C@ZnO nanorod array-based hydrazine electrochemical sensor with improved sensitivity and stability
Jinping
Liu
*a,
Yuanyuan
Li
b,
Jian
Jiang
a and
Xintang
Huang
a
aInstitute of Nanoscience and Nanotechnology, Department of Physics, Huazhong Normal University, Wuhan, 430079, P. R. China. E-mail: liujp@phy.ccnu.edu.cn
bDepartment of Electronic Science and Technology, Huazhong University of Science and Technology, Wuhan, 430074, P. R. China
First published on 12th August 2010
Abstract
ZnO nanorod array grown directly on an inert alloy substrate has been modified with carbon by a simple immersion-calcination route and further used as the working electrode to construct a hydrazine sensor. The C@ZnO nanorod array-based sensor demonstrates a very high sensitivity of 9.4 μA μM−1 cm−2 and a low detection limit of 0.1 μM. The improved electrochemical properties are proposed to result from the synergy between the carbon layer and nanorod array, which can increase the ZnO electrocatalytic activity and promote the electron transport along the one-dimensional (1D) pathway, respectively. In particular, the carbon layer on ZnO nanorods also improves the sensor stability for successive usage due to the high chemical stability of carbon. The present study demonstrates the facile design of a promising electrode material for a hydrazine sensor and sheds light on the performance optimization of other electrochemical devices.
1 Introduction
Hydrazine (N2H4) is a neurotoxin that can produce carcinogenic and mutagenic effects causing severe damage to the liver, lungs, kidneys and human central nervous system.1–3 Despite this, it is widely used in industrial applications such as corrosion inhibitors, catalysts, emulsifiers and antioxidants.2–3 Hydrazine is also a precursor in the production of some insecticides, explosive, pesticides, dyestuffs and several pharmaceutical derivatives.4–6 Therefore, it is highly necessary to establish a reliable and sensitive analytical tool for the determination and quantification of hydrazine. Up until now, various hydrazine detection techniques including ion chromatography,7 electrochemistry,8 chemiluminescence9 and spectroscopy10–11 have been proposed. Among them, the electrochemical method is promising in terms of its portability, low cost, high sensitivity and fast response. It was reported that the anodic electrochemical oxidation of hydrazine can happen on Au, Ag, Pt, Rh and Pd electrodes, but such metals are too expensive for practical applications and relatively high overpotentials are generally required.12 Large overpotential for hydrazine oxidation was also observed at carbon macroelectrodes.13 One promising method to address the above concern is the modification of electrodes with redox mediators such as various transition metal complexes,5,6,14 and pyrogallol red.15 The major problem of mediator-modified electrodes is their lack of long-term stability due to desorption of mediators from the electrode substrates.16 As an alternative, nanoparticle-modified electrodes have recently drawn much interest in hydrazine detection. In particular, nano/microelectrode ensembles have shown many advantages over conventional macroelectrodes such as increased mass transport, large surface area, fast electron transport (reduced resistance), low detection limit and high signal-to-noise ratio. Up until now, some nanostructured electrodes including noble metal nanoparticles,17–20 carbon nanotubes,21,22 boron-doped diamond,23 ZnO,8,24–26 CuO27,28 have been reported for the determination of hydrazine and have shown improved performance in terms of sensitivity and detection limit.ZnO is biocompatible, non-toxic, thermally stable and electrochemically active.29–32 Its nanostructures have been widely used for the fabrication of efficient amperometric sensors such as hydrazine chemical sensor and glucose/H2O2 biosensors.33–36 ZnO nanorods/nanowires are particularly promising for electrochemical sensing because of their excellent electron transport path along the length direction. When aligned directly on electrode substrates, the transport of electron to electrode can be further promoted.30,36,37 In addition, aligned nanorods/nanowires can serve as numerous “nanoelectrodes” to enhance the electrochemical activity while the interspacing between nanorods/nanowires provides a large surface area for electrolyte percolation. For hydrazine detection, although several ZnO nanowire/rod array electrodes have demonstrated superior performance,8,24,26 they are not sufficiently stable in PBS solution and alkaline electrolytes. This would lead to stability problems of the sensor and limit its potential in practical application.
In order to address the above issue, we propose in the present study to modify ZnO nanorods with a thin layer of carbon of several nanometre thickness since carbon is well-known to be chemically inert. The ZnO nanorod array was fabricated directly on a large-area inert alloy substrate by a very simple solution-based route at ∼75 °C.38 After the carbon modification, the conductivity of ZnO-based array can also be improved, leading to higher sensitivity for hydrazine determination when compared with pristine ZnO nanorod array. It should be pointed out that the direct use of C@ZnO nanorod array as the sensing electrode not only leads to good electrochemical performance but also avoids the conventional electrode fabrication process, which typically involves the pasting of electroactive materials onto standard glassy carbon electrode or expensive Au electrode and the use of Nafion for film immobilization.
2 Experimental
To construct the hydrazine electrochemical sensor, a ZnO nanorod array on alloy substrate was grown firstly via a mild solution method according to our previous study.38 For the carbon modification, the as-grown ZnO array was immersed into a 0.02 M glucose solution for 15 h and further annealed in a flow of argon at 500 °C for 5 h. The glucose molecules were first adsorbed onto the nanorod surface and carbonized during the annealing process, leading to the coating of nanorods with carbon layers. The obtained C@ZnO nanorod array (0.5 cm × 0.25 cm) was employed directly as the working electrode (binder-free) for the determination and quantification of hydrazine on a CHI660C electrochemical workstation (Shanghai Chenhua, China). A platinum wire and a saturated calomel electrode (SCE) were used as the auxiliary electrode and reference electrode, respectively. The electrolyte was 0.1 M pH = 8.0 phosphate buffer solution (PBS). For the amperometric experiments, drops of hydrazine were injected into the stirred electrolyte solution and each addition of hydrazine could result in a rapid increase in the current. A comparative study was also performed by using pristine ZnO nanorod array as the working electrode under otherwise similar conditions.The C@ZnO nanorod array was characterized using powder X-ray diffraction (XRD, Bruker D-8 Avance), scanning electron microscopy (SEM, JSM-6700F) and transmission electron microscopy (TEM, JEM-2010FEF).
3 Results and discussion
Typical SEM images of the C@ZnO nanorod array are shown in Fig. 1(a) and (b). It can be seen that needle-like ZnO nanostructures are grown nearly vertical to the substrate, leaving lots of void spaces between each other. The naturally generated voids of as-obtained array are beneficial to the electrolyte access, different from the traditional dense film structures. The composition of the array is confirmed by the XRD pattern depicted in Fig. 1(c), in which peaks marked with stars are from the alloy substrate.38 In order to give evidence to the existence of carbon, TEM analysis was carried out and the results are displayed in Fig. 1(d) and its inset. It is clear that there is a thin layer of carbon presented on the surface of the ZnO nanorod and the thickness of the carbon layer is ∼7 nm. The lattice fringe of 2.6 Å can be indexed to the (002) plane of ZnO, which confirms the c axis growth habit of ZnO nanorods. | ||
| Fig. 1 (a) Low, (b) high resolution SEM images and (c) XRD pattern of C@ZnO nanorod array. (d) HRTEM image of an individual nanorod clearly showing the carbon layer coating; inset figures are TEM images of three nanorods (upper) and enlarged HRTEM image revealing the single-crystal structure of ZnO (bottom). | ||
We have successfully used the C@ZnO nanorod array for hydrazine detection. The cyclic voltammograms (CVs) of C@ZnO nanorod array electrodes in a 0.1 M PBS (pH = 8.0) with 1 μM hydrazine and without hydrazine at the scan rate of 100 mV s−1 are presented in Fig. 2. It is clear that there are no obvious redox peaks observed when hydrazine is absent. In contrast, with the presence of 1 μM hydrazine, a representative anodic peak centered at ∼0.36 V with the current of 7.5 μA can be detected. Thereby, C@ZnO nanorod array serves as a very good platform to promote the electrochemical oxidation reaction of hydrazine. The oxidation of hydrazine begins at around −0.3 V, almost consistent with previous reports.24,28 Accordingly, the oxidation process of hydrazine can be described as N2H4 + 5/2OH− → 1/2N3− + 1/2NH3 + 5/2H2O + 2e−.8 There are no cathodic peaks observed in the CV curve, indicating the electrochemical response is irreversible. In addition, for C@ZnO nanorod array, the oxidation current increases as the concentration of hydrazine increases. It should be mentioned that the oxidation of hydrazine generally requires very high overpotentials of 0.6–0.8 V with a poorly-defined anodic wave characteristic at the bare electrodes.8,25,28 Thus, the use of C@ZnO nanorod array indeed reduces the overpotential and demonstrates fast electrode kinetics. Furthermore, the electrochemical oxidation of hydrazine can also happen at pristine ZnO nanorod array, as shown in Fig. 2; however, the oxidation current is smaller and the peak is ill-defined as compared to C@ZnO nanorod array. This implies that the presence of a nanosized carbon layer can enhance the electrooxidation activity, most probably due to the improved conductivity and specific surface area30 derived from carbon.
 | ||
| Fig. 2 CV curves of different electrodes in 0.1 M PBS buffer solution (pH = 8.0) in the presence of various concentrations of N2H4. Scan rate: 100 mV s−1. | ||
In order to further investigate the hydrazine sensing properties of C@ZnO and pristine ZnO nanorod arrays, amperometric response of these two electrodes upon the successive addition of hydrazine into continuously stirred 0.1 M pH = 8.0 PBS at an applied potential of 0.3 V was carried out, and the typical results are displayed in Fig. 3(a). For the C@ZnO-based sensor, the oxidation current increases gradually and then quickly with the successive introduction of 0.3, 0.8, and 1.2 μM hydrazine into the PBS, demonstrating a rapid and sensitive response upon the hydrazine concentration variation. The response time to achieve 95% of the steady-state current is within 4 s (Fig. 3(b)). There are also current changes at the pristine ZnO nanorod array electrode upon the continuous addition of hydrazine. The current variations, however, are significantly lower than those of the C@ZnO electrode when the same amount of hydrazine is introduced. In addition, the response time for pristine ZnO electrode is typically around 8 s (Fig. 3(b)). Based on the amperometric performance, we can conclude that the carbon modification on the ZnO nanorod surface is quite important and represents a promising pathway for improving the sensing capability of nanostructured arrays. Fig. 3(c) illustrates the current–time curve of C@ZnO electrode with the addition of very low hydrazine concentration. In the presence of only 0.1 μM N2H4, there is still a pronounced current increase, indicating the C@ZnO-based sensor has a very low detection limit.
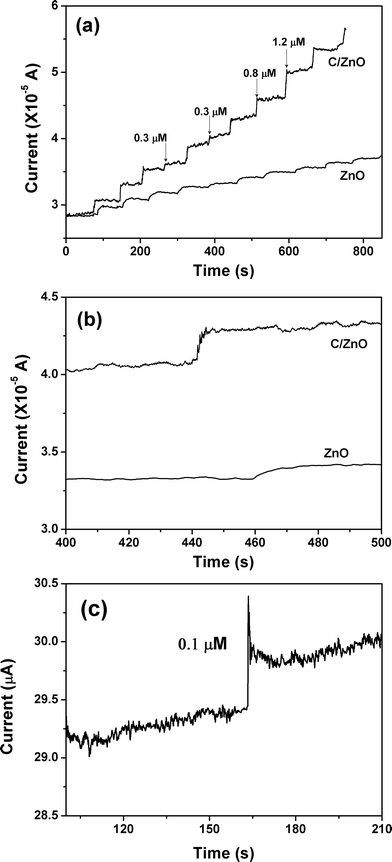 | ||
| Fig. 3 (a) Amperometric responses of the C@ZnO and pristine ZnO nanorod array electrodes at 0.30 V to the successive addition of N2H4 into the 0.1 M PBS buffer solution (pH = 8.0). (b) Representative amperometric response in the time range of 400–500 s. (c) Amperometric response to 0.1 μM N2H4 for C@ZnO nanorod array electrode. | ||
The calibration curves of the C@ZnO and pristine ZnO-based sensors derived from Fig. 3(a) are shown in Fig. 4. Both the two sensors demonstrate current increase with increasing hydrazine concentration, and the steady-state current shows a linear relationship with the hydrazine concentration in the range of 0.1–3.8 μM for C@ZnO-based sensor. The linear concentration range for pristine ZnO nanorod array-based sensor is slightly narrow. From the slopes of the calibration curves, the sensitivities of C@ZnO and pristine ZnO-based sensors can be determined as 9.4 and 4.48 μA μM−1 cm−2, respectively. The former value is nearly twice of the latter one and larger than many of the previous reported ones obtained from hydrazine sensors using ZnO nanonails/nanorods, CuO nanoflowers, ZnO hierarchical micro/nanostructures, etc.8,26,28 To compare the characteristics and performance of the present C@ZnO nanorod array hydrazine sensor with previously reported ones, their detection limit, sensitivity, and response time are summarized in Table 1. Obviously, the C@ZnO-based sensor exhibits superior performance in terms of the sensitivity and detection limit. We believe that modification of carbon onto the surface of ZnO nanorods improves not only the electronic conductivity but also the electroactivity of the array architecture.
| Electrodes | Detection limit/μM | Sensitivity/μA μM−1 cm−2 | Response time/s | Reference |
|---|---|---|---|---|
| ZnO nanorods | 2.2 | 4.76 | <10 | 26 |
| ZnO nanonails | 0.2 | 8.56 | <5 | 8a |
| Flower-like microstructure ZnO | 2.1 | 0.095 | <4 | 8b |
| Hierarchical micro/nanoarchitecture ZnO | 0.25 | 0.51 | <3 | 8b |
| ZnO nanoflower | 0.18 | 3.49 | <3 | 25 |
| CuO nanoarray | 0.17 | N.A. | <5 | 28 |
| SWCNT and catechin hydrate | 2.0 | 0.183 | N. A. | 39 |
| Hematoxylin multi-walled carbon nanotubes | 0.68 | 0.0268 | <2 | 11 |
| Carbon nanotube powder | N.A. | 0.9944 | <3 | 40 |
| Pristine ZnO nanorod array on alloy | 0.2 | 4.48 | <8 | This work |
| C@ZnO nanorod array on alloy | 0.1 | 9.4 | <4 | This work |
 | ||
| Fig. 4 Current variation versus N2H4 concentration plot derived from Fig. 3(a). | ||
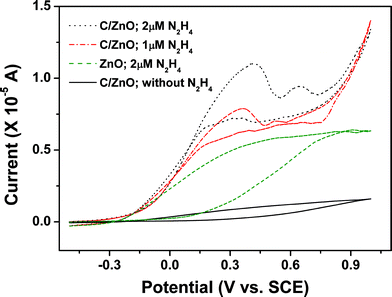
It is worth mentioning that the introduction of carbon to a pristine ZnO nanorod array could also improve the electrode stability in electrolyte. Results revealed that the CV curve of C@ZnO nanorod array electrode after successive cycling in the potential range of −0.5–1.0 V in 0.1 M PBS for 50 cycles showed negligible changes. The peak current for hydrazine oxidation retained 94% of its initial value and no detectable potential shift was observed. By contrast, only 82% of the initial oxidation current value could be recovered for a pristine ZnO nanorod array electrode after 50 times continuous cycling. It was also found that the C@ZnO-based sensor was stable over more than 30 days of continuous use, with storage in air at 4 °C when not in use. It could retain about 95% of its sensitivity after one month. The fabrication reproducibility of four electrodes, made independently, showed reproducibility with the cumulative variation of 3.0% for the detection of hydrazine. Fig. 5 shows CV curves of the four C/ZnO nanorod array electrodes in 0.1 M PBS buffer solution (pH = 8.0) in the presence of 2 μM N2H4, from which we can see the electrodes have almost the same oxidation peak current response. We believe that in addition to the chemical inertness of carbon, the carbon coating may act as an immobilization film to protect the electrode and ensure the electrode stability, even though the traditional Nafion film was not employed in our case.
 | ||
| Fig. 5 CV curves of four independent C/ZnO nanorod array electrodes in 0.1 M PBS buffer solution (pH = 8.0) in the presence of 2 μM N2H4. | ||
4 Conclusions
In summary, we have developed a novel hydrazine chemical sensor by using a C@ZnO nanorod array grown directly on an alloy substrate. The incorporation of carbon into the array is suggested to improve the specific surface area and electronic conductance of working electrode, thus leading to enhanced electrochemical performance in terms of sensitivity, detection limit, response time and stability. Our study only widens the potential applications of carbon-metal oxide nanostructure arrays but also presents a new compound electrode design for hydrazine sensing.Acknowledgements
Financially supported by self-determined research funds of CCNU from the colleges’ basic research and operation of MOE (No. CCNU09A01019), the National Natural Science Foundation of China (No. 50872039), China Postdoctoral Science Foundation (20090460996) and the Open Project Program of Key Laboratory of Quak & Lepton Physics (Huazhong Normal University), Ministry of Education, China(QLPL200902).References
- S. Garrod, M. E. Bollard, A. W. Nicholls, S. C. Connor, J. Connelly, J. K. Nicholson and E. Holmes, Chem. Res. Toxicol., 2005, 18, 115 CrossRef CAS.
- J.-W. Mo, B. Ogorevc, X. Zhang and B. Pihlar, Electroanalysis, 2000, 12, 48 CrossRef CAS.
- S. D. Zelnick, D. R. Mattie and P. C. Stepaniak, Aviat. Space Environ. Med., 2003, 74, 1285 Search PubMed.
- K. Yamada, K. Yasuda, N. Fujiwara, Z. Siroma, H. Tanaka, Y. Miyazaki and T. Kobayashi, Electrochem. Commun., 2003, 5, 892 CrossRef CAS.
- S. S. Narayanan and F. Scholz, Electroanalysis, 1999, 11, 465 CrossRef CAS.
- S. M. Colabi and H. R. Zare, J. Electroanal. Chem., 1999, 465, 168 CrossRef CAS.
- Y. Y. Liu, I. Schmeltz and D. Hoffmann, Anal. Chem., 1974, 46, 885 CrossRef CAS.
- (a) A. Umar, M. M. Rahman, S. H. Kim and Y. B. Hahn, Chem. Commun., 2008, 166 RSC; (b) Y. H. Ni, J. S. Zhu, L. Zhang and J. M. Hong, CrystEngComm, 2010 10.1039/b923857n.
- A. Safavi and M. A. Karimi, Talanta, 2002, 58, 785 CrossRef CAS.
- A. Salimi and R. Hallaj, Electroanalysis, 2004, 16, 1964 CrossRef CAS.
- A. Salimi, L. Miranzadeh and R. Hallaj, Talanta, 2008, 75, 147 CAS.
- B. K. Jena and C. R. Raj, J. Phys. Chem. C, 2007, 111, 6228 CrossRef CAS.
- B. Šljukić, C. E. Banks, A. Crossley and R. G. Compton, Electroanalysis, 2006, 18, 1757 CrossRef CAS.
- K. I. Ozoemena and T. Nyokong, Talanta, 2005, 67, 162 CrossRef CAS.
- A. A. Ensafi and E. Mirmomtaz, J. Electroanal. Chem., 2005, 583, 176 CrossRef CAS.
- D. J. Guo and H. L. Li, Electrochem. Commun., 2004, 6, 999 CrossRef CAS.
- B. Alvarez-Ruiz, R. Gomez, J. M. Orts and J. M. Feliu, J. Electrochem. Soc., 2002, 149, D35 CrossRef CAS.
- C. Bourdillon, M. Delamar, C. Demaille, R. Hitmi, H. Moiroux and J. Pinson, J. Electroanal. Chem., 1992, 336, 113 CrossRef CAS.
- N. Maleki, A. Safavi, E. Farjami and F. Tajabadi, Anal. Chim. Acta, 2008, 611, 151 CrossRef CAS.
- H. J. Zhang, J. S. Huang, H. Q. Hou and T. Y. You, Electroanalysis, 2009, 21, 1869 CrossRef.
- D. J. Guo and H. L. Li, J. Colloid Interface Sci., 2005, 286, 274 CrossRef CAS.
- L. Zheng and J. F. Song, Talanta, 2009, 79, 319 CrossRef CAS.
- C. Bathchelor-McAuley, C. E. Banks, A. O. Simm, T. G. J. Jones and R. G. Compton, Analyst, 2006, 131, 106 RSC.
- A. Umar, M. M. Rahman and Y. B. Hahn, Talanta, 2009, 77, 1376 CrossRef CAS.
- B. Fang, C. H. Zhang, W. Zhang and G. F. Wang, Electrochim. Acta, 2009, 55, 178 CrossRef CAS.
- A. Umar, M. M. Rahman, S. H. Kim and Y. B. Hahn, J. Nanosci. Nanotechnol., 2008, 8, 3216 CrossRef CAS.
- X. J. Zhang, A. X. Gu, G. F. Wang, W. Wang, H. Q. Wu and B. Fang, Chem. Lett., 2009, 38, 466 CrossRef CAS.
- G. F. Wang, A. X. Gu, W. Wang, Y. Wei, J. J. Wu, G. Z. Wang, X. J. Zhang and B. Fang, Electrochem. Commun., 2009, 11, 631 CrossRef CAS.
- H. B. Zeng, X. J. Xu, Y. Bando, U. K. Gautam, T. Y. Zhai, X. S. Fang, B. D. Liu and D. Golberg, Adv. Funct. Mater., 2009, 19, 3165 CrossRef CAS.
- J. P. Liu, C. X. Guo, C. M. Li, Y. Y. Li, Q. B. Chi, X. T. Huang, L. Liao and T. Yu, Electrochem. Commun., 2009, 11, 202 CrossRef CAS.
- H. J. Fan, Y. Yang and M. Zacharias, J. Mater. Chem., 2009, 19, 885 RSC.
- H. Zhang, D. R. Yang, Y. J. Ji, X. Y. Ma, J. Xu and D. L. Que, J. Phys. Chem. B, 2004, 108, 3955 CrossRef CAS.
- J. X. Wang, X. W. Sun, A. Wei, Y. Lei, X. P. Cai, C. M. Li and Z. L. Dong, Appl. Phys. Lett., 2006, 88, 233106 CrossRef.
- F. F. Zhang, X. L. Wang, S. Y. Ai, Z. D. Sun, Q. Wan, Z. Q. Zhu, Y. Z. Xian, L. T. Jin and K. Yamamoto, Anal. Chim. Acta, 2004, 519, 155 CrossRef CAS.
- Y. F. Li, Z. M. Liu, Y. L. Liu, Y. H. Yang, G. L. Shen and R. Q. Yu, Anal. Biochem., 2006, 349, 33 CrossRef CAS.
- A. Wei, X. W. Sun, J. X. Wang, Y. Lei, X. P. Cai, C. M. Li, Z. L. Dong and W. Huang, Appl. Phys. Lett., 2006, 89, 123902 CrossRef.
- L. Li and N. Koshizaki, J. Mater. Chem., 2010, 20, 2972 RSC.
- J. P. Liu, X. T. Huang, Y. Y. Li, X. X. Ji, Z. K. Li, X. He and F. L. Sun, J. Phys. Chem. C, 2007, 111, 4990 CrossRef CAS.
- A. Umar, S. H. Kim, J. H. Kim and Y. B. Hahn, J. Nanosci. Nanotechnol., 2007, 7, 4522 CrossRef CAS.
- Y. D. Zhao, W. D. Zhang, H. Chen and Q. M. Luo, Talanta, 2002, 58, 529 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
