 Open Access Article
Open Access ArticleA new phosphonate pendant-armed cross-bridged tetraamine chelator accelerates copper(II) binding for radiopharmaceutical applications†
Dannon J. Stigersa, Riccardo Ferdanib, Gary R. Weisman*a, Edward H. Wong*a, Carolyn J. Anderson*b, James A. Golenc, Curtis Moored and Arnold L. Rheingoldd
aDepartment of Chemistry, University of New Hampshire, Durham, New Hampshire 03824, USA. E-mail: gary.weisman@unh.edu; ehw@cisunix.unh.edu
bMallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri 63110, USA. E-mail: andersoncj@wustl.edu
cDepartment of Chemistry and Biochemistry, University of California, San Diego, La Jolla, California 92093, USA
dDepartment of Chemistry and Biochemistry, University of Massachusetts, Dartmouth, North Dartmouth, Massachusetts 02747, USA
First published on 15th December 2009
Abstract
A phosphonate pendant-armed cross-bridged cyclam chelator has been synthesized, complexed to Cu(II), radiolabeled with 64Cu under mild conditions, and its biodistribution studied.
The family of ethylene cross-bridged cyclam macrobicycles has received increasing attention as chelators in radiopharmaceutical research.1,2 An especially promising ligand for radio-copper binding is the dicarboxymethyl pendant-armed ligand 1 (CB-TE2A, Fig. 1) which imparts remarkable kinetic inertness to its Cu(II) complex resulting in superior in vivo behavior of its 64Cu-labeled bioconjugates.3,4 However, its sluggish radiolabeling kinetics has necessitated the use of relatively harsh protocols (e.g. 85 °C, pH 8, 1 h). While still practicable for bioconjugates of some biological targeting vectors,5 these severe labeling conditions have precluded the use of more sensitive peptides or biomolecules.
 | ||
| Fig. 1 Structures of ligands 1 (CB-TE2A) and 2 (CB-TE2P). | ||
Phosphonate pendant-armed cyclen and cyclam derivatives have been reported to have accelerated metal-binding kinetics relative to their carboxylate-armed analogues while retaining high thermodynamic stabilities of their complexes.6-10 We report here the synthesis and characterization of a di-methanephosphonate pendant-armed cross-bridged cyclam 2 (CB-TE2P), its Cu(II) complex as well as its 64Cu radiolabeling and in vivo biodistribution studies.
Ligand 2 was synthesized as shown in Scheme 1. Parent cross-bridged cyclam 3 was first converted to bis-diethylphosphonate 4 by a variant of the Kabachnik–Fields three-component reaction11,12 and this synthetic intermediate was then hydrolyzed to the title ligand. Thus, reaction of 3 with triethylphosphite and paraformaldehyde in anhydrous THF under nitrogen at room temperature for 4 days gave pure 4 in 94% yield after extractive workup. 4 was hydrolyzed in 6 N HCl under reflux for 24 h to give H22 in 64% yield as a hydrochloride after purification by ion-exchange chromatography.
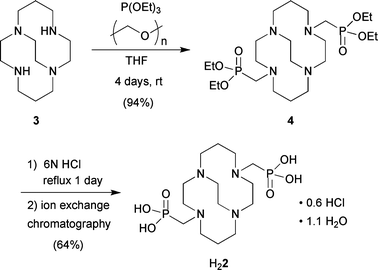 | ||
| Scheme 1 Synthesis of ligand 2. | ||
Diffusion of acetone into an aqueous solution gave crystals of H22·HCl·4H2O suitable for X-ray crystallography. The structure (Fig. 2) shows the ligand to be di-inside-protonated and the two phosphonate arms to be intramolecularly O–H⋯O hydrogen bonded. The ligand adopts a [2233]/[2233] conformation rather than the [2323]/[2323] conformation,13,14 perhaps in order to facilitate the appropriate arm–arm distance for the intramolecular H-bond.
 | ||
| Fig. 2 X-ray structure of H22·HCl·4H2O (50% thermal ellipsoids for all non-hydrogen atoms). Intramolecular hydrogen bonds are shown as dashed lines. One of the two half-occupancy symmetry-related hydrogens on O2 has been removed for clarity and so that the H-bond can be shown. | ||
The Cu(II) complex of 2 was first prepared from CuCl2 and the ligand in refluxing methanol with the pH adjusted to 8 using aqueous NaOH. Subsequently, it was found that complexation in methanol was actually complete in less than 5 min at ambient temperature. The blue complex (λmax (MeOH)/nm 639 (ε/dm3 mol−1 cm−1 35)) was studied by cyclic voltammetry in 0.1 M aq. NaOAc. It exhibited a quasi-reversible reduction at −0.96 V (Ag/AgCl), typical of Cu(II) cross-bridged cyclam complexes.15 Its acid inertness was assayed under pseudo first-order conditions in 5 M HCl at 90 °C and found to have a half life of 3.8(1) h. While this is considerably shorter than the 154 h previously reported for Cu-1, it is significantly more inert than Cu-DOTA and Cu-TETA, both of which were completely decomplexed within minutes.15
The X-ray crystal structure of Cu-2‡ is shown in Fig. 3. Full envelopment of the cation within the ligand's N4O2 donor set can be seen in its distorted octahedral coordination mode. Elongated bond lengths of 2.20 Å for N(4)–Cu(1) and 2.45 Å for Cu(1)–O(6) designate the Jahn–Teller distortion axis. An N(1)–Cu(1)–N(3) bond angle of 174.8° confirms a good fit of the cation inside the chelator cavity which can be compared to the 177.5° reported for Cu-1.16 Again the ligand adopts the [2233]/[2233] conformation rather than the distorted diamond-lattice [2323]/[2323] conformation more commonly seen in Cu(II) complexes of cross-bridged cyclams, including Cu-1.16
 | ||
| Fig. 3 X-ray structure of Cu-2 (50% thermal ellipsoids for all non-hydrogen atoms. Only one of two independent complexes is shown. Both exhibit distorted octahedral coordination at Cu and have the same basic ligand conformation. Hydrogens, sodium and chloride ions, and solvent molecules have been removed for clarity.) | ||
Even though 2 showed rapid Cu2+ complexation in methanol and ethanol, the radiolabeling experiments with 64Cu were performed in water or aqueous buffers since organic solvents are not well tolerated by animals and therefore less convenient for in vivo experiments. No-carrier-added 64Cu-2 was prepared in water by addition of a solution of 64CuCl2 in 0.1 M HCl to a 2–5 mM solution of the ligand in water. We found that radio-TLC was not adequate for determining labeling purity as it only differentiated between free and chelated 64Cu. By contrast, radio-HPLC was able to resolve whether more than one product formed. Thus at room temperature, “exo-complexes” (vide infra) were observed, while 100% formation of the fully-engulfed complex was obtained at higher temperatures (>90 °C) as confirmed by LC-MS and radio-LC-MS compared to an authentic sample. Under carrier-added conditions (molar ratio of ligand/Cu2+ = 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35) 64Cu-2 formed in a 100% yield even at room temperature.
35) 64Cu-2 formed in a 100% yield even at room temperature.
Under no-carrier-added conditions (molar ratio of ligand/Cu2+ > 105:1) radioactive peaks were observed by LC-MS at lower retention times than that of 64Cu-2. This is attributed to the slower kinetics of complex formation with extremely low concentrations of Cu2+ (nM to pM). One hypothesis is that the formation of “exo” or “out of cleft” complexes occurs initially, rather than the fully encapsulated form that is shown in the crystal structure. At this stage the Cu2+ is only bound to one or more of the pendant arms, and its coordination sphere is likely to contain water molecules, while some of the nitrogen atoms in the macrocycle remain protonated. The conversion to the thermodynamically stable final complex is a slower process that can be accelerated either by increasing copper concentration (carrier-added conditions) or by heating.
Another possibility is the presence of trace level impurities (< 1%) that bind 64Cu2+ more rapidly than 2. Adding cold copper saturates the impurities and the thermodynamically favored 64Cu-2 preferentially forms. Increasing the temperature also favors the formation of the desired complex due to its superior thermodynamic stability. A similar hypothesis was formulated in 2005 by Boswell et al.17 for the labeling of compound 1.
In order to gain an insight into the pharmacokinetics of 64Cu-2, a biodistribution study was performed on 26-day-old Lewis male rats. 64Cu-2 (50 μCi (∼1 μCi/μg) in 150 μL of saline) was injected through the tail vein, and the animals were sacrificed at 1, 2, 4 and 24 h after injection. The organs were harvested and their activity measured with a γ counter.
As shown in Fig. 4, the radiolabeled compound clears rapidly from the blood (from 0.15% ID/g at 1 h to 0.002 ID/g at 24 h) and has only minor residual uptake in organs such as liver, spleen, lung, heart and marrow (not higher than 0.03% ID/g at 24 h). Rapid clearance of 64Cu-2 from the liver is indicative of its high in vivo stability, as it is known that dissociated 64Cu is rapidly coordinated by liver proteins such as SOD, ceruloplasmin and metallothionein.18
 | ||
| Fig. 4 Biodistribution of 64Cu-2 in male Lewis rats at 1, 2, 4, and 24 h post-injection (in % Injected Dose/gram). | ||
The biodistribution data demonstrate that 64Cu-2 is rapidly excreted through the kidney, where there is 0.36% ID/g after 24 h. Not surprisingly, at earlier time points a significant percentage of radioactivity was observed in the bone, most likely due to the affinity of the methanephosphonic pendant arms for the hydroxyapatite in the bone; however, this activity is cleared several-fold by 24 h post-injection. Similar results were obtained for methanephosphonic derivatives of cyclen.9
Unlike the recently-reported 4,11-dimethyl-cyclam-1,8-bis(methylphosphonic acid),10 chelator 2 forms a sufficiently inert Cu(II) complex for radiopharmaceutical applications. Syntheses and bioconjugation of unsymmetrically functionalized monophosphonate cross-bridged cyclam are under active investigation in our laboratories and will be reported in due course.
Acknowledgements
This work was supported by National Institutes of Health (U.S.A) grant CA093375.Notes and references
- M. Shokeen and C. J. Anderson, Acc. Chem. Res., 2009, 42, 832–841 CrossRef CAS.
- T. J. Wadas, E. H. Wong, G. R. Weisman and C. J. Anderson, Curr. Pharm. Des., 2007, 13, 3–16 CrossRef CAS.
- J. E. Sprague, Y. Peng, X. Sun, G. Weisman, E. Wong, S. Achilefu and C. Anderson, Clin. Cancer Res., 2004, 10, 8674–8682 CrossRef CAS.
- L. Wei, C. Butcher, Y. Miao, F. Gallazzi, T. P. Quinn, M. J. Welch and J. S. Lewis, J. Nucl. Med., 2007, 48, 64–72 CAS.
- L. Wei, Y. Ye, T. Wadas, J. S. Lewis, M. J. Welch, S. Achilefu and C. J. Anderson, Nucl. Med. Biol., 2009, 36, 277–285 CrossRef CAS.
- S. Füzerová, J. Kotek, I. Císařová, P. Hermann, K. Binnemans and I. Lukeš, Dalton Trans., 2005, 2908–2915 RSC.
- J. Kotek, P. Lubal, P. Hermann, I. Cisarova, I. Lukes, T. Godula, I. Svobodova, P. Taborsky and J. Havel, Chem.–Eur. J., 2003, 9, 233–248 CrossRef CAS.
- I. Lukeš, J. Kotek, P. Vojtíšek and P. Hermann, Coord. Chem. Rev., 2001, 216–217, 287–312 CrossRef CAS.
- X. Sun, M. Wuest, Z. Kovacs, A. D. Sherry, R. Motekaitis, Z. Wang, A. E. Martell, M. J. Welch and C. J. Anderson, JBIC, J. Biol. Inorg. Chem., 2003, 8, 217–225 CrossRef CAS.
- I. Svobodová, J. Havlícková, J. Plutnar, P. Lubal, J. Kotek and P. Hermann, Eur. J. Inorg. Chem., 2009, 3577–3592 CrossRef CAS.
- P. Kafarski and J. Zon, in Aminophosphonic Aminophosphinic Acids, ed. V. P. Kukhar and H. R. Hudson, John Wiley & Sons, Ltd., Chichester, 2000, pp. 33-74 Search PubMed.
- M. Syamala, Org. Prep. Proced. Int., 2005, 37, 103–171 CrossRef CAS.
- J. Dale, Acta Chem. Scand., 1973, 27, 1115–1129 CrossRef CAS.
- J. Dale, in Topics in Stereochemistry, ed. N. L. Allinger and E. L. Eliel, John Wiley & Sons, New York, 1976, vol. 9, pp. 199-270 Search PubMed.
- K. S. Woodin, K. J. Heroux, C. A. Boswell, E. H. Wong, G. R. Weisman, W. Niu, S. A. Tomellini, C. J. Anderson, L. N. Zakharov and A. L. Rheingold, Eur. J. Inorg. Chem., 2005, 4829–4833 CrossRef CAS.
- E. H. Wong, G. R. Weisman, D. C. Hill, D. P. Reed, M. E. Rogers, J. S. Condon, M. A. Fagan, J. C. Calabrese, K.-C. Lam, I. A. Guzei and A. L. Rheingold, J. Am. Chem. Soc., 2000, 122, 10561–10572 CrossRef CAS.
- C. A. Boswell, P. McQuade, G. Weisman, E. Wong and C. Anderson, Nucl. Med. Biol., 2005, 32, 29–38 CrossRef CAS.
- C. Boswell, X. Sun, W. Niu, G. Weisman, E. Wong, A. Rheingold and C. Anderson, J. Med. Chem., 2004, 47, 1465–1474 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, characterization, and NMR spectra of precursor 4 and ligand 2; synthesis and characterization of the Cu(II) complex of 2; X-ray crystallographic data for H22·HCl·4H2O and Cu-2; and biodistribution data of 64Cu-2 in male Lewis rats. CCDC reference numbers 750366 & 750367. For ESI and crystallographic data in CIF format see DOI: 10.1039/b920871b |
| ‡ Crystal data for2: C14H41ClN4O10P2, monoclinic, C2, a = 11.5219(16), b = 11.7468(16), c = 9.6554(13) Å, β = 115.254(3)°, V = 1181.9(3) Å3, Z = 2, T = 100(2) K. 6044 reflections collected, 2590 unique, Bruker D8 diffractometer (Mo-Kα). R1 = 3.37%, wR2 = 8.35% (I > 2σI). Refined as a racemic (68/32) twin. Crystal data for Cu-2: C14H34Cl0.5CuNa0.5N4O9.5P2, triclinic, P |
| This journal is © The Royal Society of Chemistry 2010 |

![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ,
,