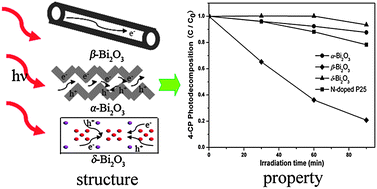
Three polymorphs of Bi2O3 were selectively synthesized via solution-based methods. The phase structures of the as-prepared samples were confirmed by X-ray powder diffraction (XRD) and X-ray photoelectron spectroscopy (XPS). UV-vis diffuse reflectance spectroscopy was employed to study the optical properties of Bi2O3 polymorphs, and the band gaps were estimated to be 2.80, 2.48, and 3.01 eV for α-Bi2O3, β-Bi2O3, and δ-Bi2O3, respectively. The photocatalytic performances of the oxides were investigated by decomposing methyl orange and 4-chlorophenol under visible irradiation at room temperature. It was observed that β-Bi2O3 displayed much higher photocatalytic performance than N-doped P25. Among the three polymorphs of Bi2O3, the photocatalytic activities followed the order: β-Bi2O3 > α-Bi2O3 > δ-Bi2O3, which was in good accordance with the photoluminescence spectra measurement results. The synergistic effect of the crystal and electronic structures on the photocatalytic performances of Bi2O3 polymorphs was investigated. The much better photocatalytic activity of β-Bi2O3 was considered to be closely related to its smaller band gap, higher crystallinity and unique tunnel structure.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...