, transport, transformation, and toxicity of man-made nanomaterials in the aquatic environment and there are, unfortunately, few effective techniques that can distinguish man-made nanoparticles from natural materials. The challenge for the analytical scientist is to develop the means by which specific types of particles can be both identified and quantified against a complex background of natural solids. This will require the development of both new techniques and procedures. The simplest environmental system to study is arguably that of atmospheric particulates and significant work and expertise has already been developed in that area. Next in line, needing increased attention, is the aquatic environment. Despite low particle concentrations, the study of water may turn out to be the most successful environmental matrix to investigate in the early stages of technique development, as it is much less complex than some other sample types (cf. soils, sediments, biota etc.). Solutions to such problems require more than just the development of new instrumentation, equally important is the complex sequence that makes up the extraction and enrichment of samples prior to their measurement. These steps become increasingly important as the complexity of the sample increases and as the concentration of the analyte particles drops.Our future ability to assess the environmental impact of man-made nanoparticles in the aquatic environment is highly dependent on the development of new analytical approaches and instrumentation. This is a major challenge to the analyst, both conceptually and instrumentally.
References
- J. H. Duffus, M. Nordberg and D. M. Templeton, Pure Appl. Chem., 2007, 79, 1153–1344 CrossRef CAS.
- IUPAC, Manual of symbols and terminology for physicochemical quantities and units appendix ii Definitions, Terminology and Symbols in Colloid and Surface Chemistry part i., IUPAC, Washington, 1971 Search PubMed.
- K. Sellers, Nanotechnology and the environment, CRC Press, Boca Raton, 2009 Search PubMed.
- S. J. Klaine, P. J. J. Alvarez, G. E. Batley, T. F. Fernandes, R. D. Handy, D. Y. Lyon, S. Mahendra, M. J. McLaughlin and J. R. Lead, Environ. Toxicol. Chem., 2008, 27, 1825–1851 CrossRef CAS.
- Y. Ju-Nam and J. R. Lead, Sci. Total Environ., 2008, 400, 396–414 CrossRef CAS.
- R. D. Handy, F. von der Kammer, J. R. Lead, M. Hassellov, R. Owen and M. Crane, Ecotoxicology, 2008, 17, 287–314 CrossRef CAS.
- M. N. Moore, Environ. Int., 2006, 32, 967–976 CrossRef CAS.
- D. A. L. Vignati, S. Valsecchi, S. Polesello, L. Patrolecco and J. Dominik, TrAC, Trends Anal. Chem., 2009, 28, 159–169 CrossRef CAS.
- E. D. Goldberg, M. Baker and D. L. Fox, Journal of Marine Research, 1952, 11, 194–203 CAS.
- O. Gustafsson and P. M. Gschwend, Limnology and Oceanography, 1997, 42, 519–528 CAS.
- N. Ketkoom and A. G. Howard, unpublished results, 2009.
- J. R. Lead and E. Smith, Environmental and human health impacts of nanotechnology, Wiley, Chichester, West Sussex, U.K.; Hoboken, N.J., 2009 Search PubMed.
- K. J. Wilkinson and J. R. Lead, Environmental colloids and particles: behaviour, separation and characterisation, John Wiley & Sons Ltd, Chichester, England; Hoboken, NJ, 2007 Search PubMed.
- R. B. Gupta and U. B. Kompella, Nanoparticle technology for drug delivery, Taylor & Francis, New York, 2006 Search PubMed.
- K. Tiede, A. B. A. Boxall, S. P. Tear, J. Lewis, H. David and M. Hassellov, Food Addit. Contam., Part A, 2008, 25, 795–821 Search PubMed.
- R. Liu and J. R. Lead, Anal. Chem., 2006, 78, 8105–8112 CrossRef CAS.
- J. C. Giddings, Sep. Sci. Technol., 1985, 20, 749–768 CrossRef CAS.
- J. R. Lead, A. De Momi, G. Goula and A. Baker, Anal. Chem., 2006, 78, 3609–3615 CrossRef CAS.
- B. D. Cullity and C. D. Graham, Introduction to magnetic materials, 2nd edn., IEEE/Wiley, Hoboken, N.J., 2009 Search PubMed.
- T. Provder, J. Texter, American Chemical Society. Division of Colloid and Surface Chemistry. and American Chemical Society. Meeting, Particle sizing and characterization, American Chemical Society: Distributed by Oxford University Press, Washington, DC, 2004.
- C. S. Xu, H. Cang, D. Montiel and H. Yang, J. Phys. Chem. C, 2007, 111, 32–35 CrossRef.
- B. J. Berne and R. Pecora, Dynamic light scattering: with applications to chemistry, biology, and physics, Dover edn., Dover Publications, Mineola, N.Y., 2000 Search PubMed.
- V. Amendola and M. Meneghetti, J. Phys. Chem. C, 2009, 113, 4277–4285 CrossRef CAS.
- P. Bowen, J. Dispersion Sci. Technol., 2002, 23, 631–662 CrossRef CAS.
- P. A. Kralchevsky and K. Nagayama, Particles at fluids interfaces and membranes: attachment of colloid particles and proteins to interfaces and formation of two-dimensional arrays, Elsevier, Amsterdam; New York, 2001 Search PubMed.
- O. Balmes, J. O. Bovin and J. O. Malm, Journal of Nanoscience and Nanotechnology, 2006, 6, 130–134 CAS.
- T. Takeuchi, Siswoyo, Z. Aspanut and L. W. Lim, Anal. Sci., 2009, 25, 301–306 CrossRef CAS.
- F. K. Liu, Chromatographia, 2007, 66, 791–796 CrossRef CAS.
- W. M. Hwang, C. Y. Lee, D. W. Boo and J. G. Choi, Bulletin of the Korean Chemical Society, 2003, 24, 684–686 CAS.
- I. Arnaud, J. P. Abid, C. Roussel and H. H. Girault, Chem. Commun., 2005, 787–788 RSC.
- E. M. Goldys, Fluorescence applications in biotechnology and life sciences, John Wiley & Sons, Hoboken, N.J., 2008 Search PubMed.
- A. Helfrich, W. Bruchert and J. Bettmer, J. Anal. At. Spectrom., 2006, 21, 431–434 RSC.
Footnotes |
| † Part of a themed issue dealing with water and water related issues. |
| ‡ The definition of a nanoparticle is subject to debate and whilst it may seem obvious that a nanoparticle should simply have a particle size measured in nanometres, many definitions limit a nanoparticle to having at least one dimension that is less than 100 nm. IUPAC give the following definition: “nanoparticle: microscopic particle whose size is measured in nanometres, often restricted to so-called nanosized particles. Ref. 1. J. H. Duffus, M. Nordberg and D. M. Templeton, Pure and Applied Chemistry, 2007, 79, 1153–1344. A colloid, on the other hand, is a dispersion of particles between 1 nm and 1 μm in size in a fluid medium. A colloid is therefore a heterogeneous mixture, not just a particle. Ref. 2. IUPAC, Manual of symbols and terminology for physicochemical quantities and units appendix ii Definitions, Terminology and Symbols in Colloid and Surface Chemistry part i., IUPAC, Washington, 1971. |
| This journal is © The Royal Society of Chemistry 2010 |
