Sustainability: the capacity of smokeless biomass pyrolysis for energy production, global carbon capture and sequestration
James Weifu
Lee†
*a,
Bob
Hawkins
b,
Danny M.
Day
c and
Donald C.
Reicosky‡
d
aOak Ridge National Laboratory, Oak Ridge, TN 37831, USA. E-mail: JLee349@jhu.edu
bBiocharConsulting, 375 Rumson Rd, Athens, GA 30605, USA
cEprida Power and Life Sciences, Inc., 6300 Powers Ferry Rd, #307, Atlanta, GA 30339, USA
dUSDA-ARS North Central Soil Conservation Research Lab, 803 Iowa Ave., Morris, Minnesota 56267, USA
First published on 7th July 2010
Abstract
Smokeless biomass pyrolysis for biochar and biofuel production is a possible arsenal for global carbon capture and sequestration at gigatons of carbon (GtC) scales. The United States can annually harvest over 1.3 Gt (gigaton) of dry biomass. Use of the smokeless (clean and efficient) biomass-pyrolysis technology would enable the United States to converts its 1.3 Gt of annually harvestable biomass to biochar products equivalent to 325 million tons of stable carbon plus significant amount of biofuels including syngas and bio-oils. Currently, the world could annually harvest more than 6.5 GtC y−1 of biomass. The 6.5 GtC y−1 of biomass could be converted to biochar (3.25 GtC y−1) and biofuels (with heating value equivalent to that of 6500 million barrels of crude oil). Because biochar is mostly not digestible to microorganisms, a biochar-based soil amendment could serve as a permanent carbon-sequestration agent in soils/subsoil earth layers for thousands of years. By storing 3.25 GtC y−1 of biochar (equivalent to 11.9 Gt of CO2 per year) into soil and/or underground reservoirs alone, it would offset the world's 8.67 GtC y−1 of fossil fuel CO2 emissions by about 38%. The worldwide maximum capacity for storing biochar carbon into agricultural soils (1411 million hectares) is estimated to be about 428 GtC. It may be also possible to provide a global carbon “thermostat” mechanism by creating biochar carbon energy storage reserves. This biomass-pyrolysis “carbon-negative” energy approach merits serious research and development worldwide to help provide clean energy and control global warming for a sustainable future of human civilization on Earth.
 James Weifu Lee | James Weifu Lee, a senior scientist, conducted energy research for more than 15 years at US Department of Energy's Oak Ridge National Laboratory (ORNL) after completed his PhD training in photosynthesis/plant physiology, physical chemistry, and biochemistry at Cornell University in 1992. At ORNL, he laid a framework of putting solidified CO2 (e.g., bicarbonate) into soil for carbon management in 1998. Subsequently, he and Danny Day co-initiated the approach of smokeless biomass pyrolysis with biochar fertilizer as soil amendment and carbon sequestration agent in 2002. Dr Lee is currently at Johns Hopkins University with continued interest in helping move this work forward. |
 Bob Hawkins | Bob Hawkins is currently a research project management consultant in the field of clean syngas production and pyrolysis. His previous activities include director of research for Eprida's Research and Development facility at UGA's BioConversion Center where he constructed, operated and maintained a biomass to hydrogen pilot plant. Bob served as project manager for research projects conducted by Eprida for US DOE and Iowa State University and conducted research on biochar production and utilization with partners such as NREL, ORNL, USDA, and University of Georgia. His work has been reported in publications including Nature, Scientific American and Time magazines. |
 Danny M. Day | Danny M. Day, President of Eprida Power and Life Sciences, is a visionary and pioneer in modern biomass pyrolysis for biochar and biofuel production. He and James W. Lee co-initiated the approach of smokeless biomass pyrolysis using biochar fertilizer as soil amendment and carbon sequestration agent through a 2002 US provisional patent application and followed by PCT patent application. He organized and sponsored the first two US biochar scientific meetings and held briefings around the world to educate and further the use of biochar. Mr Day was named one of the 25 Brave Thinkers of 2009 by The Atlantic magazine. He currently focuses on further development of the technology through providing consulting, testing services, product/system development and bench scale equipment to help move the work forward. |
 Donald C. Reicosky | Donald C. Reicosky is a retired Soil Scientist from the USDA-ARS, North Central Soil Conservation Research Laboratory, Morris, MN, and adjunct Professor in the Soil, Water and Climate Department, University of Minnesota, St Paul with degrees from Ohio State University and the University of Illinois. Early research described crop response and water use on conventional till and no-till systems with and without irrigation and use of a canopy gas exchange measurement technique to evaluate short-term tillage-induced CO2 flux from soil. Research prior to retirement showed tillage-induced losses after moldboard plowing can help explain the long-term decline in soil carbon associated with intensive cropping and explains environmental quality issues related to soil carbon management, carbon cycling, biomass removal for bio-energy and carbon sequestration as biochar. |
Broader contextSmokeless biomass pyrolysis for biochar and biofuel production is a possible arsenal for global carbon capture and sequestration at gigatons of carbon (GtC) scales. Currently, the world could annually harvest more than 6.5 GtC y−1of biomass. The 6.5 GtC y−1of biomass could be converted to biochar (3.25 GtC y−1) and biofuels (with heating value equivalent to that of 6500 million barrels of crude oil). Because biochar is resistant to microbial degradation, a biochar-based soil amendment could serve as a permanent carbon-sequestration agent in soils/subsoil earth layers for thousands of years. By storing 3.25 GtC y−1 of biochar (equivalent to 11.9 Gt of CO2) into soil and/or underground reservoirs alone, it would offset the world's 8.67 GtC y−1 of fossil fuel CO2 emissions by about 38%. The worldwide maximum capacity for storing biochar carbon into agricultural soils (1411 million hectares) is estimated to be about 428 GtC. It may be also possible to provide a global carbon “thermostat” mechanism by creating biochar carbon energy storage reserves. This biomass-pyrolysis “carbon-negative” energy approach merits serious research and development worldwide to help provide clean energy and control global warming for a sustainable future of human civilization on Earth. |
Introduction
The increasing anthropogenic CO2 emission and global warming have challenged the United States and other countries to find new and better ways to meet the world's increasing needs for energy while reducing greenhouse gases. The mean global atmospheric CO2 concentration has increased from 280 ppm in the 1700s to 380 ppm in 2005 at a progressively faster rate1 because of: (i) CO2 emissions from fossil-fuel use; and (ii) the CO2 flux from land-use change including land clearing such as “slash and burn”, agriculture and intensive tillage. In certain areas, agriculture and intensive tillage have also caused a 30 to 50 percent decrease in soil organic carbon (SOC) since many soils were brought into cultivation more than 100 years ago.2 With the CO2 emission from land-use change (1.5 GtC y−1) remaining nearly steady, the CO2 emissions from fossil-fuel use have been rapidly growing and it reached 7.9 gigatons of carbon (GtC) y−1 in 2005. The growth rate of CO2 emissions from fossil-fuel use had increased from 1.1% y−1 for 1990–1999 to >3% y−1 for 2000–2004.1 The latest data from US Department of Energy's Carbon Dioxide Information Analysis Center at Oak Ridge National Laboratory show: the CO2 emissions from fossil-fuel use in 2008 reached 8.67 GtC y−1; and the atmospheric CO2 concentration increased to 385 ppm, which represents accumulation of about 3007 gigatons of CO2 (equivalent to 820 GtC) in the atmosphere.3Therefore, the world currently faces a systematic problem of increased CO2 emissions, decreased soil-carbon content, and global-climate change (global warming). Huesemann (2006) provided a quite good critical review of the limitations and challenges in dealing with this massive problem and pointed out that, to stabilize atmospheric CO2 concentration at the current or below current levels, it would require more than 20-fold reduction (i.e., ≥95%) in per capita carbon emissions in industrialized nations within the next 50–100 years.4 Improvement on energy efficiency alone clearly could not achieve such a target. Huesemann (2006) also stated, large amounts of CO2 can potentially be removed from the atmosphere via sequestration in geologic formations and oceans, but that type of CO2 storage is not necessarily permanent and is likely to create many unpredictable environmental consequences. A serious question was then raised: “can advances in science and technology prevent global warming?” In the recent issue of Science, significant efforts on carbon capture and geologic sequestration are reported with a special devoted section and a review article.5 To solve the massive global energy and environmental sustainability problem, it likely requires a comprehensive portfolio of R&D efforts with multiple energy technologies. Here, we present a perspective overview of the smokeless (clean and efficient) biomass-pyrolysis “carbon-negative” energy approach6–9 for biochar and biofuel production, which might also be able to provide a positive answer to this question important to the well being of all people on Earth.
Possible solution: the biomass-pyrolysis “carbon-negative” energy approach
As illustrated in Fig. 1 and 2, photosynthesis captures more CO2 from the atmosphere than any other process on Earth. Each year, land-based green plants capture about 440 gigatons (Gt) CO2 (equivalent to 120 Gt C y−1) from the atmosphere into biomass.10 That is, about one-seventh of all the CO2 in the atmosphere (820 GtC) is fixed by photosynthesis (gross primary production) every year. However, biomass is not a stable form of carbon material with nearly all returning to the atmosphere in a relatively short time as CO2. Because of respiration and biomass decomposition, there is nearly equal amount of CO2 (about 120 GtC y−1) released from the terrestrial biomass system back into the atmosphere each year.11 As a result, using biomass for carbon sequestration is limited. Any technology that could significantly prolong the lifetime of biomass materials would be helpful to global carbon sequestration. As shown in Table 1, a conversion as small as 7.2% of the annual terrestrial gross photosynthetic products (120 GtC y−1) into a stable biomass carbon material such as biochar would be sufficient to offset the entire amount (nearly 8.67 GtC y−1) of CO2 emitted into the atmosphere annually from the use of fossil fuels. The approach of biomass pyrolysis provides such a possible capability to convert the otherwise unstable biomass into biofuel, and, more importantly, biochar which is suitable for use as a soil amendment and serves as a semi-permanent carbon-sequestration agent in soils/subsoil earth layers for hundreds and perhaps thousands of years. | ||
| Fig. 1 The potential benefits of the envisioned “carbon-negative” energy systems technology concept with biomass pyrolysis and ammonia carbonation for carbon dioxide capture and sequestration. As illustrated by the major CO2 arrow pointing from the top, photosynthetic biomass production on Earth (center) is the biggest process that can take CO2 from the atmosphere. Biomass pyrolysis (upper left) could produce biochar and biofuels (such as H2), which could be optionally utilized to make NH4HCO3–char and/or urea–char fertilizers. Use of biochar fertilizers could store carbon into soil and subsoil earth layers, reduce fertilizer (such as NO3−) runoff, and improve soil fertility for more photosynthetic biomass production to provide more win–win benefits including more forest, more fabric and wooden products, more food and feedstocks. It is also possible to create biochar/energy reserves (bottom right) as “global carbon thermostat” to control global warming. | ||
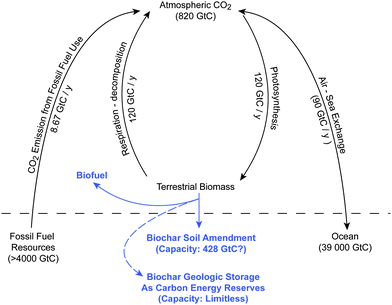 | ||
| Fig. 2 The global carbon cycle and envisioned “carbon-negative” biomass-pyrolysis energy technology concept for biofuel and biochar production, and carbon dioxide capture and sequestration. | ||
| World CO2 emissions from fossil-fuel use | Amount of CO2 captured by land-based green plants into primary photosynthetic products | Amount of CO2 released from the terrestrial biomass system because of respiration and biomass decomposition | Percentage of terrestrial photosynthetic products needed to be converted into a stable form to offset the world fossil-fuel CO2 emissions |
|---|---|---|---|
| 8.67 GtC per year | 120 GtC per year | ∼120 GtC per year | About 7.2% |
Low-temperature biomass pyrolysis is a process in which biomass such as forest waste woods and/or crop residues (e.g., corn stover) are heated to about 400 °C in the absence of oxygen and, as a result, the biomass is converted to biofuel (bio-oils and syngas) and charcoal (char)—a stable form of solid black carbon (C) material (Fig. 3). Although its detailed thermo-chemical reactions are quite complex, the biomass-pyrolysis process can be described by the following general equation:
| Biomass (e.g., lignin cellulose) → char + H2O + bio-oils + syngas |
 | ||
| Fig. 3 Photographs showing, from left to right, 10 g biochar from pyrolysis of corn stover, 10 g soil, and 10 g mixture of biochar (10% wt) and soil (90% wt). The soil sample shown here is a surface soil from 0–15 cm deep at the University of Tennessee's Research and Education Center, Milan, Tennessee, USA (358560N latitude, 888430W longitude), which is also known as the Carbon Sequestration in Terrestrial Ecosystems site (CSiTE) supported by the US Department of Energy. | ||
Since the char (charcoal), in this case, is created from pyrolysis of biomass, it is commonly called “biochar.” Typically, biochar contains mainly carbon (C) with certain amounts of hydrogen (H), oxygen (O), and nitrogen (N) atoms, plus ash. As reported in one of our previous studies,6 a typical composition of biochar on an ash- and nitrogen-free basis can be 82% C, 3.4% H, and 14.6% O. The chemical composition and the yield of biochar depend on the feedstock properties and pyrolysis conditions including temperature, heating rate, pressure, moisture, and vapor-phase residence time.12 With certain refinery process, the organic volatiles (bio-oils) and syngas (CO, CO2, and H2, etc.) from biomass pyrolysis could be used as biofuels for clean energy production.6 Typically, about 50% of the biomass C (carbon) can be converted into biochar while the remainder 50% C going to the biofuel fraction. Depending on the biomass materials, low-temperature pyrolysis process can be slightly exothermic so that once the process is started it could sustain itself with its own heat. That is, the exothermic heat evolution from biomass pyrolysis can elevate the temperature of the incoming (dry) biomass feedstock sufficiently to initiate the carbonation reactions.12 Consequently, once initiated, it is possible to convert large amounts of biomass into biochar and biofuel with minimal exogenous energy cost.
According to a recent study13 using a pilot-scale pyrolysis unit at Eprida, pyrolysis of 100 kg biomass (southern yellow pine pellets) at 482 °C can typically produce 26.3 kg of biochar. Based on energy value calculation, the 26.3 kg biochar contains 528 million joules (MJ) (28%) of the 100 kg biomass energy (1859 MJ). The remaining biomass energy (1331 MJ) exists as pyrolysis vapors (crude biofuel) and heat in the gas phase from the pyrolyzer. The addition of steam and the use of a steam-reforming process (the water gas shift reaction) can convert the pyrolysis vapors and water into syngas (126.6 kg) giving an average composition of 47.6% H2 (6.66 kg), 18.3% CO2 (55.9 kg), 2.7% CH4 (3.00 kg), 13.7% CO (26.6 kg), and 17.7% N2 (34.4 kg). The significant amount of nitrogen in the syngas was due to the N2 used to purge the lines for the sensor equipment and for pressuring the biomass feed and char discharge systems. The higher heating value (HHV) of this syngas is calculated to be 1403 MJ and the total energy cost for the pyrolysis and steam-reforming process is 787 MJ, assuming no heat recovery. The net biofuel (syngas) energy production is 616 MJ (per 100 kg biomass), which represents about 33% of the biomass energy (1859 MJ) while the biochar product contains 28% of the biomass energy (1859 MJ). The total process energy-conversion efficiency for production of biochar and biofuel combined is 61% in this case. With better process designs such as use of heat recovery techniques, the energy conversion efficiency may be further improved. Nonetheless, this example demonstrates that it is possible to produce both biochar and biofuel from biomass with reasonable energy efficiency.
In perspective of the global carbon cycle, as shown in Fig. 1 and 2, this char-producing biomass-pyrolysis approach essentially employs the existing natural green-plant photosynthesis on the planet as the first step to capture CO2 from the atmosphere; then, the use of a pyrolysis process converts biomass materials primarily into biofuel and char—a stable form of solid carbon material that is resistant to microbial degradation. The net result is the removal of CO2 from the atmosphere since the total process capturing CO2 from the atmosphere and placing it into soils and/or subsoil earth layers as a stable carbon (biochar) while producing significant amount of biofuel energy through biomass pyrolysis. Therefore, this is a “carbon negative” energy production approach.
Currently, the United States can annually harvest over 1.3 Gt (gigaton) of dry biomass, of which about 1.0 Gt is generated from the croplands and over 0.3 Gt is from a fraction of forestlands that are accessible by roads.14 If this amount of biomass (1.3 Gt y−1) is processed through controlled low-temperature pyrolysis assuming 50% conversion of biomass C to stable biochar C and 33% of the biomass energy to biofuels (syngas and bio-oils), it could produce biochar (0.325 GtC y−1) and biofuels (with heating value equivalent to that of 1300 million barrels of crude oil) to help control global warming and achieve energy independence from fossil fuel. In 2008, the US brought in 845 million barrels of crude oil, according to the Energy Information Administration, an arm of the US Department of Energy. The heating value (equivalent to that of 1300 million barrels of crude oil) of the syngas/bio-oils from biomass pyrolysis in this scenario exceeds that of the USA-imported crude oils (845 million barrels). Even if a half of the syngas/bio-oils may be consumed to cover any other energy costs in handling the biomass (such as biomass collection, drying and transport), the remainder syngas/bio-oils (equivalent to that of 650 million barrels of crude oil) is still quite significant as the net biofuel output. Therefore, if a cost-effective biofuel-refinery technology can be developed to convert the syngas/bio-oils from biomass pyrolysis into liquid transportation fuels, use of this approach could significantly help reduce the imports of foreign oil. In the immediate future before such a biofuel refinery technology is available, the syngas/bio-oils from biomass pyrolysis could be used for its heating energy by combustion to replace fossil fuels including coal, natural gas and heating oils. In addition, application of the 0.325 GtC y−1of biochar products as soil amendment and carbon sequestration agent in soil could stimulate the agriculture economy and achieve major carbon sequestration for the US to control global warming as well.
The world's annual biomass-production capacity probably exceeds 13 Gt of dry biomass, which is equivalent to about 6.5 GtC y−1 since dry biomass typically contains approximately 50% C by mass. If this amount of biomass (6.5 GtC y−1) is processed through low-temperature pyrolysis, it would produce 3.25 GtC y−1 of biochar and huge amounts of biofuel (syngas/bio-oils with a heating value equivalent to 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 million barrels of crude oil) since the pyrolysis typically converts about 50% of the biomass carbon to biochar while the remainder 50% biomass C goes to the biofuel fraction (bio-oils and syngas). Even if assuming only half of the syngas/bio-oils as the net biofuel output, it could replace fossil energy with a heating value equivalent to that of 6500 million barrels of crude oil for the world. By storing 3.25 GtC y−1 of biochar (equivalent to 11.9 Gt of CO2 per year) into soil and/or underground reservoirs alone, it would offset the 8.67 GtC y−1 of fossil fuel CO2 emissions by about 38%, which probably represents the upper limit of the capacity.
000 million barrels of crude oil) since the pyrolysis typically converts about 50% of the biomass carbon to biochar while the remainder 50% biomass C goes to the biofuel fraction (bio-oils and syngas). Even if assuming only half of the syngas/bio-oils as the net biofuel output, it could replace fossil energy with a heating value equivalent to that of 6500 million barrels of crude oil for the world. By storing 3.25 GtC y−1 of biochar (equivalent to 11.9 Gt of CO2 per year) into soil and/or underground reservoirs alone, it would offset the 8.67 GtC y−1 of fossil fuel CO2 emissions by about 38%, which probably represents the upper limit of the capacity.
For the immediate future, application of this biochar-producing biomass pyrolysis approach to turn waste biomass into valuable products could likely provide the best economic and environmental benefits. Globally, each year, there are about 6.6 Gt dry matter of biomass (3.3 GtC) such as crop stovers that are appropriated but not used.15 Development and deployment of the smokeless biomass pyrolysis technology could turn this type of waste biomass into valuable biochar and biofuel products. Even if assuming that only half amount of this waste biomass is utilized by this approach, it would produce 0.825 GtC y−1 of biochar and large amounts of biofuel (with a heating value equivalent to that of 3250 million barrels of crude oil). By storing 0.825 GtC y−1 of biochar (equivalent to 3 Gt of CO2 per year) into soil and/or underground reservoirs alone, it could offset the world's 8.67 GtC y−1 of fossil fuel CO2 emissions by 9.5%, which is still very significant. According to a recent life-cycle assessment,16 for each ton of dry waste biomass utilized through biomass pyrolysis with biochar returned to soil, it could provide a net sequestration of about 800–900 kg of CO2 emissions (per ton of dry biomass). The life-cycle assessment also indicated that the biochar-producing biomass pyrolysis technology could be operated profitably when CO2 emission reductions are valued at or above about $60 per ton of CO2 equivalent emissions. Therefore, the envisioned photosynthetic biomass production and biofuel/biochar-producing biomass-pyrolysis approach (Fig. 1) should be considered as an option to mitigate the problem of global greenhouse-gas emissions.
The need of a modern smokeless biochar-producing biomass pyrolysis process
Development and use of a smokeless biofuel/biochar-producing biomass-pyrolysis technology are essential for such a large (GtC) scale operation to avoid negative impact on air quality. Biochar can be produced by other processes including (1) “slash and burn”, (2) “slash and char”, and (3) wild fires. All of these three processes generate large amounts of hazardous smokes that can impact air quality. In the practice of “slash and burn”, trees, bushes and other green plants are cut down and burned in the field to clear the land for cropland. The burning of biomass in open fields creates large amounts of hazardous smoke similar to a wild fire; the biochar formed through the slash-and-burn techniques represents only about 1.7% of the pre-burn biomass.17 “Slash and char” is a practice to make charcoal from biomass by use of conventional charcoal kilns,17,18 which is better than the practice of “slash and burn”, but would still produce large amounts of smoke. Use of conventional charcoal kilns for charcoal production at a GtC scale would produce large amounts of smoke (pollutants including soot black-carbon particles) that are not acceptable to the environment and air quality, in addition to allowing heat, energy and valuable chemicals to escape into the atmosphere. A recent study indicates that black-carbon aerosols which can directly absorb solar radiation might have substantially contributed to the rapid Arctic warming during the past three decades.19 Therefore, a smokeless and efficient modern biomass-pyrolysis process is essential to achieve the mission of annually converting gigatons of biomass into biochar and biofuel. Use of a modern biomass-pyrolysis biofuel/biochar-producing process6 would enable collecting of the “smoke” (organic volatiles and gases) into the biofuel fraction for clean energy (e.g., hydrogen) production. Therefore, further development and use of this type of smokeless biofuel/biochar-producing biomass-pyrolysis technologies are essential for the envisioned large (GtC) scale mission in mitigating CO2 emissions, and, at same time, ensuring good air quality.Biochar fertilizers for soil amendment and carbon sequestration
Biochar materials can be placed directly into soil to serve as a carbon-sequestration agent and to improve soil fertility. To serve as an effective biochar fertilizer and carbon-sequestration agent, the biochar materials need to possess certain specific properties such as the capacity in retaining fertilizer nutrients that could be produced only under certain pyrolysis conditions. According to preliminary studies, biochar materials that were produced from biomass pyrolysis at a relatively low temperature (400 °C) appear to have a significantly higher capacity in retaining fertilizer nutrients in comparison with those created at high temperature (900 °C).6 Therefore, use of biochar produced from low-temperature (about 400 °C) biomass pyrolysis is probably better for this application.Although proper application of biochar can improve soil properties, the biochar C itself is not a crop nutrient except its ash contents which can serve as mineral nutrients for crop growth. Therefore, it is probably better to apply biochar along with certain fertilizers such as NH4HCO3 and/or urea to achieve maximal environmental and agricultural benefits.6,20 One of the options is to produce a biochar–NH4HCO3 (or urea) compound fertilizer that may make the biochar materials more suitable to stimulate plant growth and to maximally place the carbon of biochar and bicarbonate (HCO3−) into soils.7,21 The ammonia-carbonation process22 can provide an option to integrate biomass pyrolysis with major industrial combustion facilities such as a coal-fired power plant to solidify major flue-gas CO2 emission and ppm levels of NOx and SOx emissions at the smokestacks into valuable fertilizers (mainly, NH4HCO3 with trace amount of other fertilizer species such as NH4NO3 and (NH4)2SO4) with biochar particles to produce a biochar–NH4HCO3 and/or biochar–urea fertilizer (Fig. 1 and 3) which could not only benefit agriculture, but also sequester carbon into soils for protecting the global environment.7,21,23
Due to their alkaline ash contents, the pH of biochar material can sometimes be as high as about 10, which would be unfavorable for use in alkaline soils (pH above 8) such as those in the western part of the United States because addition of an alkaline material could make the alkaline soil pH worse for plant growth. NH4HCO3 can act as a pH buffer. For example, mixing (50/50 by weight) with NH4HCO3 can neutralize the pH of biochar material from 9.85 to 7.89 (unpublished data), which could make the biochar fertilizer pH more favorable to use in many soils including (but not limited to) the alkaline soils. On the other hand, biochar can effectively adsorb ammonia (NH3) and other nutrients to minimize fertilizer nutrient loss. This type of chemisorption property is typical of biochar since the biomass pyrolysis thermochemical process involves the fracture of many chemical bonds initially present in the biomass feedstock. The product biochar carbon does not go through a fluid state during the pyrolysis; consequently many of these bonds are left “dangling”.12 As described by Antal and Gronli (2003),12 these dangling bonds are believed to give rise to some of the chemisorption properties of biochar. In addition, certain polar functional groups such as hydroxyl (–OH) and carboxyl (–COOH) groups of the biochar materials may give rise to the property of cation exchange capacity, which is important also in helping retain nutrients such as ammonium and potassium ions (NH4+ and K+) in soil. Therefore, co-application of biochar and NH4HCO3 (or urea) can probably maximize the beneficial effects. Furthermore, the bicarbonate (HCO3−) of NH4HCO3 (or urea) that could be used in this manner may stay in the alkaline soils. This could also provide an option to help solve the environmental problem of nitrate (NO3−) run off from the current use of ammonium nitrate (NH4NO3) as a fertilizer in USA.21 However, more research and development efforts are still needed to test this option.
The recent discovery of “black carbon” in the pre-historic (pre-Columbian) “Terra Preta” soils in Amazonia24,25 has now provided some excellent scientific support for the envisioned application of biochar as a soil amendment and carbon sequestration agent.6,7 According to a recent analysis on the “black carbon” found in the “Terra Preta” soils,26 polycyclic aromatic structure and carboxylic groups on edges of the aromatic backbone may contribute to the nutrient-holding capacity of the materials. Studies also indicated that black carbon increases cation exchange capacity in soils27 and that the anthropogenic enrichment with carbonaceous charred residues from biomass-derived black carbon is likely to be the precursor of these recalcitrant polyaromatic structures in the Amazonian Dark Earths.28
Because biochar is not completely digestible to microorganisms, a biochar-based soil amendment could serve as a permanent carbon-sequestration agent in soils/subsoil earth layers for thousands of years. As indicated by the recent discovery of biochar particles in “Terra Preta” soils formed by pre-Columbian indigenous agriculturalists in Amazonia, biochar materials could be stored in soils as a means of carbon sequestration for hundreds and perhaps thousands of years. The longest lifetime of biochar material that has been reported with scientific evidence is about 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years, according to the carbon isotope dating of a prehistoric Cro-Magnon man's charcoal painting discovered in the Grotte Chauvet cave.12,29 The black carbon in a “Terra Preta” soil at the Acutuba site has been dated to be about 6850 years old.30 At the Jaguariuna soil site in Brazil, some high abundance of charcoal found in the summit soil was dated to occur about 9000 years ago.31 These carbon-isotope dating results all indicate how stable and permanent the biochar carbon sequestration can be. Through a 14C labeling study, the mean residence time of pyrogenic carbon in soils has now been estimated in the range of millennia.32 As recently commented by Lehman,9 although the precise duration of biochar-storage lifetime is still debatable, “it would be considered a long-term sink for the purposes of reducing carbon dioxide emissions” whether biochar can remain in soils for hundreds or thousands of years.
000 years, according to the carbon isotope dating of a prehistoric Cro-Magnon man's charcoal painting discovered in the Grotte Chauvet cave.12,29 The black carbon in a “Terra Preta” soil at the Acutuba site has been dated to be about 6850 years old.30 At the Jaguariuna soil site in Brazil, some high abundance of charcoal found in the summit soil was dated to occur about 9000 years ago.31 These carbon-isotope dating results all indicate how stable and permanent the biochar carbon sequestration can be. Through a 14C labeling study, the mean residence time of pyrogenic carbon in soils has now been estimated in the range of millennia.32 As recently commented by Lehman,9 although the precise duration of biochar-storage lifetime is still debatable, “it would be considered a long-term sink for the purposes of reducing carbon dioxide emissions” whether biochar can remain in soils for hundreds or thousands of years.
The capacity of carbon sequestration by application of biochar fertilizer in soils could be quite significant since the technology could potentially be applied in many land areas including croplands, grasslands and also a fraction of forestlands. The maximum capacity of carbon sequestration through biochar soil amendment in croplands alone is estimated to be about 428 GtC for the world (Table 2). This capacity is estimated according to: (a) the maximal amount of biochar carbon that could be cumulatively placed into soil while still beneficial to soil environment and plant growth; and (b) the arable land area that the technology could potentially be applied through biochar agricultural practice.
| World region | Arable landa/million hectares | Estimated capacity (GtC) of biochar carbon storage in soilb |
|---|---|---|
| a Arable land area (1 hectare = 2.47 acres) from the 2007 database of the Food and Agriculture Organization of The United Nations: http://faostat.fao.org/site/377/default.aspx%23ancor. b Calculated based on the theoretical biochar carbon (C) storage capacity of 303.8 ton C per hectare for the first 30 cm soil layer alone, assuming average soil density of 1.3 tons per m3, maximally 10% biochar by soil weight, and biochar material containing about 70% (wt) of its mass as the stable carbon (C). | ||
| North America | 215.5 | 65.5 |
| Europe | 277.5 | 84.3 |
| Asia | 504.5 | 153.3 |
| Africa | 219.2 | 66.6 |
| Oceania | 45.6 | 13.9 |
| Central America/Caribbean | 36.2 | 10.9 |
| South America | 112.5 | 34.2 |
| Total | 1411 | 428.7 |
Using charcoal collected from a wildfire, Gundale and DeLuca (2007) recently showed that amount of charcoal that can be applied can be as much as 10% of the weight of soil to increase plant Koeleria macrantha biomass growth without negative effect.33 The composition of a biochar material depends on its source biomass material and pyrolysis conditions. Typically, biochar material produced from low-temperature (about 400 °C) pyrolysis contains about 70% (wt) of its mass as the stable carbon (C) and the remainder as ash content, oxides and residual degradable carbon (such as bio-oil residue). The density of bulk soil is typically about 1.3 tons per m3. With this preliminary knowledge, we calculated that the maximum theoretical biochar sequestration capacity is about 303.8 ton C per hectare (1 hectare = 2.47 acres; 123 ton C per acre) in a 30 cm soil layer alone. Accordingly, about 51.6 GtC of biochar particles could be sequestered in the first 30 cm layer of US cropland (170 million hectares) soil alone.
Table 2 lists the world's regional cropland areas and their estimated capacities to store biochar carbon in the soils of croplands. The worldwide potential capacity for storing biochar carbon in agricultural soils (1411 million hectares) was estimated to be 428 GtC. This estimate (428 GtC), which is somewhat higher than that (224 GtC) estimated by Lehman et al.,34 probably represents an upper limit value. The maximal amounts of biochar carbon that could be cumulatively placed into soil while still beneficial to soil environment and plant growth are probably dependent on a number of factors including the specific biochar properties, topography, soil type, weather, and plant species. Worldwide biochar soil field tests are needed to obtain more accurate information on the capacity of biochar carbon sequestration in soils.
Table 3 lists the world's capacity of biochar carbon sequestration in soils of all possible applicable lands. The world's total land areas are 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 million hectares,35 which consist of 1411 million hectares of croplands, 1250 million hectares of temperate grasslands, 3937 million hectares of forestlands, and 6402 million hectares of others lands. In principle, in addition to the soils of croplands, it is also possible to apply biochar materials in soils of other lands including grasslands and probably also a fraction of forestlands. A maximum theoretical biochar sequestration of 303.8 ton C per hectare would calculate to 380 GtC globally for the 1250 million hectares of temperate grasslands. There are 3937 million hectares of forestlands in the world. If the forestlands could also be used to sequestrate biochar carbon, the capacity of biochar storage in the 3937 million hectares of forest soils would be 1196 GtC. If the entire world land areas could contribute to biochar carbon sequestration on average of 303.8 ton C per hectare, the global theoretical capacity for soil biochar carbon sequestration in all the lands would be 3950 GtC, which would be about sufficient to mitigate the additional carbon (4000 Gt) that is expected to be released from the burning of the remaining fossil fuel resources.4 However, for various reasons, not all the world land areas could necessarily be used for biochar soil application. Of the total land area on Earth, cropland accounts for about 11 percent, pastureland 27 percent, forested land 32 percent, and urban lands 9 percent. Most of the remaining 21 percent is unsuitable for crops, pasture, and/or forests because the soil is too infertile or shallow to support plant growth, or the climate and region are too cold, dry, steep, stony, or wet. At this point, it is probably reasonable to assume that only a fraction (about 30%?) of the world lands could be considered for using this technology in the 21st century, which would theoretically translate to a potential soil biochar carbon sequestration capacity of about 1166 GtC for the world consisting of the following: croplands (potential capacity: 428 GtC), grasslands (380 GtC), and a fraction (30%?) of forest lands (1196 GtC × 30% = 358 GtC). More studies are needed to fully determine the total potential capacity of soil biochar carbon sequestration for the various lands in the world.
000 million hectares,35 which consist of 1411 million hectares of croplands, 1250 million hectares of temperate grasslands, 3937 million hectares of forestlands, and 6402 million hectares of others lands. In principle, in addition to the soils of croplands, it is also possible to apply biochar materials in soils of other lands including grasslands and probably also a fraction of forestlands. A maximum theoretical biochar sequestration of 303.8 ton C per hectare would calculate to 380 GtC globally for the 1250 million hectares of temperate grasslands. There are 3937 million hectares of forestlands in the world. If the forestlands could also be used to sequestrate biochar carbon, the capacity of biochar storage in the 3937 million hectares of forest soils would be 1196 GtC. If the entire world land areas could contribute to biochar carbon sequestration on average of 303.8 ton C per hectare, the global theoretical capacity for soil biochar carbon sequestration in all the lands would be 3950 GtC, which would be about sufficient to mitigate the additional carbon (4000 Gt) that is expected to be released from the burning of the remaining fossil fuel resources.4 However, for various reasons, not all the world land areas could necessarily be used for biochar soil application. Of the total land area on Earth, cropland accounts for about 11 percent, pastureland 27 percent, forested land 32 percent, and urban lands 9 percent. Most of the remaining 21 percent is unsuitable for crops, pasture, and/or forests because the soil is too infertile or shallow to support plant growth, or the climate and region are too cold, dry, steep, stony, or wet. At this point, it is probably reasonable to assume that only a fraction (about 30%?) of the world lands could be considered for using this technology in the 21st century, which would theoretically translate to a potential soil biochar carbon sequestration capacity of about 1166 GtC for the world consisting of the following: croplands (potential capacity: 428 GtC), grasslands (380 GtC), and a fraction (30%?) of forest lands (1196 GtC × 30% = 358 GtC). More studies are needed to fully determine the total potential capacity of soil biochar carbon sequestration for the various lands in the world.
| Applicable world lands | Land areaa/million hectares | Estimated capacity (GtC) of biochar carbon storage in soilb |
|---|---|---|
| a Land area from the 2007 database of the Food and Agriculture Organization of The United Nations: http://faostat.fao.org/site/377/default.aspx%23ancor. b Calculated based on the maximal biochar carbon (C) storage of 303.8 ton C per hectare for the first 30 cm soil layer alone, assuming average soil density of 1.3 tons per m3, maximally 10% biochar by soil weight, and biochar material containing about 70% (wt) of its mass as the stable carbon (C). | ||
| Croplands | 1411 | 428 |
| Temperate grasslands | 1250 | 380 |
| 30% Forest lands | 1181 | 358 |
| Total | 3842 | 1166 |
Biochar carbon sequestration in soil actually also occurs naturally in the Earth's carbon cycle, in which some biochar generated from forest and grassland fires enters the soil.36 Certain agricultural use and effect of biochar were noted in an American Agriculture handbook more than 160 years ago.37 The recently discovered terra preta do Indio38 probably represents the best scientifically investigated prehistorical man-made soils with high concentrations of carbon, which provide an excellent “living” demonstration that biochar carbon can be sequestrated in soils for thousands of years with beneficial effects to soil fertility.39 Some of the farmers in certain parts of Asia including China and Japan40,41 also have a tradition to place ashes and char powders (often collected from chimneys of biomass-burning stoves and conventional charcoal-producing kilns) into soils to grow crops including rice, wheat, corn, sweet potatoes, and vegetables. With proper technical guidance and policy incentives, the envisioned application of biochar for both soil amendment and carbon sequestration could be quickly accepted around the world, especially since some of the population is probably somewhat familiar with this approach. Therefore, research for possible implementation of biochar sequestration in soils of croplands, grassland, and a marginal portion of forestlands should be initiated now. Trial application of biochar fertilizer in agricultural soil is a logical place to start. As shown in Table 2, the agricultural soils in all the world regions could contribute to biochar sequestration. There are possibilities that application of biochar might enhance certain soil–microbial activities including the glomalin producer arbuscular mycorrhizal fungi,42 found living on plant roots around the world.43 Glomalin has recently been reported as a highly stable glycoprotein that permeates organic matter, binding it to silt, sand, and clay particles. This super “soil glue” was believed to represent a third of the world's stored soil carbon.44 Not only does glomalin contain 30 to 40 percent carbon, but it also forms clumps of soil granules called aggregates that add structure to soil and keep other stored soil carbon from escaping. Therefore, soil pot studies and field trials of the biochar application in relation to soil fertility, and carbon sequestration are needed in all of these world regions to further demonstrate its feasibility.
Creation of biochar storage reservoirs as a possible global carbon thermostat
One option is to create large biochar reserves for long-term carbon storage. That is, to place large amounts of pure biochar materials produced from biomass pyrolysis into long-term storage places underground and/or above ground such as in used mines, landfills, and/or even at sea. This option is probably also essential in the long term, because the capacity (428 GtC) for storing biochar carbon into agricultural soils alone is not enough to take care of all the CO2 emissions (4000 GtC) that are expected to be released from the burning of the remaining fossil fuel resources on Earth.4 Even if other lands including grasslands and a part of forestlands are considered, the total capacity of biochar sequestration in soils is probably limited to about 1166 GtC because not all the lands in the world could necessarily be used for biochar soil application for various reasons.On the other hand, the capacity of carbon sequestration by putting concentrated biochar materials into underground and/or above ground reservoirs in a distributed manner can be limitless with the possibility of matching up or even exceeding the required capacity of 4000 GtC to offset all the additional CO2 emissions (4000 GtC) that are expected to be released from the burning of the remaining fossil fuel resources. Since the capacity of biochar storage reservoirs could be so large, the envisioned photosynthetic biomass production and biofuel/biochar-producing biomass-pyrolysis approach (Fig. 1) could be used for many years to reduce the atmospheric CO2 concentrations to any desired levels if the world population is mobilized to implement the approach. With this approach, it is possible not only to stop but also to reverse the trend of global warming if needed. That is, with this “carbon-negative” technology concept in conjunction with other energy technologies, it is possible to build a sustainable future for the human civilization on Earth.
Another advantage of this biochar storage option is that it is possible to recover the concentrated biochar materials from the reservoirs later when needed for use of its energy by combustion. This is different from the application of biochar as a soil amendment where biochar particles are mixed with soil particles in such a diluted manner (such as 10% by soil weight) that recovering of biochar materials from the mixed soils would be very difficult. The biochar materials in storage reservoirs are preferably in a pure and concentrated form so that they could be readily retrieved at any time when needed for use. Consequently, global use of biochar reservoirs in a regulated manner could provide a possible mechanism to control the atmospheric CO2 concentrations in a desirable manner. Therefore, this long-term biochar storage option could probably provide a global carbon “thermostat” mechanism in protecting the human civilization from the greenhouse-gas associated climate change on the planet Earth.
Summary of future R&D opportunities
Smokeless biomass pyrolysis for producing biofuels and biochar is a potentially revolutionary arsenal for global carbon capture and sequestration at GtC scales. Currently, the world could annually harvest more than 6.5 GtC y−1of biomass. The 6.5 GtC y−1of biomass could be converted to biochar (3.25 GtC y−1) and biofuels including syngas/bio-oils (with a heating value equivalent to that of at least 6500 million barrels of crude oil), which could help achieve energy independence from fossil fuels.Pyrolysis vapors can be burned directly as fuel for integrated heat and power production and can be combusted in existing boilers to produce heat, steam and electricity. Pyrolysis vapors excluding the non-condensable gases, can be condensed to form bio-oil which is a complex mixture of oxygenated hydrocarbons and water that can be used as low grade heating fuel. The heating value of bio-oil is about 40% to 50% of that for petroleum-based fuels45 and about 60% of ethanol,46 but has a higher energy density to compensate for the lower heating value.47 Bio-oil can be refined to be used as a source of chemical feedstock for gasoline, and can be added to petroleum refinery feedstock or combusted in raw form.48 Biomass pyrolysis allows biomass to be processed at dispersed locations where feedstocks are generated and bio-oil can be transported to a central refinery or power plant. Due to its high density, bio-oil is much more economical to transport than either biomass or hydrogen.49
Utilizing steam reforming, pyrolysis vapors can be converted to a syngas consisting of over 50% hydrogen, plus CO, CO2, and small amounts of methane, which is a clean burning, mid BTU fuel, similar to natural gas.49 This can be combusted in existing engines, generators, boilers, and turbines to produce heat, steam and electricity. Syngas is also suitable as a cooking fuel and can substitute for propane or natural gas in uses such as home heating. Hydrogen from syngas can be suitable for use in production of ammonia fertilizers. The current largest use of hydrogen in the world today is for the production of ammonia. Utilizing pyrolysis to generate hydrogen could replace natural gas as the primary feedstock required to manufacture ammonia based fertilizers. The production of ammonia using natural gas emits carbon dioxide into the atmosphere representing an opportunity to further reduce anthropogenic CO2 emissions. It is also possible to catalytically convert the biomass-derived syngas into liquid transportation fuels through the Fischer–Tropsch synthesis of hydrocarbons.50–52 However, so far, there is very little progress on large-scale pyrolysis/gasification involving biomass and there are significant hurdles here as in the conversion of pyrolysis oil to fuel. Large scale biomass to hydrocarbons via gasification on Fischer–Tropsch synthesis of hydrocarbons still has many significant technical issues to be resolved also.
Because biochar is mostly not digestible to microorganisms, a biochar-based soil amendment could serve as a permanent carbon-sequestration agent in soils/subsoil earth layers for thousands of years. By storing 3.25 GtC y−1 of biochar into soil and/or underground reservoirs alone, it would offset the world's 8.67 GtC y−1 of fossil fuel CO2 emissions by 38%. The worldwide maximum capacity for storing biochar carbon into agricultural soils (1411 million hectares) is estimated to be about 428 GtC. However, this technology concept is still in its early developing stage. Much more research and development work is needed before this approach could be considered for practical implementation.
To achieve the mission of biomass pyrolysis for energy production and carbon sequestration, a number of technical issues still need to be addressed. First, as mentioned before, the process technology must be smokeless (clean and effective) to avoid negative impact on air quality for such a large (GtC) scale operation. In other words, it is essential to fully develop a smokeless biomass-pyrolysis process to achieve the mission. A lifecycle energy and environmental health analysis including toxicology and ecology studies must also be carefully conducted to fully evaluate the potential benefits and possible risks.
Second, there are significant R&D opportunities to improve biofuel and biochar products. For example, more research is needed to further balance the biochar cation exchange capacity, volatile gradient within appropriate sized biochar particle and optimal micro/nanostructures for localized microbial interaction. There are many factors to understand about this natural process which can leverage the stable carbon biochar products as a soil amendment and carbon sequestration agent. Meanwhile, more studies are also needed on how to best utilize the biofuels (the bio-oils and syngas) co-produced from the biomass pyrolysis process. It is possible to convert the liquid bio-oils by different refinery processes into biodiesel for use as a transportation fuel. It is also possible to convert the syngas into hydrogen, electricity, or liquid fuels. However, since most require expensive catalysts and/or processing operations, other viable options (such as using bio-oils as a heating oil and non-catalytic fuel production) remain to be examined to determine trade-off between energy efficiency and costs.
Third, the maximum capacity of carbon sequestration through biochar soil amendment in world agricultural soils (1411 million hectares) is estimated to be about 428 GtC. To verify this potential capacity and demonstrate its feasibility, soil pot studies and field trials of biochar applications in relation to soil fertility and carbon sequestration are needed in all of the world regions. For the immediate future, biochar should be used to revitalize barren degraded land. This will improve the world's capacity for growing biomass thus naturally removing more CO2 from the atmosphere.
Another aspect is on the question whether it is possible to provide a global carbon “thermostat” mechanism by creating biochar carbon energy storage reserves. This is also a question whether it is possible to mitigate all the additional CO2 emissions (4000 GtC) that are expected to be released from the burning of the remaining fossil fuel resources on Earth by creating large reservoirs underground and/or above ground for any biochar not immediately used for soil restoration. Since the capacity of biochar storage reservoirs underground and/or above ground could be so large (limitless), the envisioned photosynthetic biomass production and biochar-producing biomass-pyrolysis energy-production approach (Fig. 1) could be used for many years to reduce the atmospheric CO2 concentrations to any desired levels with a flexibility to recover the concentrated biochar materials from the reservoirs later when needed for use of its energy by combustion. Consequently, global use of biochar reservoirs in a regulated manner could provide a possible mechanism to control the atmospheric CO2 concentrations in a desirable manner as well. These aspects of biochar carbon and energy storage also merit further studies.
As a conclusion, this smokeless biomass-pyrolysis “carbon-negative” energy-production approach merits a major program support for serious research and development worldwide. With further research and development, this approach could provide more efficient and cleaner biomass pyrolysis technology for producing biofuels and biochar from biomass as a significant arsenal to help achieve independence from fossil energy and to control global warming for a sustainable future on Earth.
Acknowledgements
The authors wish to thank Mac Post, Gregg Marland, and Joe Katz for stimulating discussions. This research was supported in parts by Oak Ridge National Laboratory Director's Seed Money Project Funds and by the US Department of Energy (DOE) Office of Science Young Scientist Award and the US Presidential Early Career Award for Scientists and Engineers (to J. W. Lee). Oak Ridge National Laboratory is managed by UT-Battelle, LLC, for DOE under contract No. DE-AC05-00OR22725. We are grateful also to three anonymous referees for their constructive suggestions to the manuscript.References
- M. R. Raupach, G. Marland, P. Ciais, C. L. Quere, J. G. Canadell, G. Klepper and C. B. Field, Global and regional drivers of accelerating CO2 emissions, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(24), 10288–10293 CrossRef CAS.
- W. H. Schlesinger, Changes in Soil Carbon Storage and Associated Properties with Disturbance and Recovery, in The Changing Carbon Cycle: A Global Analysis, ed. J. R. Trabalha et al., Springer Verlag, New York, NY, 1985, pp. 194–220 Search PubMed.
- The latest CO2 emission data are from US DOE's Carbon Dioxide Information Analysis Center (CDIAC) at Oak Ridge National Laboratory (http://cdiac.ornl.gov/). The 820 GtC is calculated by converting the 2008 atmospheric CO2 concentration (385 ppm) with a ppm-to-GtC conversion constant of 2.13 provided by Dr Gregg Marland of CDIAC according to the mass of the atmosphere.
- M. H. Huesemann, Can Advances in Science and Technology Prevent Global Warming?Mitigation and Adaptation Strategies for Global Change, Springer, New York, 2006, vol. 11, pp. 539–577 Search PubMed.
- R. S. Haszeldine, Carbon capture and storage: how green can black be?, Science, 2009, 325, 1647–1652 CrossRef CAS.
- D. Day, R. J. Evans, J. W. Lee and D. Reicosky, Economical CO2, SOx, and NOx capture from fossil-fuel utilization with combined renewable hydrogen production and large-scale carbon sequestration, Energy, 2005, 30, 2558–2579 CrossRef CAS.
- D. M. Day, J. W. Lee, The production and use of a soil amendment made by the combined production of hydrogen, sequestered carbon and utilizing off gases containing carbon dioxide, PCT Int. Appl., WO 2004037747 A2, 2004, 58p.
- E. Marris, Sequestration News Feature: Black is the new green, Nature, 2006, 442, 624–626 Search PubMed.
- J. Lehmann, Commentary: A handful of carbon, Nature, 2007, 447, 143–144 Search PubMed.
- R. J. Geider and E. H. Delucia, et al., Primary productivity of planet earth: biological determinants and physical constraints in terrestrial and aquatic habitats, Global Change Biol., 2001, 7(8), 849–882 CrossRef.
- D. R. Sauerbeck, CO2 emissions and C sequestration by agriculture—perspectives and limitations, Nutr. Cycling Agroecosyst., 2001, 60(1–3), 253–266 Search PubMed.
- M. J. Antal and M. Gronli, The art, science, and technology of charcoal production, Ind. Eng. Chem. Res., 2003, 42(8), 1619–1640 CrossRef CAS.
- K. C. Das, K. Singh, R. Adolphson, B. Hawkins, R. Oglesby, D. Lakly and D. Day, Steam pyrolysis and catalytic steam reforming of biomass for hydrogen and biochar production, Appl. Eng. Agric., 2010, 26(1), 137–146 Search PubMed.
- R. D. Perlack, L. L. Wright, A. Turhollow, R. Graham, B. Stokes, D. C. Erbach, Biomass as feedstock for a bioenergy and Bioproducts Industry: the technical feasibility of a billion-ton annual supply, Report ORNL/TM-2005/66 prepared by Oak Ridge National Laboratory for the US Department of energy, 2005 Search PubMed.
- F. Krausmann, K. Erb, S. Gingrich, C. Lauk and H. Haberl, Global patterns of socioeconomic biomass flows in the year 2000: a comprehensive assessment of supply, consumption and constraints, Ecol. Econ., 2008, 65, 471–487 CrossRef.
- K. Roberts, B. A. Gloy, S. Joseph, N. R. Scott and J. Lehmann, Life cycle assessment of biochar systems: estimating the energetic, economic, and climate change potential, Environ. Sci. Technol., 2010, 44, 827–833 CrossRef CAS.
- C. Steiner, Slash and Char as Alternative to Slash and Burn, Dissertation, University of Bayreuth, Germany, 2006.
- Y. Okimori, M. Ogawa, and F. Takahashi, Potential of CO2 Emission Reductions by Carbonizing Biomasswaste from Industrial Tree Plantation in South Sumatra, Indonesia, Mitigation and Adaptation Strategies for Global Change, Kluwer Academic Publishers, Netherlands, 2003, vol. 8, pp. 261–280 Search PubMed.
- D. Shindell and G. Faluvegi, Climate response to regional radiative forcing during the twentieth century, Nat. Geosci., 2009, 2, 294–300 Search PubMed; E. Kintisch, Climate change: new push focuses on quick ways to curb global warming, Science, 2009, 324, 323 CAS.
- H. Asai, B. K. Samson, H. M. Stephan, K. Songyikhangsuthor, K. Homma, Y. Kiyono, Y. Inoue, T. Shiraiwa and T. Horie, Biochar amendment techniques for upland rice production in Northern Laos 1. Soil physical properties, leaf SPAD and grain yield, Field Crops Res., 2009, 111, 81–84 CrossRef.
- J. W. Lee and R. Li, Integration of fossil energy systems with CO2 sequestration through NH4HCO3 production, Energy Convers. Manage., 2003, 44(9), 1535–1546 CrossRef CAS.
- X. Li, E. Hagaman, C. Tsouris and J. W. Lee, Removal of carbon dioxide from flue gas by ammonia carbonation in the gas phase, Energy Fuels, 2003, 17, 69–74 CrossRef CAS.
-
J. W. Lee and R. Li, Method for reducing CO2, CO, NOx, and SOx emissions, US Pat., 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 447
447![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 437 B1, 2002.
437 B1, 2002. - W. M. Denevan, A bluff meddle of river in the settlement in prehistoric Amazonia, Ann. Assoc. Am. Geogr., 1996, 86(4), 654–681 Search PubMed.
- W. I. Woods and J. M. McCann, The Anthropogenic Origin and Persistence of Amazonian Dark EarthYearbook, Conference of Latin Americanist Geographer, 1999, vol. 25, pp. 7–14 Search PubMed.
- H. Glaser and Z. Guggenberger, Naturwissenschaften, 2001, 88, 37–41 CrossRef.
- B. Liang, J. Lehmann, D. Solomon, J. Kinyangi, J. Grossman, B. O'Neill, J. O. Skjemstad, J. Thies, F. J. Luizão, J. Petersen and E. G. Neves, Black carbon increases cation exchange capacity in soils, Soil Sci. Soc. Am. J., 2006, 70, 1719–1730 CrossRef CAS.
- D. Solomon, J. Lehmann, J. Thies, T. Schäfer, B. Liang, J. Kinyangi, E. Neves, J. Petersen, F. Luizão and J. Skjemstad, Molecular signature and sources of biochemical recalcitrance of organic C in Amazonian Dark Earths, Geochim. Cosmochim. Acta, 2007, 71, 2285–2298 CrossRef CAS.
- E. Bard, Extending the Calibrated Radiocarbon Record, Science, 2001, 292, 2443–2444 CrossRef CAS.
- L. A. German, Historical contingencies in the coevolution of environment and livelihood: contributions to the debate on Amazonian Black Earth, Geoderma, 2003, 111, 307–331 CrossRef.
- S. E. M. Gouveia and L. C. R. Pessenda, et al., Carbon isotopes in charcoal and soils in studies of paleovegetation and climate changes during the late Pleistocene and the Holocene in the southeast and center west regions of Brazil, Global Planet. Change, 2002, 33(1–2), 95–106 CrossRef.
- Y. Kuzyakov, I. Subbotina, H. Chen, I. Bogomolova and X. Xu, Black carbon decomposition and incorporation into soil microbial biomass estimated by 14C labeling, Soil Biol. Biochem., 2009, 41, 210–219 CrossRef CAS.
- M. J. Gundale and T. H. DeLuca, Charcoal effects on soil solution chemistry and growth of Koeleria macrantha in the ponderosa pine/Douglas fir ecosystem, Biol. Fertil. Soils, 2007, 43, 303–311 CAS.
- J. Lehmann, J. Gaunt, and M. Rondon, Bio-char Sequestration in Terrestrial Ecosystems—A Review, Mitigation and Adaptation Strategies for Global Change, Springer, New York, 2006, vol. 11, pp. 403–427 Search PubMed.
- The 2007 World lands database of the food and agriculture Organization of the United nations, http://faostat.fao.org/site/377/default.aspx%23ancor.
- M. Fowles, Black carbon sequestration as an alternative to bioenergy, Biomass Bioenergy, 2007, 31, 426–432 CrossRef CAS.
- A. L. Allen, A Brief Compend of American agriculture, Saxton & Miles, T & M Butler, New York, Buffalo, 1846, this is a 437-page book of year 1846, digitized for Microsoft Corporation by the Internet Archive in 2007, from University of California Libraries Search PubMed.
- Amazonian Dark Earth: Exploration in Space and Time, ed. B. Glaser and W. I. Woods, Springer, Netherlands, 2004 Search PubMed.
- J. Lehmann, D. C. Kern, B. Glaser and W. I. Woods, Amazonian Dark Earths: Origin, Properties, Management, Kluwer Academic Publishers, 2004 Search PubMed.
- K. Isobe, H. Fujii and Y. Tsuboki, Effect of charcoal on the yield of sweet potato, Jpn. J. Crop Sci., 1996, 65(3), 453–459 Search PubMed.
- A. F. Islam, Y. Kitaya, H. Hirai, M. Yanase, G. Mori and M. Kiyota, Effects of placing rice husk charcoal inside soil ridges for soil aeration and growth and yield of sweet potato in wet lowland fields, J. Root Crops, 1999, 25(1), 85–97 Search PubMed.
- S. F. Wright and A. Upadhyaya, Extraction of an Abundant and Unusual Protein from soil and comparison with Hyphal Protein of arbuscular mycorrhizal fungi, Soil Sci., 1996, 161(9), 575–586 CrossRef CAS.
- C. E. Lovelock, S. F. Wright, D. A. Clark and R. W. Ruess, Soil stocks of glomalin produced by arbuscular mycorrhizal fungi across a tropical rain forest landscape, J. Ecol., 2004, 92, 278–287 CrossRef CAS.
- D. Comis, Glomalin: hiding place for a third of the World's stored soil carbon, Agric. Res. (Washington, D. C.), 2002, 50(9), 4–7 Search PubMed.
- S. Yaman, Pyrolysis of biomass to produce fuels and chemical feedstocks, Energy Convers. Manage., 2004, 45, 651–671 CrossRef CAS.
- K. Raveendran and A. Ganesh, Heating value of biomass and biomass pyrolysis products, Fuel, 1996, 75, 1715–1720 CrossRef CAS.
- D. Nguyen and D. Honnery, Combustion of bio-oil ethanol blends at elevated pressure, Fuel, 2008, 87, 232–243 CrossRef CAS.
- M. C. Samolada, W. Baldauf and I. A. Vasalos, Production of a bio-gasoline by upgrading biomass flash pyrolysis liquids via hydrogen processing and catalytic cracking, Fuel, 1998, 77, 1667–1675 CrossRef CAS.
- S. Czernik, R. Evans and R. French, Hydrogen from biomass-production by steam reforming of biomass pyrolysis oil, Catal. Today, 2007, 129, 265–268 CrossRef CAS.
- C. N. Hamelinck, A. P. C. Faaij, H. den Uil and H. Boerrigter, Production of FT transportation fuels from biomass; technical options, process analysis and optimisation, and development potential, Energy, 2004, 29, 1743–1771 CrossRef CAS.
- A. Demirbas, Progress and recent trends in biofuels, Prog. Energy Combust. Sci., 2007, 33, 1–18 CrossRef CAS.
- O. O. James, A. M. Mesubi, T. C. Ako and S. Maity, Increasing carbon utilization in Fischer–Tropsch synthesis using H2-deficient or CO2-rich syngas feeds, Fuel Process. Technol., 2010, 91, 136–144 CrossRef CAS.
Footnotes |
| † Current address: Johns Hopkins University, Whiting School of Engineering, 118 Latrobe Hall, Baltimore, MD 21218, USA. |
| ‡ Retired from USDA-ARS North Central Soil Conservation Research Lab. |
| This journal is © The Royal Society of Chemistry 2010 |
