Implementation of E.U. Water Framework Directive: source assessment of metallic substances at catchment levels†
Ho-Sik
Chon
,
Dieudonne-Guy
Ohandja
and
Nikolaos
Voulvoulis
*
Centre for Environmental Policy, Imperial College London, London, SW7 2AZ, UK. E-mail: n.voulvoulis@imperial.ac.uk; Fax: +44 (0)20 7594 9334; Tel: +44 (0)20 7594 7459
First published on 4th September 2009
Abstract
The E.U. Water Framework Directive (WFD) aims to prevent deterioration of water quality and to phase out or reduce the concentrations of priority substances at catchment levels. It requires changes in water management from a local scale to a river basin scale, and establishes Environmental Quality Standards (EQS) as a guideline for the chemical status of receiving waters. According to the Directive, the standard and the scope of the investigation for water management are more stringent and expanded than in the past, and this change also needs to be applied to restoring the level of metals in water bodies. The aim of this study was to identify anthropogenic emission sources of metallic substances at catchment levels. Potential sources providing substantial amounts of such substances in receiving waters included stormwater, industrial effluents, treated effluents, agricultural drainage, sediments, mining drainage and landfill leachates. Metallic substances have more emission sources than other dangerous substances at catchment levels. Therefore, source assessment for these substances is required to be considered more significantly to restore their chemical status in the context of the WFD. To improve source assessment quality, research on the role of societal and environmental parameters and contribution of each source to the chemical distribution in receiving waters need to be carried out.
Environmental impactThe European Union Water Framework Directive (WFD) requires member countries to change water management practices from end-of pipe solutions to management at catchment levels. Such a shift in water management requires better understanding and monitoring of emission sources. This study has identified the anthropogenic sources that can contribute to changes in concentrations of metallic substances at catchment levels. Results from this study can be used effectively to develop source management that will minimise the concentrations of metallic substances in the aquatic environment to implement the WFD. |
Introduction
The E.U. Water Framework Directive (2000/60/EC) (WFD) was introduced in October 2000 in order to restore the quality of surface and ground waters. Based on a River Basin Management Plan (RBMP) reflecting the most cost-effective option, each E.U. Member State has been compelled not only to prevent deterioration of the quality of these waters but also to achieve chemical, ecological and quantitative good status in the aquatic environment by 2015 (Article 4). This Directive causes the transformation of water management scale from local base to a river basin base. A river basin district, used as an administrative unit of management in the WFD, is decided based on geographical and hydrological characteristics. Therefore, this Directive brings about a change in water quality control from national care to international cooperation.1A list of 33 dangerous substances, called “priority substances” was selected and their Environmental Quality Standards (EQSs) were determined from various assessment of their risk to ecosystems and to the aquatic systems at European levels.2–4 According to Article 16 of the WFD, these substances are divided into following two categories: priority substances and priority hazardous substances. Substances in the former group are required to be controlled for progressive reduction of discharges, emissions and losses, whereas the latter substances are required to be removed or phased out of discharges, emissions and losses by 2020.1 In the list of priority substances, cadmium, mercury and their compounds are classified as priority hazardous substances, while lead, nickel and their compounds as priority substances.3,4 The EQSs of these metals are shown in Table 1.
| Metal | Inland surface watersa | Other surface watersb | ||
|---|---|---|---|---|
| AAc-EQS | MACd-EQS | AA-EQS | MAC-EQS | |
| a Rivers, lakes and related artificial or heavily modified water bodies. b Transitional, coastal and territorial waters. c Annual average. d Maximum allowable concentration. e The range depends on the hardness (CaCO3) of surface waters. f Not applicable. | ||||
| Cadmium and its compounds | ≤0.08–0.25e | ≤0.45–1.50e | 0.20 | ≤0.45–1.50e |
| Lead and its compounds | 7.20 | N.A.f | 7.20 | N.A. |
| Mercury and its compounds | 0.05 | 0.07 | 0.05 | 0.07 |
| Nickel and its compounds | 20.00 | N.A. | 20.00 | N.A. |
Much research has been carried out on the sources and concentrations of metals in the aquatic environment. However, most of this work has dealt with specific areas without much reference to the strategic management intended to by the WFD. Limited research has been undertaken on the relationship between the sources of metals and their concentrations in catchments.5–8 According to the implementation of the WFD, the scope of the investigation of water quality becomes more expanded and complicated than in the past. However, as a starting point, Member States are required to identify all emission sources affecting water quality at catchment levels.
Of the list of priority substances and the list of dangerous substances classified by the Directive (2006/11/EC), known as “the Dangerous Substances Directive”,9 barium (Ba), cadmium (Cd), chromium (Cr), copper (Cu), lead (Pb), mercury (Hg), nickel (Ni), titanium (Ti), vanadium (V), zinc (Zn), antimony (Sb) and arsenic (As) were selected as metallic substances considered in this study. Although chemical speciation of these substances from emission sources was not included, this study discussed its importance to manage chemical and ecological status of the aquatic systems. This paper aimed to identify and assess predominant anthropogenic sources of metallic substances in receiving waters.
Sources and emissions of metallic substances
Substantial quantities of metallic substances are released from anthropogenic sources. As the apportionment of these sources increases, their concentrations also rise in the aquatic environment.10,11 A holistic diagram showing emissions of metallic substances from potential anthropogenic sources is presented in Fig. 1.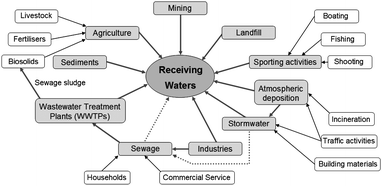 | ||
| Fig. 1 A conceptual model outlining metal emissions from anthropogenic sources. | ||
Stormwater
The effects of stormwater on metal levels in receiving waters have steadily increased with urbanisation and subsequent changes such as land-use and the volume of traffic in catchments.12 Direct discharge of stormwater without appropriate treatment is thought to bring about severe contamination in receiving waters with a significant contribution to total metal concentrations in the aquatic environment.13 Several researchers reported that atmospheric deposition, traffic activities and building materials are the most significant human-originated sources of metals in stormwater.5,7,14,15 In general, the quantity and the quality of stormwater are associated with surface runoffs. Road and roof runoffs serve as reactants or carriers of pollutants, and cause various total metal concentrations in stormwater. Ackerman and Schiff16 calculated a runoff coefficient (a ratio of runoff volume to rainfall volume) in catchments with different land-use. They found that commercial and industrial areas had runoff coefficients of 0.61 and 0.64 respectively. These coefficients were higher than those measured in other types of land-use, suggesting that the quantity of runoffs was affected by a dimension of impervious surface.Metals in the atmosphere can directly influence their total concentrations in stormwater. They exist as particulates in dry conditions and as dissolved phases in rainfall in wet conditions. Total metal concentrations in the atmosphere depend on seasonal variations and human-related activities, such as fossil fuel combustion, waste incineration, industrial emissions from plating, smelting and refining operations in a given area. In terms of metal emissions from the atmosphere in catchments, Sabin et al.17 reported that wet deposition was responsible for 1–10% of annual total deposition (wet + dry). Results of their analysis of dissolved metal levels are summarised in Table 2. Although, this previous study concluded that the contribution of atmospheric deposition was significant in terms of total metal concentrations in stormwater, atmospheric impact on water bodies is understood to be negligible compared to other metal sources.18,19 Sörme and Lagerkvist7 reported that about 3–4% of total cadmium and lead, 2% of total zinc and less than 1% of total chromium, copper and nickel in influents to Henriksdal wastewater treatment plant (WWTP) in Stockholm were derived from atmospheric depositions and this trait was also found in other research.8
| Metal | Annual metal fluxes (range) (µg m−2 year−1) | |
|---|---|---|
| Wet conditions | Dry conditions | |
| Cr | 18 (0–45) | 440 (250–620) |
| Cu | 200 (0–520) | 3211 (1800–4600) |
| Pb | 29 (0–74) | 2000 (390–3600) |
| Ni | 38 (0–96) | 1300 (0–2700) |
| Zn | 1500 (0–3900) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (4900–22 000 (4900–22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) 000) |
Traffic activities represent the most crucial source affecting stormwater quality and their impacts increase along with the rise in traffic density.20,21 Sörme and Lagerkvist7 reported that about 20% of total wastes from brake abrasion and 40% from tyre abrasion could be transported into stormwater, whereas Göbel et al.14 mentioned that 5–20% of total released chemicals in stormwater were from road runoffs. Traffic wastes produced by combustion operations, tyre, brake lining and motorway abrasion, de-icing agents, lubrication losses and road dust resuspension could generate substantial metal levels in road runoffs.5,7,20,22–27 Legret and Pagotto28 estimated that abrasion of tyres and brake linings in a light vehicle were 68 mg km−1 and 29 mg km−1, respectively. In terms of motorway abrasion, Brinkmann29 mentioned that metals from road pavements in winter could be four times higher than those observed in summer due to the use of de-icing agents. Of metals released from traffic activities, zinc levels are the most significant in road runoffs, followed by copper, lead and cadmium in sequence.5,30,31 With regard to particulate size, Lough et al.25 demonstrated that coarse-sized particles (1–18 µm) were mainly associated with road dust resuspension and tyre and brake abrasion, while fine-sized particles (less than 0.1 µm) with combustion products. However, even small particles released from combustion operations could aggregate themselves or adhere to other particles, forming larger complexes.32 Furthermore, Legret and Pagotto28 showed that 91% of total lead was in particulate forms in road runoffs, while 60%, 56% and 54% of total zinc, copper and cadmium, respectively, were in dissolved forms. In particular, cadmium and zinc emitted from traffic activities have higher mobility and bioavailability compared to others.20,23 Emission sources and related metals are presented in Table 3. Few studies have been undertaken for quantitative evaluation of metal emissions from specific traffic activities. Hjortenkrans et al.,33 for example, calculated that metals from brake lining abrasion were 710 kg year−1 of antimony, 0.064 kg year−1 of cadmium, 3800 kg year−1 of copper, 35 kg year−1 of lead and 1000 kg year−1 of zinc, whereas there were 0.54 kg year−1 of antimony, 0.47 kg year−1 of cadmium, 0.76 kg year−1 of chromium, 5.3 kg year−1 of copper, 3.7 kg year−1 of lead, 1.4 kg year−1 of nickel and 4200 kg year−1 of zinc from tyre abrasion in Stockholm in 2005.
| Emission sources | Metal | References | |
|---|---|---|---|
| Abrasion | Brake lining | Sb, Ba, Cd, Cr, Cu, Pb Ni, Ti, Zn | 5,7,20–22,25 |
| Motorway | Cd, Cr, Cu, Pb, Ni, Zn | 7 | |
| Tyre | Cd, Cu, Pb, Ti, Zn | 5,20–22,28 | |
| Combustion | Engine oil | Sb, Pb, Zn | 5,7,20,21,25 |
| Diesel-fuel additive | Ba | 35 | |
| Fossil fuel | Pb, V, Zn | 20,25,36 | |
| De-icing agent | Zn | 20 | |
| Road dust (Crustal dust resuspension) | Cr, Cu, Pb, Ni, Ti, V, Zn | 7,11,21,25 | |
Building materials used for structured frames, roof coverings and building sidings (façades) considerably affect total metal concentrations in stormwater. Förster34 reported that roof runoffs contained higher metal levels than rainwater, while Thévenot et al.8 showed that roof runoffs could cause about 55% of annual lead and zinc loads in stormwater. Metals in these runoffs are largely from corrosion products of building materials. When these materials are exposed to the atmospheric environment, they start rusting or corroding. Corrosion layers featuring higher porosity and cracks, then, dispatch contained substances through chemical reaction with rainwater.
Precipitation conditions can be one of the main factors affecting metal concentrations in roof runoffs and include: rainwater pH, the total rainfall and its residence time, rainfall intensity, and antecedent dry period.37 Among them, lower pH value of rainwater is the most significant factor in an increase in metal emissions.38 Chemical properties of building materials, building types and their histories are also found to influence metal concentrations in roof runoffs.5,6,8,26,39 Rule et al.6 reported that the mean total concentrations of cadmium and copper in the runoffs sampled in new town areas were higher than those in old town areas, whereas He et al.37 showed that total copper concentrations in roof runoffs increased as copper roofing aged. Some roof materials, including ceramic tiles and cement sheets, can release more metals than others,26 while building bricks and painted wood also have the potential to emit metals.5 For example, efflorescence phenomena in which crystalised salts are formed on the surface of bricks causes the exposure of crystal components such as titanium and vanadium to the rainwater or related runoffs.40 Building materials from demolition and construction activities contribute to metal levels in stormwater.41 The crustal dust generated from these activities contains metals and their complexes which can settle on the ground surface of activity sites and surrounding road pavement. These pollutants are washed off by rains, and cause an increase in metal concentrations in surface runoffs. Amato et al.42 reported metal concentrations in road dust particulates (PM10) sampled from demolition and construction sites in Barcelona, Spain with the average of 76 ± 72 mg kg−1 of antimony, 17 ± 5 mg kg−1 of arsenic and 491 ± 470 mg kg−1 of copper.
Copper, lead and zinc are the major metals emitted from building materials. These metals are widely used to make roof coverings and fittings, gutters and down pipes.8,14 In a 12-month study carried out in Stockholm, Persson and Kucera43 found that dissolved copper concentrations in roof runoffs sampled from copper roofs were about 3575 ± 1425 (standard deviation) µg L−1, whereas dissolved zinc was contained in roof runoffs sampled from galvanised and painted steel with the average of 6758 ± 1879 µg L−1 and 2100 ± 100 µg L−1, respectively. Zinc is more readily emitted from building materials than copper,14 whereas lead is used for paint materials and copper for wood preservatives.5 Arsenic and cadmium are also found in roof runoffs. They are included in zinc-bearing materials as impurities, being released after reaction with acid rainwater.8
The contribution of stormwater to metal increase in water bodies is considerable. Table 4 compares metal concentrations in stormwater with those in raw sewage and WWTP effluents. It can be seen that the concentrations of cadmium, lead, copper and zinc in stormwater are as high as those found in raw sewage and WWTP effluents. The contributions of these metals found in stormwater depend on specific characteristics of a given catchment and such characteristics include the volume of stormwater, sewerage systems (combined or separated systems), the extent of direct discharge into receiving waters, etc.44
| Source | Average total metal concentrations (µg L−1) | Remarks | Reference No. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Pb | Hg | Ni | Zn | |||
| a No evidence of the state of metal concentrations. b Median metal concentrations (no evidence of the state of metal concentrations). c Average value of previous studies (no evidence of the state of metal concentrations). | ||||||||||
| Raw sewage | — | 6.0 | 290.0 | 310.0 | 230.0 | 7.0 | 330.0 | 240.0 | — | 53 |
| — | 38.0 | 173.0 | 226.0 | 101.0 | 0.6 | 120.0 | 723.0 | — | 54 | |
| — | 0.8 | 12.4 | 77.8 | 25.3 | 0.5 | 14.2 | 155.4 | — | 45 | |
| — | 0.2 | 6.9 | 17.3 | 37.4 | 0.1 | - | 79.2 | — | 55 | |
| WWTP effluents | — | 1.0 | 60.0 | 80.0 | 15.0 | <1.0 | 270.0 | 560.0 | — | 53 |
| — | 8.5 | 55.0 | 27.5 | 48.5 | 0.1 | 116.0 | 103.5 | — | 56 | |
| — | 0.2 | 3.0 | 12.4 | 2.6 | — | 7.8 | 46.7 | — | 57 | |
| — | 1.5 | 20.0 | 33.0 | 27.0 | — | 430.0 | 270.0 | — | 58 | |
| — | 0.1 | 5.7 | 9.7 | 22.6 | 0.1 | — | 43.6 | — | 55 | |
| Stormwater | 3.0 | 0.5 | 4.6 | 12.0 | 12.0 | 0.2 | 5.4 | 73.0 | Residential area | 59 |
| 2.4 | 0.9 | 6.0 | 17.0 | 18.0 | 0.2 | 7.0 | 150.0 | Commercial area | 59 | |
| 4.0 | 2.0 | 14.0 | 22.0 | 25.0 | 0.2 | 16.0 | 210.0 | Industrial area | 59 | |
| 2.4 | 1.0 | 8.3 | 34.7 | 25.0 | — | 9.0 | 200.0 | Motorway | 59 | |
| 4.0 | 0.4 | 5.4 | 10.0 | 10.0 | — | — | 40.0 | Open space | 59 | |
| 22.3 | 2.6 | 23.5 | 157.4 | 189.4 | 0.9 | 47.0 | 717.4 | — | 12 | |
| — | 2.3 | 16.0 | 48.0 | 118.0 | — | 22.6 | 275.0 | — | 57 | |
Industrial effluents
For a long time, industrial effluents have been reported to be one of the most important contributors affecting water quality.18 Some researchers, Thévenot et al.8 for example, still estimate the considerable contribution of industrial effluents to metal levels in a river basin. However, other studies reported that industrial effluents are less significantly associated with metal concentrations in receiving waters. Rule et al.45 and Bergbäck et al.46 reported that the dominant sources of metals in receiving waters were not industry-related wastes (point sources) but non-point sources. Such divergence is due to the fact that each E.U. Member State has implemented its own regulations on the level on treatment of industrial effluents prior to their discharge into receiving water bodies.47,48 Depending on country, industrial effluents are first treated in industry-owned treatment facilities, and then disposed of either directly into receiving waters or into public sewerage systems for further treatment. Thus, the relative contribution of industrial effluents will depend on the discharge route and effluent consent of industries.Chemical precipitation is a method predominantly used to reduce metals in industrial effluents. By adding lime, caustic and sulphate, for example, with controlling of pH values, metals are precipitated as hydroxide, carbonate or sulphide.49,50 Some substances, such as arsenic and cadmium, are effectively treated by coprecipitation with iron or alum floc. As an additional process, filtration is applied after precipitation and coprecipitation to remove metal particles or flocs suspended in a clarifer.49 Ion exchange is an alternative to precipitation which can treat even low concentrations of metals in industrial wastewater.51 Moreover, metals can be decreased by adsorption on activated carbon, aluminium oxides, silica, clays and synthetic materials such as zeolite and resins.49 Types of industry/operation emitting metallic substances and treatment methods with their efficiencies are summarised in Table 5.
| Metal | Type of industry or operation | Treatment method | Metal concentration (mg L−1) | |
|---|---|---|---|---|
| Influent | Effluent | |||
| a All information is compiled from the following ref. 49,50. | ||||
| As | Metallurgical industry | Precipitation as the hydroxide | 0.20 | 0.03 |
| Glassware production | 0.50 | 0.03 | ||
| Ceramic production | Precipitation as the sulphide | — | 0.05 | |
| Tannery operation | Coprecipitation with iron floc | 0.31–0.35 | 0.003–0.006 | |
| Dyestuff manufacture | Coprecipitation with alum floc | 0.35 | 0.003–0.005 | |
| Pesticide manufacture | Adsorption (activated carbon) | 0.50 | 0.30 | |
| Organic chemical manufacture | Ion exchange | 2.30 | 0.52 | |
| Inorganic chemical manufacture | ||||
| Petroleum refining operation | ||||
| Cd | Metallurgical industry | Precipitation as the hydroxide | — | 1.00 |
| Ceramic manufacture | 0.54 | |||
| Pigment manufacture | 4.00 | 0.20 | ||
| Electroplating operation | Precipitation as the sulphide | 2200.00 | 1.20 | |
| Textile printing operation | Coprecipitation with iron floc | — | 0.05 | |
| Photography | — | 0.04 | ||
| Chemical industry | Coprecipitation with alum floc | 0.70 | 0.39 | |
| Ion exchange | ||||
| Reverse osmosis | ||||
| Cr6+ | Ink, pigment manufacture | Preicipiation | 140.00 | 1.00 |
| Industrial dye manufacture | 16.00 | 0.06–0.15 | ||
| Aluminium anodizing operation | 47.00–52.00 | 0.30–1.50 | ||
| Metal cleaning operation | 26.00 | 0.44–0.86 | ||
| Metal plating operation | Ion exchange | 17.90 | 1.80 | |
| Electroplating operation | 10.00 | 1.00 | ||
| Wood preservative treatment | 9.00 | 0.20 | ||
| Cu | Metal cleaning operation | Precipitation as the hydroxide | — | 0.20–2.30 |
| Metal plating operation | — | 1.40–7.80 | ||
| Wood preservative manufacture | 0.25–1.10 | 0.10–0.35 | ||
| Fertiliser manufacture | ||||
| Petroleum refining operation | ||||
| Paint, pigment manufacture | ||||
| Steel manufacture | ||||
| Car/aircraft industry | ||||
| Integrated circuit manufacture | ||||
| Metal finishing operation | ||||
| Pb | Storage-battery manufacture | Precipitation as the carbonate | — | 0.01–0.03 |
| Explosive manufacture | Precipitation as the sulphide | — | 0.01 | |
| Textile dyeing operation | Ion exchange | 0.10 | 0.01 | |
| Petroleum industry | ||||
| Ink, pigment manufacture | ||||
| Hg | Electrical/electronic industry | Precipitation as the sulphide | — | 0.01–0.02 |
| Explosive manufacture | Coprecipitation with iron floc | — | <0.001–0.005 | |
| Photographic industry | Coprecipitation with alum floc | — | 0.001–0.01 | |
| Chemical industry | Ion exchange | — | 0.001–0.005 | |
| Petrochemical industry | Adsorption (activated carbon) | — | <0.001–0.02 | |
| Preservative manufacture | ||||
| Pesticide manufacture | ||||
| Ni | Metal-processing industry | Precipitation | 46.00 | 0.80 |
| Car/aircraft industry | 5.60 | 0.60 | ||
| Chemical industry | 7.70 | 2.50 | ||
| Metal finishing operation | 16.30 | 1.00 | ||
| Steel manufacture | ||||
| Printing | ||||
| Zn | Steel manufacture | Precipitation as the hydroxide | 70.00 | 3.00–5.00 |
| Textile industry | 46.00 | 2.90 | ||
| Wood-pulp production | 18.40 | 2.00 | ||
| Metal-processing industry | — | 2.90 | ||
| Metal plating operation | Reverse osmosis | 1.70 | 0.03 | |
| Petroleum refining operation | 7.20 | 0.14 | ||
Effluents from WWTPs
Most E.U. Member States have been using combined sewerage systems for wastewater discharges. In these systems, domestic, commercial and industrial effluents and part of stormwater are combined, and treated in WWTPs before discharging into corresponding receiving waters. Combined sewage contributes significantly to a wide range of total metal concentrations.Several kinds of discarded household products such as detergents, cosmetics, medicine and drinking (tap) waters emit metals.6,7,15,19,52 In general, the quality of domestic sewage is accounted for by population equivalents, being associated with urban development and the lifestyle of people in catchments. Of potential sources, drinking waters are one of the most dominant metal-emitting factors in sewage. Metal concentrations in drinking waters seem to depend not only on regional and geological conditions, but also on plumbing systems and purification processes. Particular elements which provide metals are water pipes and settled sludge in sewage pipes.6,12Table 6 shows the daily flux of metals from drinking water into sewage in Germany.15
| Element (mg cap−1 day−1) | Cd | Cr | Cu | Pb | Zn |
|---|---|---|---|---|---|
| Drinking water | 0.02 | 0.07 | 26.00 | 0.80 | 79.00 |
Copper emissions are caused by the corrosion of drinking water pipes.7,15 Bergbäck et al.46 estimated that copper from drinking waters (4300 kg year−1) was higher than those from brake lining abrasion (3900 kg year−1) in Stockholm. Although their estimation is associated with uncertainty, the results show the relative importance of water pipes as a copper source. Copper can also come from detergents, pesticides, cosmetics, polish, pigments and medicine.19
Chromium and nickel are employed as ingredients of finishing processes in metal products. Rule et al.6 reported that these metals were used to produce stainless steel and household appliances, such as washing machines and dishwashers. The same authors, however, remarked that it was difficult to estimate metal release from these products as well as their potential risk. In addition, chromium is found in drinking waters, pigments, detergents and pesticides, whereas nickel proceeds from food processing, sanitary facilities, cosmetics and pigments.6,15,19,52,60
In terms of other metals, zinc is a metal which is widely used to make numerous household products, including batteries, cosmetics, medicine, caulking compounds, paints, pigments, etc.6,19,52 Arsenic is found in detergents, medicines and pesticides, while cadmium is released from drinking waters, pigments, cosmetics, pesticides, detergents and fertilisers. Lead is emitted from cosmetics, pesticides, fuel, lubricants, paints and pigments, while mercury is derived from amalgams, cosmetics, medicines, paints, pigments, disinfectants and pesticides.7,10,15,19,52
Commercial effluents also enter sewerage systems with quantities of metals. In particular, car washing is reported to dispose of a large quantity of metals. Metals are from traffic waste and atmospheric depositions settled on the surface of vehicles and from detergents used for cleaning. Sörme and Lagerkvist7 reported that about 30%, 9%, 5%, 35%, 4% and 22% of total cadmium, chromium, copper, lead, nickel and zinc, respectively, measured in WWTP influents originated from this business in Stockholm in 1999.
Sewerage systems themselves also give rise to unexpected metal inputs. Sewage pipes, mainly made of copper, are corroded or chemically react with combined wastewater, releasing metals.7,15 Moreover, sewage sediments which settle inside the pipes and delay the transport of sewage into WWTPs have the potential to affect metal concentrations in sewage. These sediments are able to resuspend metals when they have sufficient time to react with sewage. Rule et al.6 showed that stagnant waters in pipes contained higher levels of metals than flowing waters from monitoring influent sewage from domestic, commercial and industrial sources. Gromaire-Mertz et al.61 also performed a sewage sediment-related study, demonstrating that these sediments are strongly associated with a source of particles and organic compounds in sewage. Moreover, Thévenot et al.8 considered sewage sediments as a source of metals in the River Seine. It is impossible to estimate the volume of sewage sediments in the pipes and their impacts on the quality of combined sewage. However, Sörme and Lagerkvist7 reported that sewage sediments were one of the main elements increasing metal concentrations in WWTP influents.
WWTPs are designed to remove suspended solid (SS), biochemical oxygen demand (BOD), chemical oxygen demand (COD) and nutrients from the incoming wastewater.55,62,63 Treated effluents should meet or be below the permissible standards set in regulations. Similar to organic substances, the levels of metals in influent wastewater decrease in WWTPs; however, their reduction is merely an additional advantage during treatment processes.58 Until now, some E.U. Member States, the U.K. for example, have not established metal removal efficiency of WWTPs, whereas they set stringent discharge limits for SS, BOD and COD. This allows WWTP effluents with a substantial quantity of metals to be discharged into receiving waters. With regard to metal speciation in treated effluents, Oliver and Cosgrove53 showed that conventional wastewater treatment is effective for removal of suspended metals compared to that of dissolved metals. Results of their studies are shown in Fig. 2. It can be seen that the percentage of dissolved phases in total metal concentrations increases in effluents through treatment processes. Although WWTPs reduce total metal levels in their incoming wastewater, a large proportion of dissolved metals remains untreated and is disposed of with effluents, affecting the chemical and ecological status in receiving water bodies. Moreover, metal catalysts or additives such as nickel, chromium and zinc used in wastewater treatment also influence the concentrations of these metals in treated effluents.7
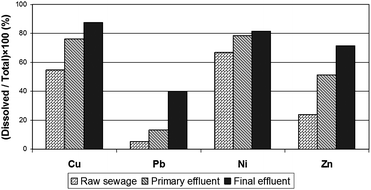 | ||
| Fig. 2 Fractions of dissolved metal concentrations to total metal concentrations in effluents.53 | ||
Metal removals depend on the layout of WWTPs (Table 7). Differences in metal removals in WWTPs result from influent concentrations and chemical metal speciation.64 Operational parameters of WWTPs, such as design of and retention time in sedimentation tanks, aeration and hydraulic retention time, are important factors affecting the degree of metal reduction. In Table 7, it can be seen that nickel is the least removed, and has the highest variation in removal efficiencies. These characteristics are associated with the solubility of nickel. Fig. 2 shows that most of the nickel found in WWTP influents exists in dissolved forms. This has also been reported by previous research.56,64,65 Because nickel is highly soluble, relatively low removal of this metal occurs in WWTPs where the sedimentation and accumulation are the predominant processes for metal reduction.
| Treatment process | Removal efficiency (%) | Reference No. | ||||||
|---|---|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Pb | Hg | Ni | Zn | ||
| Primary sedimentation | 60 | 55 | 33 | 66 | 60 | 15 | 54 | 53 |
| 37 | 38 | 45 | 58 | 56 | 33 | 44 | 64 | |
| 57 | 60 | 40 | 60 | 40 | 8 | 57 | 66 | |
| Activated sludge | 50 | 54 | 60 | 79 | >62 | 1 | 50 | 53 |
| 47 | 82 | 77 | 53 | 72 | 41 | 60 | 56 | |
| 62 | 60 | 43 | 61 | — | 9 | 57 | 67 | |
| 7 | 50 | 25 | 30 | — | 15 | 38 | 68 | |
| 33 | 33 | 86 | 78 | — | 61 | 78 | 69 | |
| 49 | 66 | 68 | 53 | 22 | 25 | 62 | 70 | |
| 85 | 84 | 84 | 82 | 76 | 34 | 81 | 54 | |
| 49 | 57 | 62 | 59 | 63 | 27 | 55 | 71 | |
| 92 | 95 | 90 | 95 | 90 | 33 | 92 | 66 | |
Agricultural activities
Agriculture is considered the most predominant source of metals in catchments in the U.S.A.18,72,73 Ackerman and Schiff16 showed that 0.16 kg km−2 of cadmium, 3.85 kg km−2 of chromium, 5.64 kg km−2 of copper, 1.61 kg km−2 of lead, 2.89 kg km−2 of nickel and 8.28 kg km−2 of zinc were released by runoffs in agricultural lands in Southern California, U.S.A. By carrying out experiments in different cultivation environments, Azimi et al.74 found that a relatively higher portion of copper could be contained in agricultural drainage, compared to other elements such as cadmium, nickel, lead and zinc during overall cultivation process. Several studies reported metals from fertilisers and livestock farming and their pathways into the environment.8 Phosphate fertilisers, for example, are responsible for metal contents in agricultural drainage. They are produced with lower levels of metals; however, appreciable amounts of cadmium and zinc contained in phosphate fertilisers are reported to be released.8,20,74,75 In addition, although the use of mercury-containing pesticides and fungicides has been banned, mercury is still found in the environment, causing contamination in soils and waters.76Sewage sludge is taken into consideration as an alternative to chemical fertilisers. Many E.U. Member States have applied this sludge under related regulations defining the maximum permissible concentrations of total metals in sludge (Table 8). However, the chemical status of metals and their mobilising abilities are more important factors in estimating the toxicity of sewage sludge in the environment rather than their total concentrations.77 In fact, the mobilisation of metals is strongly related not only to their bioavailability but also to the probability of them being released into water bodies during precipitation events. Several researchers have identified that substantial proportions of nickel, cadmium and zinc exist in the form of exchangeable, carbonate and reducible fractions. This characteristic allows these metals to be readily bioavailable and to mobilise in sludge.77–79 Moreover, Zufiaurre et al.80 reported the possibility of metal modification during the addition of sewage sludge to soils. They mentioned that adding sludge caused a change in the physico-chemical conditions of the receiving soils and this could affect metal speciation.
| Country | Maximum metal concentrations (mg kg−1, dry weight) | |||||
|---|---|---|---|---|---|---|
| Cd | Cr | Cu | Pb | Ni | Zn | |
| E.U. | 20–40 | — | 1000–1750 | 750–1200 | 300–400 | 2500–4000 |
| Austria | 3 | 250 | 500 | 250 | 100 | 1200 |
| Belgium | 5 | 200 | 500 | 1000 | 100 | 1500 |
| Denmark | 0.4 | — | — | 120 | 30 | — |
| France | 20 | 1000 | 1000 | 800 | 200 | 3000 |
| Germany | 10 | 900 | 800 | 900 | 200 | 2500 |
| Greece | 20–40 | — | 1000–1750 | 750–1200 | 300–400 | 2500–4000 |
| Holland | 1 | 50 | 75 | 100 | 30 | 200 |
| Italy | 10 | 600 | 600 | 500 | 200 | 2500 |
| Luxembourg | 1.5 | 100 | 100 | 150 | 50 | 400 |
| Slovenia | 5 | 500 | 600 | 500 | 80 | 2000 |
| Spain | 10 | 400 | 50 | 300 | 120 | 1100 |
Other potential sources
Apart from the sources mentioned above, there are other factors which can increase metal concentrations in river basins. Sediments, for example, can influence the chemical quality of their overlying waters. The origin of sediments is associated with natural mechanisms, such as weathering and erosion of minerals, organic materials and surface soils.81 However, the influence of human-related activities results in increasing levels of dangerous substances in corresponding waters. Under hydrological conditions, the sedimentation of these substances, then, occurs based on physico-chemical reactions such as accumulation and sorption to organic materials or suspended solids.82,83 As such, the components of river sediments, especially in urban areas, include anthropogenic influences.84 Total metal concentrations of sediments in Europe compiled by Fraunhofer-Institut2 are presented in Table 9.| Metal | Total metal concentrations in sediments | |||
|---|---|---|---|---|
| Median (µg L−1) | Mean (µg L−1) | S.D.a | No. of sample | |
| a Standard deviation of mean value. | ||||
| As | 8.0 | 14.4 | 19.1 | 2708 |
| Cd | 2.0 | 12.4 | 91.0 | 2873 |
| Cr | 50.0 | 73.7 | 29.3 | 3409 |
| Cu | 38.3 | 127.1 | 1269.0 | 3153 |
| Pb | 60.0 | 140.1 | 265.8 | 3091 |
| Hg | 1.0 | 2.4 | 3.1 | 2735 |
| Ni | 27.0 | 31.8 | 7.5 | 2909 |
| Zn | 219.3 | 565.1 | 638.8 | 2833 |
Although sediments are an integral part of the aquatic system, several studies have identified them as a separate potential source of metals and other dangerous substances.81,85,86 This is due to resuspension causing release of entrapped soluble substances and oxidation of solid compounds in sediments. Sediment resuspension is induced by natural sediment transport, strong currents, flooding and dredging and by bioturbation and bioirrigation.85,87 Simpson et al.87 studied effects of anoxic sediment resuspension on dissolved metal concentrations in river waters and sea waters. They demonstrated that emission levels of dissolved copper and nickel adsorbed to the surface of sulphides increased with a rise in resuspension time. Su et al.88 calculated bioaccumulation loads of chemicals in several benthic species during the dredging process in the Passaic River, New Jersey, U.S.A. They showed that due to an increase in dissolved metal concentrations in river waters affected by dredging, benthic species had more metal uptake than they took in ordinary river conditions. Moreover, from their direct exposure, sediments can chemically react with overlying waters, releasing metals in solution. In the sediment–water interface, Audry et al.86 estimated the contribution of sediments to dissolved metal concentrations in overlying waters to be about 20%, 30% and 10% of total dissolved cadmium, copper and zinc loads, respectively.
Sediment depth remobilisation associated with the exposure of historically accumulated metals to overlying waters is another important factor affecting metal levels. The characteristics and quantities of chemicals in sediments depend on the depth of sediments.89–91 Since the industrial revolution, substantial quantities of metal-bearing suspended sediments have been settled, and steadily accumulated along with natural chemical components in sediments.92 Continuous natural effects such as erosion and anthropogenic activities such as dredging can disturb sediment environments, causing the removal of recent (or surface) sediments and the consequent exposure of old sediments to overlying waters. As a result, buried particulate compounds with high metal levels can unveil and affect water quality.93,94
The mining industry has the potential to emit metals in the environment. Mine waste, such as tailing, disposed rock and mine waters, is produced during mining, mineral processing (crushing, grinding, separation, flotation, etc.) and metallurgical extraction, causing serious metal contamination in the environment.95,96 In particular, dewatering processes, acid mine drainage (AMD), generated from mine waste, metal ore and coal seams, and the transportation or the dissolution of metals by rainwater at mine waste areas contribute to water contamination. As a contributory factor, oxidising sulphide mineral, especially pyrite (FeS2), has been reported to be the main process in accelerating metal release from minerals as well as in producing acid waters at mine sites.96 If mining areas are situated within a river basin, they cause significant metal levels in receiving waters.
Landfills are used as a method to dispose of municipal and industrial solid waste. In the U.K., for example, 77% of total municipal solid waste was landfilled in 2001 to 2002.97 Solid waste going to landfills is classified as a hazardous or a non-hazardous material prior to disposal.98 However, waste disposal to landfills can result in serious environmental problems. When metal-bearing solid waste, mainly in the form of sulphides, hydroxides, carbonates and organic matters, is oxidised by waters, it releases metals or its compounds.99 Rainwater percolating through the layer of waste in landfills creates landfill leachates.100 These leachates contain high levels of dangerous substances, causing groundwater or surface water contamination.101,102 The mobility, toxicity and bioavailability of metals in landfill leachates depend on their origin, retention time and exposure conditions.103 Although methods such as leachate transfer, biological, physical and chemical treatment and membrane processes have been developed to minimise the levels of metals and other dangerous substances in landfill leachates,101 these are still considered to be one of the potential metal carriers in receiving waters.
Sporting activities are also known to be a source of metal contamination at catchment levels. Boating is one emitting metals in receiving waters. Schiff et al.104 reported that antifouling paints, sacrificial anodes, motor exhausts and the leakage of hazardous materials released metals into the aquatic environment. Among them, antifouling paints used for preventing growth of aquatic organisms on vessel hulls are considered to be the most crucial factor.105–107 Copper is the main biocidal component of antifouling paints, which is in the form of cuprous oxide (Cu2O). This metal can exist as a dissolved phase in waters or as a paint fragment in sediments.107 Schiff et al.104 tested antifouling paints with different coating methods to estimate their monthly copper emission rates. The study found that hard vinyl and modified epoxy coating released 3.7 µg cm−2 day−1 and 4.3 µg cm−2 day−1 of copper, respectively. Some researchers reported that zinc was also released from antifouling paints and sacrificial anodes.107 Meanwhile, other sporting activities emitting metals in receiving waters include shooting and fishing. Bullets (pellets), disposed sinkers and jigs from both activities can cause a lead increase in the aquatic environment.108–110
Discussion
The implementation of the WFD results in unifying the policy of water management and quality levels of priority substances in Europe.111 In the context of the Directive, E.U. Member States develop their statutes, and set RBMPs within the boundary of a river basin district. Considering geographical and hydrological status and natural background levels, they should also address contaminants of national concern. If contaminants are proved to cause risk to the ecological and the aquatic environment, their EQSs should be established and used as national levels.1In the England and Wales, the WFD was transposed into regulations in 2003, named “the Water Environment (Water Framework Directive) (England and Wales) Regulations 2003”.112 The Environment Agency divided the area of England and Wales into 11 River Basin Districts, and designed the direction of management process.113 For each River Basin District, a draft of a RBMP was proposed in December 2008 to meet the requirement set by the WFD.114 To identify toxic constituents at national levels, the U.K. Technical Advisory Group (UKTAG) has been assessing pollutants and their EQSs, evaluating that arsenic, chromium, copper and zinc are a group of substances classified as toxic constituents in fresh and salt waters in the U.K.115 Italy is another country making an effort to fulfill the requirement of the WFD. The country established environmental regulations, “the Legislative Decree April 3, 2006 no. 152 (D.L. 152/2006)”, to maintain surface water quality based on the WFD, adding chromium, copper and zinc in the list of micro-contaminants required to be assessed to define the status of water quality.116 As a candidate member of the E.U., Turkey has also developed regulations including “Law of Environment (1983/2872)” and “Regulation of Water Pollution Control (2004/25687)” recently. Six River Basin Districts have been determined so far to establish RBMPs in line with their topographical and hydrological conditions. However, the country shows limited progress on the implementation of the WFD compared to other E.U. Member States.117
According to the WFD, there is a great need for designing a sustainable management plan for receiving water quality. In this plan, source assessment should be considered as an important issue and be carried out as a fundamental stage. Emission sources cannot show the same degree of impact on the aquatic environment at every investigation period. In this case, variation in contamination patterns caused by changes in source contribution should be estimated and reflected in water management, which will be complex and site-specific. From source assessment, authorities responsible for water management can identify main sources and their contributions to substance emissions at catchment levels. The outcome of this assessment is, then, used in deciding the best management option for restoring the quality of receiving waters.
Metals have more emission sources than other dangerous substances at catchment levels. Therefore, the significance of source assessment for these substances is much greater to restore their chemical status in the context of the WFD. In the absence of particular metal producing factors, for example, mining and related waste, the major sources of metals may not differ significantly, and their contributions may be decided by the types of land-use and environmental conditions. The development of statistical and simulation models for metal source ascriptions improves the quality of source assessment. To date, some statistical methods, such as Factor Analysis (FA) and Principal Component Analysis (PCA), have been introduced to estimate emission sources and their contributions to metal concentrations in receiving waters,118,119 whereas the Storm Water Management Model (SWMM), one of the computerised models, has been used to carry out proper stormwater management.120 However, they are not considered as optimised methods.121 Experimental work alone cannot explain the whole process of water contamination at catchment levels. Better formulated models will contribute to estimating the total levels of metals and their pathways, and result in upgraded management processes.
Many other issues need to be addressed to improve the quality of source assessment at catchment levels. Further investigation is necessary to find unknown sources of metals. Sörme et al.122 reported that over 30% of the total metal concentrations in WWTP influents were from unknown sources in Sweden (Fig. 3). Undoubtedly, potential sources discussed in this paper are significant factors influencing the quality of receiving waters. However, there are still unrecognised sources of metals, and these sources can cause an impediment to source assessment and the design of water management at catchment levels.
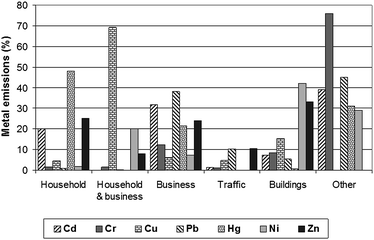 | ||
| Fig. 3 Source contributions to the levels of metals in Henriksdal WWTP influents in Stockholm in 1999.122 | ||
A study of source contributions to the chemical distribution of metals in receiving waters also needs to be performed for proper source assessment. Depending on sources and reaction during transport, metals exist in various speciation and present different physico-chemical status.123 Chemical metal speciation impacts metal mobility, bioavailability and toxicity resulting in the diversity of the extent of metal contaminations in receiving water bodies. This means that the emitting forms of these substances influence the degree of their impacts on the quality of receiving waters. Little work has been undertaken so far in order to understand chemical forms of metals originating from anthropogenic sources in the aquatic environment.
The role of parameters affecting the degree of metal emissions should be elucidated. Each river basin has specific societal and environmental conditions. Depending on changes in these conditions, emission sources, especially non-point sources, show different relative contributions to the levels of metals in river basins. Some research demonstrates that the levels of metals in water bodies depend on the characteristics of each catchment,29 whereas it is suggested that total rainfall, drainage and impervious area, land-use and annual climatic conditions can change the quality of stormwater and as a result affect the levels of metals in receiving waters.37,124 However, previous studies to estimate effects of these societal and environmental conditions and their correlation have provided inconsistent results.
In this paper, relative source contributions to the levels of metals in receiving waters are not considered. Source contributions cannot be generalised at catchment levels in that they are highly dependent on natural and anthropogenic factors as mentioned above. It is certain that each catchment shows a different trend of source contributions to metal emissions. Due to this reason, relative source contributions should be estimated on a case-by-case basis.
Conclusion
This paper reviewed predominant anthropogenic sources of metallic substances at catchment levels. It also discussed factors which need to be taken into account in order to improve the quality of source assessment. This study found that stormwater, industrial and WWTPs effluents, agricultural drainage, sediments, mining drainage and landfill leachates were the major sources of metals in the aquatic environment.The impact of metals varies according to societal and environmental conditions. For these substances, source assessment should be performed at a fundamental stage to monitor and reduce the concentrations at catchment levels under the WFD. Statistical and simulation models also need to be developed, not only to support source assessment and experimental studies but also to maintain chemical status in the aquatic environment. To improve the quality of source assessment, more research is needed to determine the role of societal and environmental parameters in conjunction with an investigation of chemical distribution of metals in receiving waters caused by each emission source. Based on this, the relative source contributions to metal emissions need to be performed on a case-by-case basis.
References
- European Commission, Official Journal of the European Communities, 2000, L327, 321–372 Search PubMed.
- Fraunhofer-Institut, Revised Proposal for a List of Priority Substances in the Context of the Water Framework Directive(COMMPS Procedure): Final Report (Declaration ref.: 98/788/3040/DEB/E1), http://ec.europa.eu/environment/water/water-framework/preparation_priority_list.htm, Accessed 22 March 2009.
- European Commission, Official Journal of the European Communities, 2001, L331, 331–335 Search PubMed.
- European Commission, Official Journal of the European Union, 2008, L348, 384–397 Search PubMed.
- A. P. Davis, M. Shokouhian and S. Ni, Chemosphere, 2001, 44, 997–1009 CrossRef.
- K. L. Rule, S. D. W. Comber, D. Ross, A. Thornton, C. K. Makropoulos and R. Rautiu, Chemosphere, 2006, 63, 64–72 CrossRef CAS.
- L. Sörme and R. Lagerkvist, Sci. Total Environ., 2002, 298, 131–145 CrossRef CAS.
- D. R. Thévenot, R. Moilleron, L. Lestel, M. C. Gromaire, V. Rocher, P. Cambier, P. Bonté, J. L. Colin, C. de Pontevès and M. Meybeck, Sci. Total Environ., 2007, 375, 180–203 CrossRef CAS.
- European Commission, Official Journal of the European Union, 2006, L64, 52–59 Search PubMed.
- D. Jenkins, Water Environ. Res., 1998, 70, 980–983 CrossRef CAS.
- S. Sindern, R. F. S. Lima, J. Schwarzbauer and R. A. Petta, Environ. Geol., 2007, 52, 731–737 CrossRef CAS.
- UKWIR, Development of a protocol for estimating effluent emissions of pollution inventory substances from sewage treatment works, Volume I: main report Report Ref. No. 02/WW/25/1, UK Water Industry Research, London, 2002 Search PubMed.
- C. S. C. Wong, X. Li and I. Thornton, Environ. Pollut., 2006, 142, 1–16 CrossRef.
- P. Göbel, C. Dierkes and W. G. Coldewey, J. Contam. Hydrol., 2007, 91, 26–42 CrossRef CAS.
- M. Koch and W. Rotard, Water Sci. Technol., 2001, 43, 67–74 Search PubMed.
- D. Ackerman and K. Schiff, J. Environ. Eng., 2003, 129, 308–317 CrossRef CAS.
- L. D. Sabin, J. H. Lim, K. D. Stolzenbach and K. C. Schiff, Water Res., 2005, 39, 3929–3937 CrossRef CAS.
- J. R. Miller and S. M. O. Miller, Contaminated Rivers: A Geomorphological-Geochemical Approach to Site Assessment and Remediation, Springer, 2007 Search PubMed.
- T. Stephenson, in Heavy Metals in Wastewater and Sludge Treatment Processes: Sources, Analysis and Legislation, ed. J. N. Lester, CRC Press, Florida, 1987, vol. I, pp. 31–63 Search PubMed.
- T. B. Councell, K. U. Duckenfield, E. R. Landa and E. Callender, Environ. Sci. Technol., 2004, 38, 4206–4214 CrossRef CAS.
- J. Sternbeck, Å. Sjödin and K. Andréasson, Atmos. Environ., 2002, 36, 4735–4744 CrossRef CAS.
- K. Adachi and Y. Tainosho, Environ. Int., 2004, 30, 1009–1017 CrossRef CAS.
- S. Charlesworth, M. Everett, R. McCarthy, A. Ordóñez and E. de Miguel, Environ. Int., 2003, 29, 563–573 CrossRef CAS.
- A. W. Gertler, J. A. Gillies and W. R. Pierson, Water, Air, Soil Pollut., 2000, 123, 203–214 CrossRef CAS.
- G. C. Lough, J. J. Schauer, J. S. Park, M. M. Shafer, J. T. Deminter and J. P. Weinstein, Environ. Sci. Technol., 2005, 39, 826–836 CrossRef CAS.
- K. Skarżyńska, Ż. Polkowska, J. Namieśnik and A. Przyjazny, Crit. Rev. Anal. Chem., 2007, 37, 91–105 CrossRef CAS.
- A. Wróbel, E. Rokita and W. Maenhaut, Sci. Total Environ., 2000, 257, 199–211 CrossRef CAS.
- M. Legret and C. Pagotto, Sci. Total Environ., 1999, 235, 143–150 CrossRef CAS.
- W. L. F. Brinkmann, GeoJournal, 1985, 11, 277–283.
- M. E. Barrett, L. B. Irish, J. F. Malina and R. J. Charbeneau, J. Environ. Eng., 1998, 124, 131–137 CrossRef CAS.
- J. J. Sansalone and S. G. Buchberger, J. Environ. Eng., 1997, 123, 134–143 CrossRef CAS.
- J. M. Ondov, W. H. Zoller and G. E. Gordon, Environ. Sci. Technol., 1982, 16, 318–328 CrossRef CAS.
- D. S. T. Hjortenkrans, B. G. Bergbäck and A. V. Häggerud, Environ. Sci. Technol., 2007, 41, 5224–5230 CrossRef CAS.
- J. Förster, Water Sci. Technol., 1996, 33, 39–48 CrossRef.
- H. Stechmann and W. Dannecker, J. Aerosol Sci., 1990, 21, 287–290.
- T. A. Pakkanen, K. Loukkola, C. H. Korhonen, M. Aurela, T. Mäkelä, R. E. Hillamo, P. Aarnio, T. Koskentalo, A. Kousa and W. Maenhaut, Atmos. Environ., 2001, 35, 5381–5391 CrossRef CAS.
- W. He, I. Odnevall Wallinder and C. Leygraf, Water, Air, Soil Pollut. Focus, 2001, 1, 67–82 Search PubMed.
- A. U. Leuenberger-Minger, M. Faller and P. Richner, Mater. Corros., 2002, 53, 157–164 CrossRef CAS.
- J. Förster, Water Sci. Technol., 1999, 39, 137–144 CrossRef.
- A. Andrés, M. C. Díaz, A. Coz, M. J. Abellán and J. R. Viguri, J. Eur. Ceram. Soc., 2009, 29, 1869–1877 CrossRef CAS.
- J. C. Chow, J. G. Watson, L. L. Ashbaugh and K. L. Magliano, Atmos. Environ., 2003, 37, 1317–1340 CrossRef CAS.
- F. Amato, M. Pandolfi, M. Viana, X. Querol, A. Alastuey and T. Moreno, Atmos. Environ., 2009, 43, 1650–1659 CrossRef CAS.
- D. Persson and V. Kucera, Water, Air, Soil Pollut. Focus, 2001, 1, 133–150 Search PubMed.
- USEPA, Report to Congress: impacts and control of CSOs and SSOs, US Environmental Protection Agency, Washington, D.C., 2004 Search PubMed.
- K. L. Rule, S. D. W. Comber, D. Ross, A. Thornton, C. K. Makropoulos and R. Rautiu, Water Environ. J., 2006, 20, 177–184 Search PubMed.
- B. Bergbäck, K. Johansson and U. Mohlander, Water, Air, Soil Pollut. Focus, 2001, 1, 3–24 Search PubMed.
- T. A. Kurniawan, G. Y. S. Chan, W. H. Lo and S. Babel, Sci. Total Environ., 2006, 366, 409–426 CrossRef CAS.
- A. Oltmann, U. Scherer and S. Fuchs, Proceedings of the 7th International Conference on Diffuse Pollution & Basin Management, Dublin, Ireland, 2003 Search PubMed.
- W. W. Eckenfelder, Industrial Water Pollution Control, 3rd edn., McGraw-Hill, 2000 Search PubMed.
- J. W. Patterson, Industrial Wastewater Treatment Technology, 2nd edn., Butterworth Publishers, Boston, London, Sydney, Wellington, Durban, Toronto, 1985 Search PubMed.
- F. Woodard, Industrial Waste Treatment Handbook, Butterworth-Heinemann, 2001 Search PubMed.
- L. Sörme, B. Bergbäck and U. Lohm, Water, Air, Soil Pollut. Focus, 2001, 1, 213–227 Search PubMed.
- B. G. Oliver and E. G. Cosgrove, Water Res., 1974, 8, 869–874 CrossRef CAS.
- USEPA, Fate of priority pollutants in publicly owned treatment works, Final report, Volume I, US Environmental Protection Agency, Washington, D.C., 1982 Search PubMed.
- A. D. Oliveira, A. Bocio, T. M. B. Trevilato, A. M. M. Takayanagui, J. L. Domingo and S. I. Segura-Muñoz, Environ. Sci. Pollut. Res., 2007, 14, 483–489 CrossRef CAS.
- K. Y. Chen, C. S. Young, T. K. Jan and N. Rohatgi, J. Water Pollut. Control Fed., 1974, 46, 2663–2675 Search PubMed.
- H. Brombach, G. Weiss and S. Fuchs, Water Sci. Technol., 2005, 51, 119–128 Search PubMed.
- M. Karvelas, A. Katsoyiannis and C. Samara, Chemosphere, 2003, 53, 1201–1210 CrossRef CAS.
- R. Pitt, A. Maestre and R. Morquecho, The National Stormwater Quality Database (NSQD, version 1.1)http://rpitt.eng.ua.edu/Research/ms4/Paper/Mainms4paper.html, Accessed 11 August 2008.
- ICON, Pollutants in urban wastewater and sewage sludge, Final report, http://www.environmental-expert.com/resultEachArticle.aspx%3F%20cid%20%3D%208819%26codi%20%3D%202586%26idproducttype%20%3D%206, Accessed 22 March 2009.
- M. C. Gromaire-Mertz, G. Chebbo and M. Saad, Water Sci. Technol., 1998, 37, 35 CrossRef CAS.
- DEFRA, Sewage treatment in the UK: UK implementation of the EC Urban Waste Water Treatment Directive, http://www.defra.gov.uk/environment/water/quality/uwwtd/report02/pdf/uwwtreport2.pdf, Accessed 22 March 2009.
- C. Gagnon and I. Saulnier, Environ. Pollut., 2003, 124, 47–55 CrossRef CAS.
- J. N. Lester, in Heavy Metals in Wastewater and Sludge Treatment Processes: Treatment and Disposal, ed. J. N. Lester, CRC Press, Florida, 1987, vol. II, pp. 1–14 Search PubMed.
- A. C. Rossin, R. M. Sterritt and J. N. Lester, Water, Air, Soil Pollut., 1982, 17, 185–198 CAS.
- WERF, Chemical characteristics and solids uptake of heavy metals in wastewater treatment, Water Environment Research Foundation, 2000 Search PubMed.
- J. A. Davis and J. Jacknow, J. Water Pollut. Control Fed., 1975, 47, 2292–2297 Search PubMed.
- P. Roberts, H. R. Hegi, A. Weber and H. R. Krähenbähl, Prog. Water Technol., 1977, 8, 301–306 Search PubMed.
- J. N. Lester, R. M. Harrison and R. Perry, Sci. Total Environ., 1979, 12, 13–23 CrossRef CAS.
- S. E. Esmond, A. C. Petrasek Jr., H. W. Wolf and D. C. Andrews, The removal of metals and viruses in advanced wastewater treatment sequences, US Environmental Protection Agency, Cincinnati, 1980 Search PubMed.
- J. N. Lester, in Heavy Metals in Wastewater and Sludge Treatment Processes: Treatment and Disposal, ed. J. N. Lester, CRC Press, Florida, 1987, vol. II, pp. 15–40 Search PubMed.
- R. Keirle and C. Hayes, Water Environ. J., 2007, 21, 208–216 Search PubMed.
- D. W. Moeller, Environmental Health, 3rd edn, Harvard University Press, 2005 Search PubMed.
- S. Azimi, P. Cambier, I. Lecuyer and D. Thévenot, Water, Air, Soil Pollut., 2004, 157, 295–313 CrossRef CAS.
- M. J. McLaughlin, K. G. Tiller, R. Naidu and D. P. Stevens, Aust. J. Soil Res., 1996, 34, 1–54 CrossRef CAS.
- Q. Wang, D. K. Kim, D. D. Dionysiou, G. A. Sorial and D. Timberlake, Environ. Pollut., 2004, 131, 323–336 CrossRef CAS.
- A. Fuentes, M. Lloréns, J. Sáez, M. I. Aguilar, J. F. Ortuño and V. F. Meseguer, Bioresour. Technol., 2008, 99, 517–525 CrossRef CAS.
- E. A. Álvarez, M. C. Mochón, J. C. J. Sánchez and M. T. Rodríguez, Chemosphere, 2002, 47, 765–775 CrossRef CAS.
- J. Ščančar, R. Milačič, M. Stražar and O. Burica, Sci. Total Environ., 2000, 250, 9–19 CrossRef CAS.
- R. Zufiaurre, A. Olivar, P. Chamorro, C. Nerín and A. Callizo, Analyst, 1998, 123, 255–259 RSC.
- J. Brils, Annali dell'Istituto Superiore di Sanità, 2008, 44, 218–223 Search PubMed.
- G. A. Burton Jr, Limnology, 2002, 3, 65–76 CrossRef CAS.
- P. Wright and C. F. Mason, Sci. Total Environ., 1999, 226, 139–156 CrossRef CAS.
- G. H. Old, G. J. L. Leeks, J. C. Packman, B. P. G. Smith, S. Lewis and E. J. Hewitt, Sci. Total Environ., 2006, 360, 98–108 CrossRef CAS.
- SedNet, Contaminated sediments in European river basins, Final draft, http://www.sednet.org/download/Sednet_booklet_final_draft.pdf, Accessed 22 March 2009.
- S. Audry, J. Schäfer, G. Blanc, C. Bossy and G. Lavaux, Appl. Geochem., 2004, 19, 769–786 CrossRef CAS.
- S. L. Simpson, S. C. Apte and G. E. Batley, Environ. Sci. Technol., 1998, 32, 620–625 CrossRef CAS.
- S. H. Su, L. C. Pearlman, J. A. Rothrock, T. J. Iannuzzi and B. L. Finley, Environ. Manage., 2002, 29, 234–249 CrossRef.
- A. J. Horowitz, A Primer on Sediment-Trace Element Chemistry, 2nd edn, Lewis Publishers, 1991 Search PubMed.
- R. C. Swartz, D. W. Schults, J. O. Lamberson, R. J. Ozretich and J. K. Stull, Mar. Environ. Res., 1991, 31, 215–225 CrossRef CAS.
- A. S. Hursthouse, J. M. Matthews, J. E. Figures, P. Iqbal-Zahid, I. M. Davies and D. H. Vaughan, Environ. Geochem. Health, 2003, 25, 171–203 Search PubMed.
- A. J. de Groot, in Metal Contaminated Aquatic Sediments, ed. H. E. Allen, Ann Arbor Press, 1995, pp. 1–20 Search PubMed.
- A. B. Cundy, I. W. Croudace, A. Cearreta and M. J. Irabien, Appl. Geochem., 2003, 18, 311–325 CrossRef CAS.
- A. J. Plater, J. Ridgway, P. G. Appleby, A. Berry and M. R. Wright, Mar. Pollut. Bull., 1999, 37, 343–360 CrossRef.
- J. S. Lee, S. W. Lee, H. T. Chon and K. W. Kim, J. Geochem. Explor., 2008, 96, 231–235 CrossRef CAS.
- B. G. Lottermoser, Mine Wastes: Characterization, Treatment and Environmental Impacts, Springer-Verlag, Berlin Heidelberg, 2003 Search PubMed.
- DEFRA, Review of Environmental and Health Effects of Waste Management: Municipal Solid Waste and Similar Wastes, http://www.defra.gov.uk/environment/waste/research/health/pdf/health-report.pdf, Accessed 22 March 2009.
- J. Scott, D. Beydoun, R. Amal, G. Low and J. Cattle, Crit. Rev. Environ. Sci. Technol., 2005, 35, 239–332 CrossRef CAS.
- M. Östman, O. Wahlberg, S. Ågren and A. Mårtensson, Waste Manage. (Oxford), 2006, 26, 29–40 Search PubMed.
- P. Kjeldsen, M. A. Barlaz, A. P. Rooker, A. Baun, A. Ledin and T. H. Christensen, Crit. Rev. Environ. Sci. Technol., 2002, 32, 297–336 CrossRef CAS.
- S. Renou, J. G. Givaudan, S. Poulain, F. Dirassouyan and P. Moulin, J. Hazard. Mater., 2008, 150, 468–493 CrossRef CAS.
- R. J. Slack, J. R. Gronow and N. Voulvoulis, Sci. Total Environ., 2005, 337, 119–137 CrossRef CAS.
- J. K. Øygard, E. Gjengedal and H. J. Mobbs, J. Hazard. Mater., 2008, 153, 751–758 CrossRef CAS.
- K. Schiff, D. Diehl and A. Valkirs, Mar. Pollut. Bull., 2004, 48, 371–377 CrossRef CAS.
- S. D. W. Comber, M. J. Gardner and A. B. A. Boxall, J. Environ. Monit., 2002, 4, 417–425 RSC.
- J. A. Nichols, Environ. Manage., 1988, 12, 243–247 CrossRef.
- A. Turner, S. Fitzer and G. A. Glegg, Environ. Pollut., 2008, 151, 176–181 CrossRef CAS.
- J. R. Craig, J. D. Rimstidt, C. A. Bonnaffon, T. K. Collins and P. F. Scanlon, Bull. Environ. Contam. Toxicol., 1999, 63, 312–319 CrossRef CAS.
- M. Migliorini, G. Pigino, N. Bianchi, F. Bernini and C. Leonzio, Environ. Pollut., 2004, 129, 331–340 CrossRef CAS.
- A. M. Scheuhammer and S. L. Norris, Ecotoxicology, 1996, 5, 279–295 CrossRef CAS.
- M. Kaika and B. Page, Eur. Env., 2003, 13, 314–327 CrossRef.
- U. K. Legislation, The Water Environment (Water Framework Directive) (England and Wales) Regulations 2003, http://www.opsi.gov.uk/si/si2003/20033242.htm, Accessed 4 June 2009.
- DEFRA, River Basin Planning Guidance, DEFRA, London, 2006 Search PubMed.
- Environment Agency, Water Framework Directive – Have your say on water: draft River Basin Management Plans consultation, http://www.environment-gency.gov.uk/research/planning/33106.aspx, Accessed 4 June 2009.
- UKTAG, Proposals for environmental quality standards for annex VIII substances, Final, http://www.wfduk.org/stakeholder_reviews/stakeholder_review_1-2007/LibraryPublicDocs/final_specific_pollutants, Accessed 22 March 2009.
- V. Naddeo, T. Zarra and V. Belgiorno, Environ. Sci. Policy, 2007, 10, 243–249 CrossRef.
- M. Moroglu and M. S. Yazgan, Desalination, 2008, 226, 271–278 CrossRef CAS.
- J. Huang, P. Du, C. Ao, M. Ho, M. Lei, D. Zhao and Z. Wang, Bull. Environ. Contam. Toxicol., 2007, 79, 650–654 CrossRef CAS.
- H. Pekey, D. Karakaş and M. Bakoğlu, Mar. Pollut. Bull., 2004, 49, 809–818 CrossRef CAS.
- L. A. Rossman, Storm Water Management Model: user's manual (version 5.0) (EPA/600/R-05/040), http://www.epa.gov/ednnrmrl/models/swmm/epaswmm5_manual.pdf, Accessed 23 March 2009.
- D. M. Revitt, L. Scholes and J. B. Ellis, Water Sci. Technol., 2008, 57, 1257–1264 Search PubMed.
- L. Sörme, A. Lindqvist and H. Söderberg, Environ. Manage., 2003, 31, 421–428 CrossRef.
- J. W. Moore and S. Ramamoorthy, Heavy Metals in Natural Waters, Springer-Verlag, New York, 1984 Search PubMed.
- P. L. Brezonik and T. H. Stadelmann, Water Res., 2002, 36, 1743–1757 CrossRef CAS.
Footnote |
| † Part of a themed issue dealing with water and water related issues. |
| This journal is © The Royal Society of Chemistry 2010 |
