In situ electrochemical monitoring of reactive oxygen and nitrogen species released by single MG63 osteosarcoma cell submitted to a mechanical stress†
Ren
Hu
ab,
Manon
Guille
a,
Stéphane
Arbault‡
a,
Chang Jian
Lin
b and
Christian
Amatore
*a
aUMR CNRS-ENS-UPMC 8640 “PASTEUR” and LIA CNRS XiamENS, Département de Chimie, École Normale Supérieure, 75231 Paris, France. E-mail: Christian.Amatore@ens.fr; Tel: 33-1-4432-3388
bState Key Laboratory of Physical Chemistry of Solid Surfaces and LIA CNRS XiamENS, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, Fujian 361005, China
First published on 24th June 2010
Abstract
The oxidative stress responses of single MG63 osteosarcoma cells submitted to a brief mechanical stress have been investigated by amperometry at platinized carbon fiber electrodes for monitoring and characterizing the nature and the amounts of the various reactive oxygen (ROS) and reactive nitrogen species (RNS) released. It was thus shown that, on average, a single MG63 cell released prominent amounts of reactive nitrogen species (17 fmol NO˙, 6 fmol ONOO−, and 5 fmol NO2−) together with a comparatively small quantity of H2O2 (2 fmol). These species resulted from the primary production of 13 fmol for O2˙− and 28 fmol for NO˙ per single cell as reconstructed from the stoichiometries of the ROS and RNS releases. The high NO˙/H2O2 and NO˙/O2˙− ratios thus found are perfectly consistent with previous claims that the malignant bone formation ability of the osteosarcoma cells is related to a specific high production of NO˙ associated to a small one of O2˙−.
Introduction
Superoxide anion (O2˙−) and nitric oxide (NO˙) are generated by most living aerobic cells and give rise to a series of secondary reactive species, including H2O2, ONOO−, NO2−, etc., which are ultimately emitted by cells and collectively designed by reactive oxygen and nitrogen species (RONS).1 RONS play a vital role in cell and tissue activities. For instance, H2O2 and NO˙, contribute to signal transduction.2 Conversely, excessive levels of RONS may destroy proteins, membrane lipids, DNA, etc., in the cell itself or in adjacent ones. For example, H2O2 and ONOO−, two highly reactive species, are generated by non specific immune cells to fight pathogens.3 Hence, to maintain a healthy state, cells and tissues need to keep a proper balance between the positive and negative effects of RONS by controlling their nature and concentration within an adequate range (homeostasis) as well as to remedy their deleterious consequences.To this object, both enzymatic and non-enzymatic antioxidant strategies are in operation in cells to avoid the damaging effects of RONS while keeping the benefits of low controlled doses. However, under some circumstances the homeostatic balance may be overloaded by an excessive RONS quantity and a situation termed oxidative stress emerges. Oxidative stress is thus involved in many human diseases, such as cancer, AIDS, neurodegeneration, etc., so that its monitoring and control have gained much attention in view of understanding and assessing the pathogenesis and progression of human diseases.4,5 In particular, it has recently been suggested that beyond the intrinsic quantity of RONS produced, the ratio between the concentrations of NO˙ and of the other RONS, in particular the NO˙/H2O2 one, offers a strong indicator of cellular dysfunctions.
In the following work we are interested in this very issue in connection with malignant bone cells. In fact, bones are living complex tissues submitted during their functions to constant mechanical stresses which damage persistently their mineral micro-architectures. Thus, bones undergo constant local reconstruction and remodeling throughout life. As described first by Frost,6 proper bone repairing/remodeling bears upon a regulated balance between the activities of two, amongst the five types of bone cells, main cell types: osteoblasts and osteoclasts (Fig. 1). Osteoblasts initiate the process of forming the bone tissue by producing osteoid. The osteoid is then mineralized so that, together with entrapped bone cells, it develops into new bone tissues that maintain bones strength. Conversely, osteoclasts prevent too much bone tissue from producing so that bones may retain their proper shape. They act by removing the mineralized bone matrix and eliminating the organic components. The antagonist action of these two cell types allows a proper bone growth and maintenance but also helps regulating the amounts of minerals (e.g., calcium) in blood by storing them in bones or by recirculating them. For example, failure to maintain such proper regulation may lead to important diseases among which is osteoporosis.
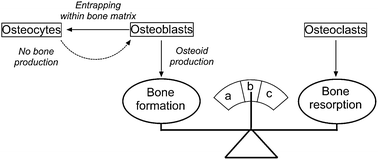 | ||
| Fig. 1 Schematic diagram illustrating the role of osteoblasts and osteoclasts in maintaining a proper bone remodeling balance: (a) excessive bone growth; (b) normal bone remodeling; (c) bone loss. | ||
This overall process is regulated by signaling pathways which control bone cell–cell communications and are ultimately enabling the further involvement of rapid-acting transcription factors, such as NF-κB, which lead to cellular differentiation and are found to be chronically activated in many inflammatory responses following an initial activation of RONS pathways.7 In fact, RONS are essential primary actors of inflammatory responses and have been shown to play crucial roles depending on their exact nature so that the generic term RONS is somewhat misleading. For example nitric oxide has been evidenced to favor bone formation by inducing osteoclasts apoptosis and inhibiting their activities.8 Conversely, reactive oxygen species production (ROS) is suggested to enhance osteoclast activities in bones.9,10 ROS9,11,12 and nitric oxide13 have thus been recognized in recent years as important modulation factors regulating bone metabolism, and, to some extent, the NO˙/ROS ratio, particularly NO˙/H2O2, has been suggested to reflect the bone formation ability.
Yet, to our best knowledge, RONS measurements in single bone cells have never been reported, all the above suggestions about their involvement being then based on indirect evidences. Indeed, the evanescent nature of the RONS and their extremely minute production at a single cell level (generally between a few attomoles and a few femtomoles at most) bring about high analytical challenges to their measurement,14,15 particularly when real time sensitive detection is required.16,17
Conversely, the introduction of the “artificial synapse” concept based on platinized carbon fiber microelectrodes has allowed the detection of RONS at the single cell level released by many cell types.18–39 Placing a microelectrode with a platinized electroactive surface (ca. 1–10 μm in diameter depending on the cell type) in the very vicinity of a cell membrane (see Fig. 2a below) allows collecting, detecting and quantifying with the proper sensitivity and in real time electroactive species emitted from a normal or stimulated cell.19,20 The validity of this strategy has been established by the detection at the single cell level of RONS emission by fibroblasts,19–23 keratinocytes,24 macrophages,25–30 neuronal31,32 or myeloid33 cells, and used for the assessment of the effect of drugs and candidate drugs on single cell oxidative stress.34–39
 | ||
| Fig. 2 (a) Schematic view of the “artificial synapse” configuration showing the placement of the platinized carbon fiber electrode and of the micropipette sealed tip used to induce a brief mechanical stress onto an adherent single MG63 osteosarcoma cell. (b–d) Optical microphotographs illustrating different phases of the experimental protocol (see text). | ||
This prompted us to examine if the artificial synapse concept could be adapted to monitor and characterize RONS released by MG63 human osteosarcoma single cells following a brief mechanical stress. Osteosarcoma is the most common and malignant histological form of primary bone cancer.40 It is an aggressive cancerous neoplasm arising from primitive transformed mesenchymal cells exhibiting osteoblastic differentiation and leading to malignant uncontrolled bone formation via osteoid production.41 Hence, establishing that RONS release by MG63 osteosarcoma single cells submitted to a brief mechanical stress may be characterized and quantified in real time is expected to offer an innovative strategy for the quantitative assessment of these malignant cells properties as well as a general method for understanding the delicate balance of bone cell activities (Fig. 1).
Stimulation by a brief mechanical stress involving a micrometric puncture of the cell membrane was selected to induce oxidative stress responses in MG63 single cells. In our view, even if a precise mimicking of pressure stresses and possible membrane punctures experienced by bone cells after shocks (bone maintenance) or created by excessive bone growth (osteosarcoma) is impossible to achieve in the absence of detailed information on such processes it seems to us that the stimulation procedure retained in this work bears some relationship to the physical stresses imposed on bone cells. Furthermore, since the brief puncturing of the cell membrane may also induce a local depolarization and entrance of several ions, particularly of calcium which is a known effector of the main enzymatic pools responsible for RONS production,23 this stimulation may in fact closely mimic the case of bone cells aggressed by membrane micropunctures due to adjacent bone microstructures. Indeed, natural bones have delicate hierarchical micro- and mesostructures which provide them with their macroscopic mechanical supporting plastic functions.42 In turn, mechanical stresses experienced by bones, even when they are not sufficient to lead to their macroscopic rupture, provoke local disruptions of their fine mineral structures which need constant repairing to avoid progressive bone weakening and destruction as, e.g., occurs during osteoporosis. For this reason, the behavior of bone cells submitted to designed mechanical stress has attracted much attention.43–45 The corresponding studies pointed out to the existence of mechanical stress responsive groups of gene encoded enzymes, including NO˙ synthase.46 This again stigmatized the close connection between osteosarcoma and bone reconstruction ability on the one hand and, on the other hand, suggested that a specific production of NO˙ amongst other RONS occurred in response to mechanical stresses.
Experimental
Cell cultures
The MG63 human osteosarcoma cell line was purchased from ATCC (Catalog No. CRL-1427). The cells were cultured in Eagle's Minimal Essential medium (EMEM, ATCC catalog No. 30–2003) supplemented with 10% heat-inactivated fetal bovine serum, 100 U mL−1 penicillin, 100 mg mL−1 streptomycin, and were incubated at 37 °C in a humidified atmosphere with 5% CO2. Monolayers of confluent cells were harvested by trypsination (trypsin-EDTA). 1000 to 5000 cells were then re-suspended in each plastic Petri dish (35 mm diameter) and analyzed 24 h later; meanwhile cells spontaneously adhered to the Petri dish surface. Just before experiments, cells were washed three times with PBS and the measurements were performed with isolated cells to avoid any interference between products possibly released by the neighboring cells during the oxidative bursts.Microelectrodes preparation
Platinized carbon fiber microelectrodes were prepared according to already published methods.18–39 Individual carbon fibers (10 μm diameter, Thornel P-55S, Amoco Performance Products, Greenville, SC, USA) were aspirated into 1.2 mm diameter glass capillary tubing (GC120F-10, Clark Electromedical Instruments, Harvard Apparatus, Edenbridge, UK), each capillary was then pulled with a microelectrode puller (Model PB7, Narishige, Tokyo, Japan) and the carbon fiber protruding from the tip was insulated by a thin coating of poly(oxyphenylene) electropolymerized according to a previously described method.47 The protruding carbon fiber was thus immersed into a solution water–methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) containing 0.4 M allylamine, 0.23 M 2-allylphenol and 0.23 M 2-butoxyethanol, and submitted to a 4 V potential step vs. a platinum counter electrode for 3 min. The microelectrode was then washed in distilled water and cured for 3 h at 150 °C to allow reticulation and insulation of the polymer coating.
1, v/v) containing 0.4 M allylamine, 0.23 M 2-allylphenol and 0.23 M 2-butoxyethanol, and submitted to a 4 V potential step vs. a platinum counter electrode for 3 min. The microelectrode was then washed in distilled water and cured for 3 h at 150 °C to allow reticulation and insulation of the polymer coating.
A fresh cross-section of the coated fiber was then exposed by a simple cutting of the fiber tip. The polymer-coated carbon fiber was then polished on a diamond particle whetstone microgrinder (Model EG-4, Narishige, Tokyo, Japan) at an angle of 45° for 3 min to expose a fresh and regular elliptic carbon surface area of ca. 100 μm2. Polished microelectrodes were then platinized by reducing hydrogen hexachloroplatinate (25 mM H2PtCl6 in presence of 0.5 mM lead acetate in PBS) at −60 mV vs. SSCE. The quantity of deposited black platinum was followed by integrating the electrodeposition current, the process being interrupted when a charge of 30 μC had been passed. After the deposition the electrodes were manipulated in air since they were designed to perform in aerated solutions owing to the requirements imposed by the aerobic cells. In this work or previous ones which used the same preparation and storage procedure no change of the Pt-black electroactivity was noted even when the electrodes were stored for different numbers of days before their use. This points out to the Pt-Black surface stability after reaching some equilibrium with the atmosphere.
As tested in previous works19,20,30 using flow micro-cell injections this procedure led to reasonably reproducible electrodes, i.e., with response times (less than 0.5 s) and sensitivities that were adequately constant (standard deviation 20%). In this respect it needs to be emphasized that in these experiments the electrode acts as a collector and its placement at ca. 5 μm distance from the cell membrane ensures a 100% collection efficiency as established in former works.23 On the other hand, since, except at their very beginning, oxidative stress bursts signals varied smoothly with time (compare Fig. 3 below) any variations in the response times around their above mean value were irrelevant.
 | ||
| Fig. 3 Average current responses detected at four different potentials (+300, +450, +650, and +850 mV vs. SSCE) featuring the release of RONS by a single MG63 osteosarcoma cell following a brief mechanical stress. Each current–time response results from averaging of 30 independent measurements performed on different MG63 cells using several different microelectrodes. | ||
Membrane mechanical stress
Single cell release of RONS was stimulated by a quick mechanical stress imposed by means of a brief poking of its membrane.23 This was exerted by a rapid vertical movement of the sealed tip of a ca. 1 μm diameter glass micro-pipette (1 mm glass rod, GR100-10, Clark Electromedical Instruments, pulled with a PB7 puller, Narishige) exerted with a hand-controlled micromanipulator (MHW-103, Narishige). The vertical movement implied a back-and-forth displacement of the tip over ca. 2 μm (compare Fig. 2 below) performed in less than one second, yet its exact duration was impossible to determine except by the fact that the cellular responses were evoked less than one second after the vertical movement of the micro-tip began (compare Fig. 3 above).Experimental protocol
Experiments were performed at controlled room temperature (22 ± 1 °C) on the stage of an inverted microscope (Axiovert 135, Carl Zeiss, Germany) placed in a Faraday cage. The Petri dish (35 mm diameter, Nunc, Denmark) containing adherent MG63 cells was washed three times to eliminate the culture medium and filled with PBS buffer immediately prior to experiments. All experiments were performed with PBS exposed to air to maintain proper aerobic conditions as required by the cells. The microelectrode tip and the glass micro-tip were precisely positioned with two micromanipulators (MHW-103 and Narishige, respectively) according to the configuration shown in Fig. 2a and c. The electrode potential was set with a Potentiostat (PRG-DEL, TACUSSEL electronique, France) and the current–time variations due to the RONS species oxidizable at the selected potential were stored in a computer through a Powerlab/4SP analog to digital converter using Chart 5.0 software (ADInstruments, USA) for further processing.Reconstruction of the individual RONS fluxes
By definition the current signals of each individual electroactive RONS at a given potential are detected additively so that each individual RONS released flux cannot be monitored directly. In particular, were any other oxidizable species present besides those investigated here they would be monitored as well. However, previous works26,29,30 established that was never the case so that monitoring sequentially the currents at 300, 450, 650, and 850 mV vs. SSCE, allowed a proper reconstruction of the individual fluxes of the four electroactive RONS of interest here. This was performed through solving a set of four linear equations relating these individual fluxes to the four currents monitored at these four potentials. These linear relationships follow from the observation that when the platinized carbon fiber electrodes used here were poised at 300 mV, the oxidation current for H2O2 achieved 77% of its limiting current plateau while that for ONOO− reached only 32% of its plateau; conversely, at 450 mV, the two limiting current plateaus of two species were monitored without any interference of NO˙ or NO2− which were not electroactive at this potential; at 650 mV, H2O2, ONOO− and NO˙ current plateaus were determined collectively but not that of NO2−; finally, at 850 mV the sum of all four RONS current plateaus was monitored. It then follows that the current plateau of each species (viz., proportional to the released species flux) may be reconstructed at any time from the four experimental measurements by application of the following relationships:| iH2O2 = 2.22 × (i300 mV −0.32 × i450 mV) | (1) |
| iONOO− = 2.22 × (0.77 × i450 mV − i300 mV) | (2) |
 | (3) |
| iNO2− = i850 mV −i850 mV | (4) |
Results
Characterization of RONS in oxidative stress responses of MG63 cells
Oxidative stress responses have been elicited from single MG63 cells mechanically stressed with a sealed glass micropipette tip (Fig. 2a). The corresponding oxidative stress responses were detected with total collection efficiencies (viz., 100%)23 by a platinized carbon fiber microelectrode positioned above the cell so as to create an artificial synapse configuration (Fig. 2a). The microelectrode was positioned at 10 μm above the Petri dish bottom, thus leaving a sufficient spacing above the cell apex (about 5 μm) for inserting the sealed tip of the micropipette. The microtip was rapidly moved downward to poke the cell membrane and was retracted in less than one second using the z axis controller of the micromanipulator (see Experimental section).20,23 Though the exact duration of the contact between the sealed micropipette tip and the cell membrane could not be known independently, it is of interest to note that the oxidative bursts raised sharply within less than one second after the poking movement was initiated (origin of time in Fig. 3).The microphotographs in Fig. 2b and c illustrate the different phases of the experiment. Briefly, in Fig. 2b, a single MG63 cell was selected and the microelectrode as well as the micro-tip were moved into its vicinity; in Fig. 2c, the microelectrode and the micro-tip were positioned as sketched in Fig. 2a to create an artificial synapse configuration; this allowed a brief stress (<1s) to be exerted with the microtip and the current–time signal to be collected. Fig. 2d is a post-control showing the same cell after the experiment when the microelectrode and the micro-tip had been moved away. This control view evidenced that the investigated cell retained its morphology after its mechanical stimulation and after the few minutes course of the RONS release (see Fig. 3).
A similar series of experiments was repeated over different cells (N = 30 for each potential) with the electrode potential poised at one of the four following values, 300, 450, 650, and 850 mV vs. SSCE (see Experimental section). For each selected potential value, the time-dependent courses of the currents were monitored from 30 different cells using different microelectrodes. Finally, these 30 individual responses were averaged to obtain a statistically significant current–time course for each potential value (Fig. 3).20 Such averaging was required to circumvent the cellular variability; note in this respect that since the collection efficiency was quantitative23 microelectrode and the diffusional flight across the artificial synaptic gap (ca. 5 μm) negligible (a few tens of milliseconds at most) using different microelectrodes had no effect on the signal variability but ensured that detection occurred at fresh platinized surfaces.
It is immediately clear from this figure that the current featuring hydrogen peroxide and peroxynitrite oxidations detected at 450 mV (see Experimental section) resulted in being much smaller than that at 650 mV which summed up the currents for NO˙, H2O2 and ONOO−. Also the current at 850 mV (though higher than that at 650 mV because it included also that due to NO2−) did not differ drastically from that at 650 mV. Finally, a comparison between currents at 300 mV where H2O2 is the main species responsible for the current and at 450 mV where both H2O2 and ONOO− are detected evidenced qualitatively that the flux of hydrogen peroxide is lesser than that of peroxynitrite. This evidenced qualitatively that NO˙ was the dominant RONS released by MG63 cells, being thus in agreement with previous suggestions.
Following the procedure detailed in the Experimental section, the analysis of the four currents shown in Fig. 3 provided the average total charges due to each individual RONS: QH2O2 = 360 pC, QONOO− = 580 pC,  and QNO2− = 870 pC (N = 30, standard deviations: 18%). Note that these standard deviations reflected mostly the cellular variability, since the electrode collection efficiencies and time constants did not vary sufficiently to affect the monitored current intensities and time course (see Experimental). Using Faraday’s law, viz., QRONS = nRONSFΘRONS where nRONS is the electron stoichiometry of the electrooxidation of a given RONS, ΘRONS its total released quantity and F the Faraday constant, this showed that an average stimulated MG63 cell produced 2 fmol of H2O2 (n = 2), 6 fmol of ONOO− (n = 1), 17 fmol of NO˙ (n = 1) and 5 fmol of NO2− (n = 2). This quantitatively confirmed the above conclusions drawn qualitatively from the compared magnitudes of the currents determined at each potential in Fig. 3.
and QNO2− = 870 pC (N = 30, standard deviations: 18%). Note that these standard deviations reflected mostly the cellular variability, since the electrode collection efficiencies and time constants did not vary sufficiently to affect the monitored current intensities and time course (see Experimental). Using Faraday’s law, viz., QRONS = nRONSFΘRONS where nRONS is the electron stoichiometry of the electrooxidation of a given RONS, ΘRONS its total released quantity and F the Faraday constant, this showed that an average stimulated MG63 cell produced 2 fmol of H2O2 (n = 2), 6 fmol of ONOO− (n = 1), 17 fmol of NO˙ (n = 1) and 5 fmol of NO2− (n = 2). This quantitatively confirmed the above conclusions drawn qualitatively from the compared magnitudes of the currents determined at each potential in Fig. 3.
Reconstruction of the initial production of O2˙− and NO˙ by MG63 cells
As sketched in Fig. 4,20 three of the species, viz., H2O2, ONOO−, and NO2−, detected in the oxidative stress bursts of aerobic cells result from the spontaneous or catalyzed reactions of two primary species, O2˙− and NO˙ which are produced by specific enzymatic pools involving NADPH-oxidases29 and/or xanthine oxidases48 for O2˙−, and constitutive and/or inducible NO-synthases for NO˙.26,29 | ||
| Fig. 4 Reaction scheme describing the origin of the four RONS detected in the oxidative bursts of MG63 osteosarcoma cells following their mechanical stimulation, from the primary production of superoxide ions and nitric oxide (see text). | ||
H2O2 is formed by the extremely fast (spontaneous or catalyzed by superoxide dismutase) disproportionation of O2˙− (viz., 2 O2˙− per H2O2).49 ONOO− results from the diffusion-limited addition of NO˙ onto O2˙−.50 NO2− is the electroactive decomposition product of ONOO− under our conditions (viz., 1 NO2− per ONOO−).51 Note that all these reactions are sufficiently rapid to occur within the cell cytoplasm before the detected efflux was released in the extracellular space. Similarly the diffusion length (<5 μm) was too small to lead to any diffusional filtering of the signals.23 Also the collection efficiencies of the electrodes remained quantitative. Hence, the kinetics observed from the current traces may represent only the outcome of their competition within the cell cytoplasm and the progressive slowing-down production rates of O2˙− and NO˙. This is in perfect agreement with the fact that all current–time amperometric curves displayed in Fig. 3 display the same apparent kinetics.
The overall stoichiometry of each reaction enables reconstructing the primary quantities of O2˙− and NO˙ produced by an average MG63 cell in response to its brief mechanical stimulation. This evidences that MG63 cells produce about twice more NO˙ (28 fmol) than O2˙− (13 fmol). Though this is coherent with previous suggestions that NO˙ is an important contributor of bone cells responses to mechanical stress,46 such result diverges strongly from the usual observations made by some of us on many normal cellular types for which the production rates of O2˙− and NO˙ were generally found extremely close. For example, the ratio NO˙/H2O2 in cells as different as human fibroblasts or murine macrophages ranged between 1 and 3 while it reached nearly 9 for MG63 cells. This points to the existence of specific pathways in these bone cells focused towards NO˙ production, possibly involving constitutive and/or inducible NO-synthases since both have been identified in MG63.52,53 In this relation, it is worth noting that constitutive NO-synthases have been reported to produce nitric oxide in response to cell mechanical stress.46,54
Conversely, reactive oxygen species (ROS) stem from the primary production of superoxide anion (see Fig. 4). In all aerobic cells small steady fluxes of O2˙− are produced by side-reactions of the oxygen metabolism. For example, side-routes in mitochondria electron transport chain contributes constantly to the generation of a few percent at most of superoxide anion per dioxygen intake. Yet, these side-reactions occur in rather modest capacity as compared to the large fluxes produced during oxidative stress bursts. These latter generally involve specific enzymatic pools activated purposely to produce intense bursts and involve generally NADPH-oxidases and/or xanthine oxidases. Yet, NADPH-oxidases (NOX) appear to be absent in MG63 cells. In agreement with this purported absence, PMA (a NOX activator) was unable to affect MG63 cell oxidative stress burst intensities during our experiments (data not shown). Thus the involvement of NADPH-oxidases, especially of NOX2, in the oxidative stress responses of MG63 cells may be excluded, a fact which certainly explains the relatively feeble production of O2˙− by these cells. Conversely, the presence of xanthine oxidase, an enzyme which is also postulated to contribute to ROS-dependent activation of NF-κB, has been reported in MG63 cells10 and may then be the main source of the relatively modest fluxes of ROS monitored in this work.
In most aerobic cells the major pathway for H2O2 production involves O2˙− disproportionation catalyzed by superoxide dismutase (SOD) which is ca. 100 times faster than its spontaneous route in physiological conditions.49 Yet, O2˙− competitively adds to NO˙ at a much larger rate (ca. ten times faster50 than the SOD-catalyzed disproportionation upon considering identical concentrations of the two reactants). The lower antioxidant enzymes expression reported in MG63 cells,55 suggests that H2O2 is mostly produced by the rather slow spontaneous disproportionation of O2˙− in these cells. This, associated to the high production rate of NO˙, implies that only a small fraction of O2˙− may be converted into H2O2 unless the two species are generated in fully distinct cellular compartments. This is in perfect agreement with the results obtained here, viz., only ca. 2 fmol of H2O2 detected in the RONS release vs. an initial production of 13 fmol of O2˙−. Yet, the total production of peroxynitrite (viz., including that of nitrite ions, its sub-product) amounts to ca. 11 fmol, i.e. only five times that of H2O2. Nonetheless, H2O2 is still released despite the larger amount of the primary NO˙ flux and the low rate of the spontaneous O2˙− disproportionation the rate constant of which is ca. one thousandth of that of its diffusion-limited with NO˙. This suggests that the superoxide ion and nitric oxide enzymatic sources are located in different compartments of the MG63 cell so that a significant fraction of O2˙− may react through its uncatalyzed disproportionation before being quenched upon reaching the comparatively larger flux of NO˙.
Anyway, because of matter conservation, the maximum amount of peroxynitrite (including that of nitrite ions) that may be generated is ultimately limited by the primary flux of O2˙− which is about half of that of NO˙. Hence, the present observation of a dominant release of NO˙ by MG63 cells in the extracellular fluid (i.e., ca. 60% of all RONS) ultimately originates from a weaker production of O2˙− associated to a much larger primary production of NO˙.
Hence, the present quantitative results fully support the suggestion that the high value of the NO˙/RONS ratio, more particularly that of NO˙/H2O2, observed in oxidative bursts produced by mechanically stimulated MG63 osteosarcoma cells is specifically linked to the pathways which ultimately lead to an uncontrolled osteoid production characteristic of these cells.41
Conclusion
Mechanical stimuli have been reported to be able to modulate bone cell activities essential for maintaining a constant bone remodeling rate through a series of biochemical reactions.56–58 Osteocytes are likely to be the primary cells which sense the mechanical stimuli in the first instance so as to modulate the activity of their surrounding bone cells, especially osteoblasts in a paracrine manner.59In this study, we used a micropipette sealed tip to stress mechanically MG63 osteosarcoma single cells and evidenced that this provoked the release of RONS. One single MG63 osteosarcoma cell oxidative burst was composed in an average of 5 fmol NO2−, 17 fmol NO˙, 6 fmol ONOO−, and 2 fmol H2O2. This RONS cocktail stemmed from the primary production of 13 fmol O2˙− and 28 fmol NO˙, a fact which confirms quantitatively the purported dominant production of NO˙ by MG63 osteosarcoma cell lines. This leads to an uncommonly high NO˙/H2O2 release ratio, which owing to the different actions of these two signal transducers, validates quantitatively at the single cell level that this feature characterizes the state of bone cancer cells while leading to malignant uncontrolled bone formation.
Acknowledgements
In Paris, this work was supported in parts by CNRS, ENS and UPMC (UMR 8640 and LIA CNRS XiamENS), ANR (μPhysChemBio, ANR-06-BLAN-029) as well as by the European Community (Nanoscale CP-FP214566-2). NSFC (20620130427, 20773100), MOST (2007DFC40440) and the 973-Program (2007CB935603) are gratefully acknowledged for their support in Xiamen. Dr Ren Hu also acknowledges the French Ministry of Research (MESR) for the award of a postdoc fellowship in ENS.References
- M. Valko, C. J. Rhodes, J. Moncol, M. Izakovic and M. Mazur, Chem.-Biol. Interact., 2006, 160, 1–40 CrossRef CAS.
- V. Adler, Z. M. Yin, K. D. Tew and Z. Ronai, Oncogene, 1999, 18, 6104–6111 CrossRef CAS.
- B. Fadeel and V. E. Kagan, Redox Rep., 2003, 8, 143–150 Search PubMed.
- R. A. Roberts, D. L. Laskin, C. V. Smith, F. M. Robertson, E. M. G. Allen, J. A. Doorn and W. Slikkerk, Toxicol. Sci., 2009, 112, 4–16 CrossRef CAS.
- D. Giustarini, I. Dalle-Donne, D. Tsikas and R. Rossi, Crit. Rev. Clin. Lab. Sci., 2009, 46, 241–281 Search PubMed.
- P. Courpon, P. Meunier and G. Vignon, Nouvelle Presse Médicale, Masson, Paris, 1975, vol. 4, pp. 421–424 Search PubMed.
- H. Okamoto, T. P. Cujec, H. Yamanaka and N. Kamatani, FEBS J., 2008, 275, 4463–4470 CrossRef CAS.
- R. J. VantHof and S. H. Ralston, J. Bone Miner. Res., 1997, 12, 1797–1804 CrossRef CAS.
- A. Sontakke and R. S. Tare, Clin. Chim. Acta, 2002, 318, 145–148 CrossRef CAS.
- X. C. Bai, D. Lu, A. L. Liu, Z. M. Zhang, X. M. Li, Z. P. Zou, W. S. Zeng, B. L. Cheng and S. Q. Luo, J. Biol. Chem., 2005, 280, 17497–17506 CrossRef CAS.
- H. Kim, I. Y. Kim, S. Y. Lee and D. Jeong, FEBS Lett., 2006, 580, 5661–5665 CrossRef CAS.
- R. Moseley, R. J. Waddington, G. Embery and S. G. Rees, Connect. Tissue Res., 1998, 37, 13–28 CrossRef CAS.
- R. J. van't Hof and S. H. Ralston, Immunology, 2001, 103, 255–261 CrossRef CAS.
- M. M. Tarpey, D. A. Wink and M. B. Grisham, Am. J. Physiol.-Regulat. Integr. Compar. Physiol., 2004, 286, R431–R444 Search PubMed.
- M. M. Tarpey and I. Fridovich, Circ. Res., 2001, 89, 224–236 CrossRef CAS.
- S. Borgmann, Anal. Bioanal. Chem., 2009, 394, 95–105 CrossRef CAS.
- P. Manning, M. R. Cookson, C. J. Eggett, C. M. Tolias, S. J. Read, A. J. Hunter, M. Tsatmali, A. J. Thody, E. W. Hillhouse, P. J. Shaw and C. J. McNeil, Analusis, 2000, 28, 493–505 CrossRef CAS.
- C. Amatore, S. Arbault, M. Guille and F. Lemaitre, Chem. Rev., 2008, 108, 2585–2621 CrossRef CAS.
- S. Arbault, P. Pantano, J. A. Jankowski, M. Vuillaume and C. Amatore, Anal. Chem., 1995, 67, 3382–3390 CrossRef CAS.
- C. Amatore, S. Arbault, D. Bruce, P. de Oliveira, M. Erard and M. Vuillaume, Faraday Discuss., 2000, 116, 319–303 RSC.
- S. Arbault, P. Pantano, N. Sojic, C. Amatore, M. Best-Belpomme, A. Sarasin and M. Vuillaume, Carcinogenesis, 1997, 18, 569–574 CrossRef CAS.
- S. Arbault, N. Sojic, D. Bruce, C. Amatore, A. Sarasin and M. Vuillaume, Carcinogenesis, 2003, 25, 509–515 CrossRef.
- C. Amatore, S. Arbault and M. Erard, Anal. Chem., 2008, 80, 9635–9641 CrossRef CAS.
- I. Tapsoba, S. Arbault, P. Walter and C. Amatore, Anal. Chem., 2010, 82, 457–460 CrossRef CAS.
- A. Lachgar, N. Sojic, S. Arbault, D. Bruce, A. Sarasin, C. Amatore, B. Bizzini, D. Zagury and M. Vuillaume, J. Virol., 1999, 73, 1447–1452 CAS.
- C. Amatore, S. Arbault, C. Bouton, K. Coffi, J.-C. Drapier, H. Ghandour and Y. Tong, ChemBioChem, 2006, 7, 653–661 CrossRef CAS.
- C. Amatore, S. Arbault, Y. Chen, C. Crozatier and I. Tapsoba, Lab Chip, 2007, 7, 233–238 RSC.
- D. C. M. Ferreira, M. O. F. Goulart, I. Tapsoba, S. Arbault and C. Amatore, Electrochem. Soc. Trans., 2007, 29, 3–11 Search PubMed.
- C. Amatore, S. Arbault, C. Bouton, J. C. Drapier, H. Ghandour and A. C. W. Koh, ChemBioChem, 2008, 9, 1472–1480 CrossRef CAS.
- C. Amatore, S. Arbault and A. Koh, Anal. Chem., 2010, 82, 1411–1419 CrossRef CAS.
- C. Amatore, S. Arbault, Y. Bouret, B. Cauli, M. Guille, A. Rancillac and J. Rossier, ChemPhysChem, 2006, 7, 181–187 CrossRef CAS.
- A. Rancillac, M. Guille, X. K. Tong, H. Geoffroy, E. Hamel, C. Amatore, S. Arbault, J. Rossier and B. Cauli, J. Neurosci., 2006, 26, 6997–7006 CrossRef CAS.
- Y. Verchier, B. Lardy, M. V. C. Nguyen, F. Morel, S. Arbault and C. Amatore, Biochem. Biophys. Res. Commun., 2007, 361, 493–498 CrossRef CAS.
- S. Arbault, M. Edeas, S. Legrand-Poëls, N. Sojic, C. Amatore, J. Piette, M. Best-Belpomme, A. Lindenbaum and M. Vuillaume, Biomed. Pharmacother., AIDS Sc. Sec., 1997, 51, 430-438 Search PubMed.
- C. Amatore, S. Arbault, D. C. Melo Ferreira, I. Tapsoba and Y. Verchier, J. Electroanal. Chem., 2008, 615, 34–44 CrossRef CAS.
- D. C. M. Ferreira, I. Tapsoba, S. Arbault, Y. Bouret, M. S. Alexandre Moreira, A. Ventura Pinto, M. O. F. Goulart and C. Amatore, ChemBioChem, 2009, 10, 528–538 CrossRef CAS.
- M. R. Filipovic, A. Koh, S. Arbault, C. Amatore, M. Mojovic, V. Niketic and I. Ivanovic-Burmazovic, Free Radical Res., 2009, 43, 51–52.
- C. Amatore, S. Arbault, G. Jaouen, A. C. W. Koh, W. K. Leong, S. Top, M. A. Valleron and C. H. Wo, ChemMedChem, 2010, 5, 296–301 CrossRef CAS.
- M. R. Filipović, A. C. W. Koh, S. Arbault, V. Niketić, A. Debus, U. Schleicher, C. Bogdan, M. Guille, F. Lemaitre, C. Amatore and I. Ivanović-Burmazović, Angew. Chem. DOI:10.1002/anie.200905936.
- N. Marina, M. Gebhardt, L. Teot and R. Gorlick, Oncologist, 2004, 9, 422–441 CrossRef.
- L. B. Rozeman, A. M. Cleton-Jansen and P. C. W. Hogendoorn, Int. Orthopaed., 2006, 30, 437–444 Search PubMed.
- S. Weiner and H. D. Wagner, Annu. Rev. Mater. Sci., 1998, 28, 271–298 CrossRef.
- R. G. Bacabac, T. H. Smit, M. G. Mullender, S. J. Dijcks, J. Van Loon and J. Klein-Nulend, Biochem. Biophys. Res. Commun., 2004, 315, 823–829 CrossRef CAS.
- U. A. Gurkan and O. Akkus, Ann. Biomed. Eng., 2008, 36, 1978–1991 CrossRef.
- A. D. Bakker, K. Soejima, J. Klein-Nulend and E. H. Burger, J. Biomech., 2001, 34, 671–677 CrossRef CAS.
- J. Klein-Nulend, M. H. Helfrich, J. G. H. Sterck, H. MacPherson, M. Joldersma, S. H. Ralston, C. M. Semeins and E. H. Burger, Biochem. Biophys. Res. Commun., 1998, 250, 108–114 CrossRef CAS.
- K. T. Kawagoe, J. A. Jankowski and R. M. Wightman, Anal. Chem., 1991, 63, 1589–1594 CrossRef CAS.
- T. Ardan, J. Kovaceva and J. Cejková, Acta Histochem., 2004, 106, 69–75 CrossRef CAS.
- C. Von Sonntag, The Chemical Basis of Radiation Biology, Taylor & Francis, London, 1987 Search PubMed.
- R. Kissner, T. Nauser, P. Bugnon, P. Lye and W. H. Koppenol, Chem. Res. Toxicol., 1997, 10, 1285–1292 CrossRef CAS.
- C. Amatore, S. Arbault, D. Bruce, P. de Oliveira, M. Erard and M. Vuillaume, Chem.–Eur. J., 2001, 7, 4171–4179 CrossRef CAS.
- H. MacPherson, B. S. Noble and S. H. Ralston, Bone, 1999, 24, 179–185 CrossRef CAS.
- M. Hukkanen, F. J. Hughes, L. D. Buttery, S. S. Gross, T. J. Evans, S. Seddon, V. Riveros- Moreno, I. Macintyre and J. M. Polak, Endocrinology, 1995, 136, 5445–5453 CrossRef CAS.
- S. Nomura and T. Takano-Yamamoto, Matrix Biol., 2000, 19, 91–96 CrossRef CAS.
- B. Grigolo, G. Lisignoli, S. Toneguzzi, I. Mazzetti and A. Facchini, Anticancer Res., 1998, 18, 1175–1180 CAS.
- J. H. Chen, C. Liu, L. D. You and C. A. Simmons, J. Biomech., 2010, 43, 108–118 CrossRef.
- T. M. Skerry, Arch. Biochem. Biophys., 2008, 473, 117–123 CrossRef CAS.
- S. Judex, S. Gupta and C. Rubin, Orthodont. Craniofacial Res., 2009, 12, 94–104 Search PubMed.
- P. J. Ehrlich and L. E. Lanyon, Osteoporosis Int., 2002, 13, 688–700 Search PubMed.
Footnotes |
| † Dedicated to Prof. Shaojun Dong in honor of her 80th birthday. |
| ‡ Present address: Université de Bordeaux 1, Institut des Sciences Moléculaires, UMR5255, ENSCPB, 16 avenue Pey Berland, 33607 PESSAC, France. |
| This journal is © the Owner Societies 2010 |
