A europium complex with enhanced long-wavelength sensitized luminescent properties†
Fumin
Xue
a,
Yan
Ma
a,
Limin
Fu
b,
Rui
Hao
a,
Guangsheng
Shao
a,
Minxian
Tang
a,
Jianping
Zhang
b and
Yuan
Wang
*a
aBeijing National Laboratory for Molecular Sciences, State Key Laboratory for Structural Chemistry of Unstable and Stable Species, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China. E-mail: wangy@pku.edu.cn; Fax: (+86) 10-6276-5769
bDepartment of Chemistry, Renmin University of China, Beijing 100872, China
First published on 10th February 2010
Abstract
We report a new complex Eu(tta)3·bpt (tta = thenoyltrifluoroacetonate; bpt = 2-(N,N-di-ethylanilin-4-yl)-4,6-bis(pyrazol-1-yl)-1,3,5-triazine) with excellent long-wavelength sensitized luminescent properties, in which four hydrogen atoms replace the methyl groups at the 3,3′- and 5,5′-positions of the pyrazolyl rings in a previously reported complex Eu(tta)3·dpbt. Upon visible-light excitation (λex = 410 nm) at 295 K, the quantum yield (ΦLnL) of Eu3+ luminescence of Eu(tta)3·bpt is higher by 23% than that of Eu(tta)3·dpbt. Different from the case of Eu(tta)3·dpbt, ΦLnL of Eu(tta)3·bpt increases linearly with the decrease in temperature. Because of the different coordination environments around Eu3+ ion, the fine structure of the hypersensitive 5D0 → 7F2 emission band of Eu(tta)3·bpt is quite different from that of Eu(tta)3·dpbt, with the strongest emission line locating at 620 nm rather than 613 nm where the strongest emission line of Eu(tta)3·dpbt appears. The excitation window of Eu(tta)3·bpt is much broader than that of Eu(tta)3·dpbt with a red edge extending up to 450 nm in a dilute toluene soluton (1.0 × 10−5 M) and 500 nm in a toluene solution (1.0 × 10−2 M). Eu(tta)3·bpt also exhibits excellent two-photon-excitation luminescent properties.
1. Introduction
Luminescent lanthanide complexes are increasingly used as luminescent labels for bioimaging1–12 and fluorescence immunoassays13–17 because of their long luminescence lifetimes, large Stokes shifts and narrow-line emission.18,19 For developing high-sensitive, deep-penetrating and less-harmful luminescent labels of biological samples, luminescent europium complexes capable of being efficiently sensitized by long-wavelength light are attractive functional building blocks because of their relative high emission capability and the acceptable red-light penetrability of biological samples. On the other hand, two-photon sensitization of lanthanide luminescence provides a promising manner for greatly extending the excitation window of lanthanide complexes to the long-wavelength region. However, examples for Eu3+ complexes with excellent visible light-sensitized20–31 and two-photon-sensitized30–36 luminescence properties are still limited. Previously, we reported a complex Eu(tta)3·dpbt (dpbt = 2-(N,N-diethylanilin-4-yl)-4,6-bis(3,5-dimethylpyrazol-1-yl)-1,3,5-triazine) (Scheme 1a),29,30 in which the Eu3+ luminescence can be sensitized by visible light through a singlet energy transfer pathway, in which the excitated triplet state of dpbt is not involved. Eu(tta)3·dpbt exhibits excellent visible-light-sensitized and two-photon-sensitized luminescent properties. By introducing two methyl groups at the 2,6-positions of the phenyl ring in the “antenna” ligand dpbt, we synthesized another Eu3+ complex Eu(tta)3·dmbpt (dmbpt = 2-(N,N-diethyl-2,6-dimethylanilin-4-yl)-4,6-bis(3,5-dimethylpyrazol-1-yl)-1,3,5-triazine) (Scheme 1b).31 Compared with Eu(tta)3·dpbt, Eu(tta)3·dmbpt exhibits better two-photon-sensitized luminescence properties, with a markedly red-shifted two-photon excitation (TPE) window, but much lower extinction coefficient in the wavelength range over 400 nm as shown by its UV-vis absorption spectrum. These results encourage us to further modify the “antenna” ligand structure on the basis of dpbt to improve the long-wavelength sensitized luminescent properties of europium complexes and to understand the interaction between the chromophore ligand and Eu3+ ion. | ||
| Scheme 1 Molecular structures of Eu(tta)3·dpbt (a), Eu(tta)3·dmbpt (b), Eu(tta)3·bpt (c) and Eu(tta)3·mpbt (d). | ||
Herein, we report an interesting complex Eu(tta)3·bpt (bpt = 2-(N,N-diethylanilin-4-yl)-4,6-bis(pyrazol-1-yl)-1,3,5-triazine) (Scheme 1c), in which, four hydrogen atoms replace the methyl groups on the pyrazolyl rings in Eu(tta)3·dpbt. This change in structure endows Eu(tta)3·bpt with enhanced long-wavelength sensitized luminescent properties and a quite different emission spectrum. The strongest emission line of Eu(tta)3·bpt locates at 620 nm, while that of Eu(tta)3·dpbt appears at 613 nm. To find the reason for the different splitting patterns of 5D0 → 7F2 transitions between Eu(tta)3·bpt and Eu(tta)3·dpbt, a new complex Eu(tta)3·mpbt (mpbt = 2-(N,N-diethylanilin-4-yl)-4,6-bis(3-methylpyrazol-1-yl)-1,3,5-triazine) (Scheme 1d) was synthesized by introducing two methyl groups at the 3,3-positions of the pyrazolyl rings of Eu(tta)3·bpt. Upon excitation at 410 nm, the Eu3+ luminescence quantum yield (ΦLnL) of Eu(tta)3·bpt increases linearly with the decrease in temperature in the range from 338 K to 283 K, and it is higher by 23% than that of Eu(tta)3·dpbt at 295 K. In addition, the excitation window of Eu(tta)3·bpt is much broader than that of Eu(tta)3·dpbt, and its TPE luminescence property is also better than that of Eu(tta)3·dpbt in the long-wavelength region.
2. Experimental section
2.1 Materials
Eu(tta)3·3H2O (purity >95%), pyrazole (98.5%) were purchased from Acros, 3-methylpyrazole (97%) were purchased from Alfa Aesar, and other chemicals of analytical grade were purchased from Beijing Chemical Corporation.2.2 Synthesis of bpt
N,N-diethyl-4-(4,6-dichloro-[1,3,5]-triazine-2-yl)-aniline (dta) was synthesized by a previously reported method.29 Potassium (0.131 g, 3.36 mmol) was added to a stirred solution of pyrazole (0.322 g, 4.74 mmol) in dry THF under Ar at 363 K. After the metal dissolved, the obtained colorless solution was cooled to room temperature, and dta (0.40 g, 1.35 mmol) was added to the mixture, producing a pale yellow solution. The reaction mixture was stirred at room temperature for 1 h and then heated at 353–363 K for 8 h. The solution was concentrated under reduced pressure, and then the residue was purified by column chromatography on silica gel with anhydrous diethyl ether and acetone as eluents, and subsequently by recycling preparative HPLC (LC-9101) with methanol as the eluent. After distillation of the eluent, bpt was obtained as a yellowish powder (0.19 g, 40%). Mp 433–436 K. FTIR (KBr): ν 3148, 3102, 3066 (N–H), 2977, 2939, 2912 (C–H), 1606 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1565 (C
N), 1565 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1484, 1434 (C
N), 1484, 1434 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1409 (C
N), 1409 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1390, 1347, 1275, 1218, 1189, 1109, 1078, 1034, 1012, 983, 949, 807, 777, 765 cm−1. 1H NMR (400 MHz, CDCl3): δ 8.82 (2H, t, J 1.66 Hz, Pz–H), 8.56 (2H, d, J 9.0 Hz, Ph–H), 7.94 (2H, d, J 0.89 Hz, Pz–H), 6.78 (2H, s, Ph–H), 6.57 (2H, q, J 1.2 Hz, Pz–H), 3.49(4H, q, J 7.1 Hz, NCH2CH3), 1.25 (6H, t, J 7.0 Hz, NCH2CH3). EI MS: m/z = 360 M+. Anal. calcd for C19H20N8: C, 63.33; H, 5.56; N, 31.1. Found: C, 63.23; H, 5.58; N, 31.0. Crystal data for bpt: C19H20N8, M = 360.43, monoclinic, P21/c, a = 5.8827(12) Å, b = 17.349(3) Å, c = 18.563(5) Å, the unit-cell angle β = 103.90(3)°, V = 1839.0(7) Å3, T = 296(2) K, Z = 4, 9616 reflections measured, 3208 unique (Rint = 0.1029). The final wR(F2) was 0.1885 (all data).†
N), 1390, 1347, 1275, 1218, 1189, 1109, 1078, 1034, 1012, 983, 949, 807, 777, 765 cm−1. 1H NMR (400 MHz, CDCl3): δ 8.82 (2H, t, J 1.66 Hz, Pz–H), 8.56 (2H, d, J 9.0 Hz, Ph–H), 7.94 (2H, d, J 0.89 Hz, Pz–H), 6.78 (2H, s, Ph–H), 6.57 (2H, q, J 1.2 Hz, Pz–H), 3.49(4H, q, J 7.1 Hz, NCH2CH3), 1.25 (6H, t, J 7.0 Hz, NCH2CH3). EI MS: m/z = 360 M+. Anal. calcd for C19H20N8: C, 63.33; H, 5.56; N, 31.1. Found: C, 63.23; H, 5.58; N, 31.0. Crystal data for bpt: C19H20N8, M = 360.43, monoclinic, P21/c, a = 5.8827(12) Å, b = 17.349(3) Å, c = 18.563(5) Å, the unit-cell angle β = 103.90(3)°, V = 1839.0(7) Å3, T = 296(2) K, Z = 4, 9616 reflections measured, 3208 unique (Rint = 0.1029). The final wR(F2) was 0.1885 (all data).†
2.3 Synthesis of Eu(tta)3·bpt
A solution of Eu(tta)3·3H2O (24.4 mg, 28.1 mmol) in THF (10 mL) was added to a solution of bpt (10.2 mg, 28.3 mmol) in THF (10 mL) to produce instantaneously a yellow solution with bright red luminescence in daylight. After evaporation of the solvent, the residue was dissolved in a small amount of diethyl ether. Addition of n-hexane to the solution led to the precipitation of Eu(tta)3·bpt as an orange powder (28.9 mg, 87%). Mp 413–416 K. FTIR (KBr): ν 3138 (N–H), 2976, 2930 (C–H), 1604, 1566 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1535, 1499, 1451, 1411 (C
N), 1535, 1499, 1451, 1411 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1386, 1351, 1299, 1231, 1176, 1135, 1076, 1045, 997, 966, 933, 916, 858, 807 (C
N), 1386, 1351, 1299, 1231, 1176, 1135, 1076, 1045, 997, 966, 933, 916, 858, 807 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 782, 769, 717, 679 cm−1. 1H NMR (400 MHz, C6D6): δ 19.22 (2H, s, Pz–H), 9.98 (2H, d, J 8.4 Hz, Pz–H), 9.84 (2H, s, Pz–H), 8.57 (2H, s, Ph–H), 7.16 (2H, d, J 8.5 Hz, Ph–H), 6.49 (3H, s, Th–H), 5.91 (3H, t, J 3.68 Hz, Th–H), 5.71 (3H, s, Th–H), 3.32 (4H, q, J 6.9 Hz, NCH2CH3), 1.18 (6H, t, J 7.1 Hz, NCH2CH3), 1.00 (3H, s, CH); MALDI-TOF MS: m/z = 1092.7 [M-thiophene]+; Anal. calcd for EuC43H32N8F9O6S3: C, 43.90; H, 2.72; N, 9.53. Found: C, 43.79; H, 2.56; N, 9.71.
N), 782, 769, 717, 679 cm−1. 1H NMR (400 MHz, C6D6): δ 19.22 (2H, s, Pz–H), 9.98 (2H, d, J 8.4 Hz, Pz–H), 9.84 (2H, s, Pz–H), 8.57 (2H, s, Ph–H), 7.16 (2H, d, J 8.5 Hz, Ph–H), 6.49 (3H, s, Th–H), 5.91 (3H, t, J 3.68 Hz, Th–H), 5.71 (3H, s, Th–H), 3.32 (4H, q, J 6.9 Hz, NCH2CH3), 1.18 (6H, t, J 7.1 Hz, NCH2CH3), 1.00 (3H, s, CH); MALDI-TOF MS: m/z = 1092.7 [M-thiophene]+; Anal. calcd for EuC43H32N8F9O6S3: C, 43.90; H, 2.72; N, 9.53. Found: C, 43.79; H, 2.56; N, 9.71.
2.4 Synthesis of mpbt
Compound mpbt was synthesized with a similar procedure as described for bpt. The product was purified and the possibly formed by-product 2-(N,N-diethylanilin-4-yl)-4,6-bis(5-dime-thylpyrazol-1-yl)-1,3,5-triazine via an isomeric reaction of 3-methylpyrazole37 was removed by the following processes. The reaction-solution was concentrated under reduced pressure, and then the residue was purified by column chromatography on silica gel with anhydrous diethyl ether, acetone/methanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and methanol as eluents, respectively, subsequently by recycling preparative HPLC (LC-9101) with methanol as the eluent. After distillation of the eluent, mpbt was obtained as a yellowish powder. Yield: 20%. Mp 428–433 K. FTIR (KBr): ν 3414, 3090 (N–H), 2968, 2916 (C–H), 1617 (C
1), and methanol as eluents, respectively, subsequently by recycling preparative HPLC (LC-9101) with methanol as the eluent. After distillation of the eluent, mpbt was obtained as a yellowish powder. Yield: 20%. Mp 428–433 K. FTIR (KBr): ν 3414, 3090 (N–H), 2968, 2916 (C–H), 1617 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1555, 1528 (C
N), 1555, 1528 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1402 (C
N), 1402 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1349, 1267, 1186, 1151, 1085, 1033, 956, 795, 756 cm−1. 1H NMR (400 MHz, CDCl3): δ 8.69 (2H, d, J 2.8 Hz, Pz–H), 8.53 (2H, d, J 9.0 Hz, Ph–H), 6.74 (2H, s, Ph–H), 6.35 (2H, q, J 2.7 Hz, Pz–H), 3.47(4H, q, J 7.0 Hz, NCH2CH3), 2.46 (6H, s, Pz-CH3) 1.24 (6H, t, J 7.1 Hz, NCH2CH3), EI MS: m/z = 388 M+. Anal. calcd for C21H24N8: C, 64.92; H, 6.22; N, 28.84. Found: C, 64.40; H, 6.25; N, 28.75.
N), 1349, 1267, 1186, 1151, 1085, 1033, 956, 795, 756 cm−1. 1H NMR (400 MHz, CDCl3): δ 8.69 (2H, d, J 2.8 Hz, Pz–H), 8.53 (2H, d, J 9.0 Hz, Ph–H), 6.74 (2H, s, Ph–H), 6.35 (2H, q, J 2.7 Hz, Pz–H), 3.47(4H, q, J 7.0 Hz, NCH2CH3), 2.46 (6H, s, Pz-CH3) 1.24 (6H, t, J 7.1 Hz, NCH2CH3), EI MS: m/z = 388 M+. Anal. calcd for C21H24N8: C, 64.92; H, 6.22; N, 28.84. Found: C, 64.40; H, 6.25; N, 28.75.
2.5 Synthesis of Eu(tta)3·mpbt
Complex Eu(tta)3·mpbt was synthesized using mpbt and Eu(tta)3·3H2O as the starting materials with the procedure described for complex Eu(tta)3·bpt. Yield: 81%. Mp 414–419 K. FTIR (KBr): ν 3100 (N–H), 2974, 2930 (C–H), 1609, 1570 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1537, 1504, 1454, 1409 (C
N), 1537, 1504, 1454, 1409 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1353, 1301, 1237, 1188, 1139, 1057, 981, 966, 855(C
N), 1353, 1301, 1237, 1188, 1139, 1057, 981, 966, 855(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 779, 718, 681 cm−1. 1H NMR (400 MHz, C6D6): δ 25.38 (6H, s, Pz-CH3), 11.87 (2H, s, Pz–H), 9.83 (2H, s, Pz–H), 7.931 (2H, s, Ph–H), 6.33 (2H, d, J 5.04 Hz, Th–H), 6.00 (2H, s, Ph–H), 5.36 (3H, s, Th–H), 4.29 (3H, s, Th–H), 2.83 (4H, q, J 6.7 Hz, NCH2CH3), 0.66 (6H, t, J 7.1 Hz, NCH2CH3). The NMR spectrum of Eu(tta)3·mpbt exhibited a chemical shift of 25.38 for the six hydrogen atoms of the two methyl groups on the pyrazolyl rings, which confirmed that the chromophore ligand in the synthesized complex was the desired 2-(N,N-diethylanilin-4-yl)-4,6-bis(3-dimethylpyrazol-1-yl)-1,3,5-triazine. ESI MS: m/z = 983.1 [M-tta]+. Anal. calcd for EuC45H36N8F9O6S3: C, 44.89; H, 3.01; N, 9.31. Found: C, 45.21; H, 3.39; N, 9.72.
N), 779, 718, 681 cm−1. 1H NMR (400 MHz, C6D6): δ 25.38 (6H, s, Pz-CH3), 11.87 (2H, s, Pz–H), 9.83 (2H, s, Pz–H), 7.931 (2H, s, Ph–H), 6.33 (2H, d, J 5.04 Hz, Th–H), 6.00 (2H, s, Ph–H), 5.36 (3H, s, Th–H), 4.29 (3H, s, Th–H), 2.83 (4H, q, J 6.7 Hz, NCH2CH3), 0.66 (6H, t, J 7.1 Hz, NCH2CH3). The NMR spectrum of Eu(tta)3·mpbt exhibited a chemical shift of 25.38 for the six hydrogen atoms of the two methyl groups on the pyrazolyl rings, which confirmed that the chromophore ligand in the synthesized complex was the desired 2-(N,N-diethylanilin-4-yl)-4,6-bis(3-dimethylpyrazol-1-yl)-1,3,5-triazine. ESI MS: m/z = 983.1 [M-tta]+. Anal. calcd for EuC45H36N8F9O6S3: C, 44.89; H, 3.01; N, 9.31. Found: C, 45.21; H, 3.39; N, 9.72.
2.6 Photophysical measurements
The UV-vis absorption and photoluminescence measurements were carried out on an absorption spectrometer (Lambda 35, Perkin Elmer), a fluorescence spectrophotometer (F-4500, Hitachi) (for the measurement of luminescence quantum yields), and a Steady State Spectrometer FLS920 equipped with a μF900 lamp (λex = 397 nm) (for measuring the high resolution emission spectra). FTIR spectra were recorded on an infrared spectrometer (Magna-IR 750, Nicolet) with samples prepared as KBr pellets. 1H NMR spectra (presented as δ in ppm and J in Hz) were recorded with a Bruker Avance DRX 400 MHz spectrometer.Lifetimes of Eu3+ were measured at 295 K with Edinburgh Instruments FLS920 based on the time correlated single photon counting technology upon the excitation at 410 and 402 nm for Eu(tta)3·bpt and Eu(tta)3·dpbt, respectively. A microsecond flash lamp was used as the excitation source and the signals were detected with a photomultiplier (Hamamatsu r955). The radiative lifetimes (τrad) of Eu(tta)3·bpt and Eu(tta)3·dpbt were measured at 77 K by directly populating the 5D2 level of Eu3+ upon the excitation at 457 nm. For recording the kinetics of luminescence, a PMT (R298, Hamamatsu) attached to a polychromator (Spectrapro-2300i, Acton) was used as the detector and the signal was sent to a digital oscilloscope (HP 54503A, HP).
Luminescence quantum yields were determined by the method described by Demas and Crosby38 and the results were obtained according to [eqn (1)], in which subscripts S and R denote the sample and the reference, respectively, A represents the absorbency, D represents the intensity of fluorescence, and n represents the refractive index of the solvents:
 | (1) |
Two photon absorption (TPA) cross-section (δ) was determined from two-photon-induced fluorescence using rhodamine B as a reference with a known δ value.40 A regenerative amplifier (Spitfire, Spectra Physics) seeding with a mode-locked Ti:Sapphire laser (Tsunami, Spectra Physics) generated ∼120 fs laser pulses at the wavelength of 795 nm, which were used to drive an optical parameter amplifier (OPA-800CF, Spectra Physics) to obtain tunable laser in the range of 720∼870 nm. The laser beam was focused into a quartz cuvette having an optical path length of 10 mm. The two-photon-induced luminescence collected with a right-angle geometry was detected with a liquid-nitrogen-cooled CCD detector (SPEC-10-400B/LbN, Roper Scientific) attached to a polychromator (Spectropro-550i, Acton). To reject the interference of stray laser light, a saturated aqueous solution of CuSO4 (10 mm in thickness) was placed in front of the entrance slit of the polychromator. Ligand bpt and complex Eu(tta)3·bpt were dissolved in toluene with a concentration of 10−4 M, and rhodamine B was dissolved in methanol at the same concentration. The TPA cross-sections were obtained according to [eqn (2)],41 in which subscripts S and R denote the sample and the reference, respectively, F represents the intensity of two-photon-induced fluorescence, Φ is the fluorescence quantum yield, c denotes the concentration, and n represents the refractive index of the solvent:
 | (2) |
3. Results and discussion
3.1 Molecular structure and luminescence properties of chromophore bpt
bpt was synthesized for the first time according to the method reported previously29 with some modification. The single crystal of bpt was obtained by crystallization in CH2Cl2.† The molecular structure of bpt in a crystal grain was determined by X-ray analyses. An ORTEP drawing with the corresponding atom labeling scheme is displayed in Fig. 1, which shows that in the crystal, one pyrazolyl ring of bpt has a markedly-twisted conformation with a dihedral angle of 160.3° for N(6)–N(5)–(12)–N(3), while the twisting extent of another pyrazolyl ring in bpt is not high, with a dihedral angle of 8.96° for N(8)–N(7)–C(13)–(3). The crystal structure of bpt is quite different from that of dpbt as reported previously.31†‡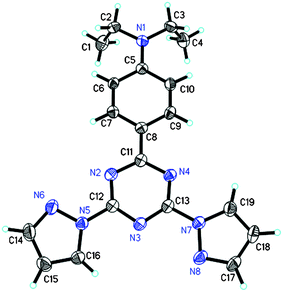 | ||
| Fig. 1 ORTEP plot of bpt with atomic labelling scheme obtained from a crystal grain of 0.39 × 0.30 × 0.152 mm. All the non-hydrogen atoms were drawn at 50% thermal ellipsoid probability level. | ||
As shown in Fig. 2, the absorption peak of bpt in toluene has a red-shift of 8 nm relative to that of dpbt, which may be derived from the absence of the methyl groups on the electron-withdrawing moiety. The fluorescence quantum yield of bpt was measured to be 0.90, as listed in Table 1.
 | ||
Fig. 2 UV-vis absorption spectra of bpt (—) and dpbt (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ) in toluene (1 × 10−5 M). ) in toluene (1 × 10−5 M). | ||
| Compounds | λ A/nm | ε max × 10−4/L mol−1 cm−1 | ΦLnL | λ T/nm | δ max /GMb | (δmax × ΦLnL)/GM |
|---|---|---|---|---|---|---|
| a The experimental uncertainty on δ is 10–15%. b 1 GM = 10−50 cm4 s photon−1 molecule−1. | ||||||
| dpbt | 375 | 4.7 | 0.75 | 745 | 136 | 102 |
| bpt | 383 | 5.5 | 0.90 | 770 | 108 | 97.2 |
| mpbt | 380 | 5.7 | 0.69 | — | — | — |
| Eu(tta)3·dpbt | 402 | 6.3 | 0.35 | 808 | 242 | 84.7 |
| Eu(tta)3·bpt | 410 | 6.9 | 0.43 | 815 | 185 | 79.6 |
| Eu(tta)3mpbt | 403 | 9.7 | 0.31 | — | — | — |
3.2 Formation and luminescence properties of complex Eu(tta)3·bpt
Eu(tta)3·bpt was synthesized by the reaction between bpt and Eu(tta)3·3H2O followed by a purifying process. In the 1H NMR spectrum of free ligand bpt, three distinct signals, locating at 8.82, 7.94 and 6.57 ppm, corresponding to the three kinds of protons for the six hydrogen atoms on the two pyrazolyl rings could be observed. In the 1H NMR spectrum of Eu(tta)3·bpt (see ESI, Fig. S2),† the proton signals assignable to the six hydrogen atoms on the two pyrazolyl rings of coordinated bpt shifted to 19.22, 9.98 and 9.84 ppm. It is well known that EuIII ions usually significantly influence the chemical shifts of nearby protons via a paramagnetic effect.42 The same chemical shifts of the protons locating at the equivalent positions of the two pyrazolyl rings in Eu(tta)3·bpt suggest that the conformation of bpt in Eu(tta)3·bpt is different from that in the bpt crystal. Therefore, in the formation process of Eu(tta)3·bpt, the rotation of the pyrazolyl moieties upon coordination occurred and the coordination manner of Eu(tta)3·bpt can be expressed by Scheme 1c.In the UV-vis absorption spectra of Eu(tta)3·bpt (curve 1) and Eu(tta)3·dpbt (curve 3) as shown in Fig. 3, the maximal absorption wavelengths of Eu(tta)3·bpt (410 nm) and Eu(tta)3·dpbt (402 nm) red-shift 27 nm relative to those of free bpt and dpbt in toluene, respectively, indicating that the polarization effects of the electric field of Eu3+ ion on the two ligands are similar. Since the absorption peak of bpt red-shifts 8 nm relative to that of dpbt, the absorption peak of Eu(tta)3·bpt red-shifts 8 nm compared with that of Eu(tta)3·dpbt. The adsorption red-edge of Eu(tta)3·bpt in a dilute toluene solution extends up to 450 nm.
 | ||
| Fig. 3 Normalized UV-vis absorption spectra and normalized fluorescence excitation spectra (λem = 620 nm) in toluene. (1) and (3) are the UV-vis absorption spectra of Eu(tta)3·bpt and Eu(tta)3·dpbt, respectively; (2) and (4) are the fluorescence excitation spectra of Eu(tta)3·bpt and Eu(tta)3·dpbt, respectively. The concentration of each of the two complexes was 1 × 10−5 mol L−1. | ||
The excitation spectra of Eu(tta)3·bpt (curve 2) and Eu(tta)3·dpbt (curve 4) (Fig. 3) match well the absorption spectra of the corresponding complexes in the long-wavelength region. The excitation window of Eu(tta)3·bpt in a dilute toluene solution (1.0 × 10−5 M) is broader than that of Eu(tta)3·dpbt. When the concentration of Eu(tta)3·bpt increased to ∼1.0 × 10−2 M, the luminescence excitation spectrum of Eu(tta)3·bpt in toluene exhibited a tailing excitation reaching to 500 nm.
As shown in Fig. 4, for Eu(tta)3·bpt and Eu(tta)3·dpbt, the 5D0 → 7F0 emission bands of Eu3+ show a single-peak, while the 5D0 → 7F1 transitions show three peaks, indicating the low symmetry of the coordination environments around europium ions in these complexes.43 It is noteworthy that the splitting numbers and the strongest emission line positions of the 5D0 → 7F2 transitions (magnified in the insets of Fig. 4) are clearly different between Eu(tta)3·bpt and Eu(tta)3·dpbt. For Eu(tta)3·bpt, the 5D0 → 7F2 transition splits into three peaks and the strongest emission line locates at 620 nm. However, the 5D0 → 7F2 transition of Eu(tta)3·dpbt exhibits only two peaks, with the strongest emission line locating at 613 nm, which indicates the different surrounding symmetry around Eu3+ between Eu(tta)3·bpt and Eu(tta)3·dpbt.44
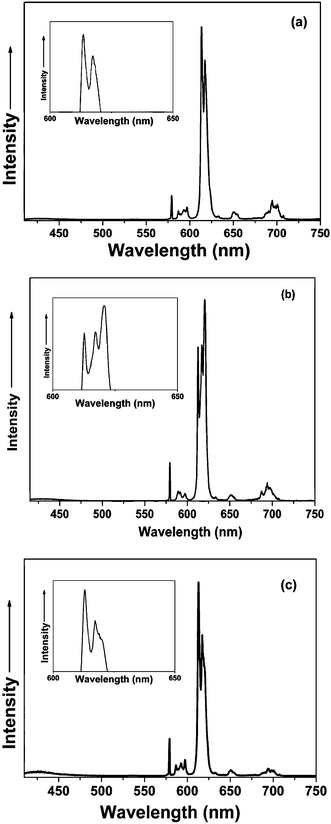 | ||
| Fig. 4 Emission spectra (λex = 410 nm) of Eu(tta)3·dpbt (a), Eu(tta)3·bpt (b), and Eu(tta)3·mpbt (c). The insets show the fine structures of the 5D0 → 7F2 transition emission bands. | ||
It is known that the probabilities of magnetic dipole 5D0 → 7F1 transitions of Eu3+ are independent of the coordination sphere around the ion, and the 5D0 → 7F2 transition is a hypersensitive transition. The intensity ratio of the 5D0 → 7F2 transition to the 5D0 → 7F1 transition (I7F2/I7F1) reflects the nature and symmetry of the first coordination sphere.45–49 The I7F2/I7F1 values are 18.1 and 13.7 for Eu(tta)3·bpt and Eu(tta)3·dpbt, respectively, indicating that the local field symmetry around Eu3+ in Eu(tta)3·bpt is different from that in Eu(tta)3·dpbt. The symmetry around Eu3+ ions may be influenced by the distance between the coordination atoms in the chromophores and Eu3+, the interactions between the different chromophore ligands and tta, the conformations of the pyrazolyl rings in the two complexes, and the distribution of electronic cloud density on the sensitization ligands in the two complexes, which may also result in the different coordination stabilities of the complexes. Unfortunately, we failed in our efforts to obtain crystals of these complexes to study the details.
To further understand the reason for the different splitting patterns of 5D0 → 7F2 transitions between Eu(tta)3·bpt and Eu(tta)3·dpbt, a new complex Eu(tta)3·mpbt (Scheme 1d) was synthesized by introducing two methyl groups at the 3,3′-positions of the pyrazolyl rings of Eu(tta)3·bpt. The emission spectrum corresponding to the 5D0 → 7F2 transitions of Eu(tta)3·mpbt (Fig. 4c), characterized by two apparent bands with a shoulder at 619 nm and the strongest emission line centered at 613 nm, is close to that of Eu(tta)3·dpbt but quite different from that of Eu(tta)3·bpt. Therefore, it is reasonable to deduce that substituent groups at the 3,3′-positions of the pyrazolyl rings markedly influence the symmetry around Eu3+, which causes changes in the splitting of 7F2 levels of Eu3+ in the complexes.
Upon excitation at 410 nm, the luminescence quantum yield of Eu(tta)3·bpt (ΦLnL) in toluene was measured at 295 K to be 0.43 using DCM in n-propanol (Φ = 0.57) as a reference, increasing by 23% relative to that of Eu(tta)3·dpbt at the same temperature. The ΦLnL can be expressed as follows:18,50
| ΦLnL = η × ΦLnLn | (3) |
| ΦLnLn = τobs/τrad | (4) |
As shown in Fig. 5, the luminescence decay profiles of Eu(tta)3·bpt and Eu(tta)3·dpbt, measured in toluene at 77 K upon the excitation at 457 nm54 and at 295 K upon the excitation at positions of maximal absorption, followed a single-exponential decay. The nonlinear least-squares fitting gave the τ77K values of 0.54 and 0.72 ms for Eu(tta)3·bpt and Eu(tta)3·dpbt, respectively, which were used as the τrad values. And the τobs values at 295 K were measured to be 0.40 ms for Eu(tta)3·bpt and 0.51 ms for Eu(tta)3·dpbt.
 | ||
| Fig. 5 Decay curves of luminescence of Eu(tta)3·bpt and Eu(tta)3·dpbt at 614 nm in toluene. | ||
As calculated using eqn (3) and (4), the η and ΦLnLn values of Eu(tta)3·bpt (0.58, 0.74) are both higher than the corresponding values of Eu(tta)3·dpbt (0.49, 0.71) by 18% and 4%, respectively. This indicated that by replacing dpbt in Eu(tta)3·dpbt with bpt, the energy transfer process was promoted while the non-radiative deactivation processes of the luminescent state were depressed at 295 K.
Recently, Borisov and coworkers reported an interesting temperature-sensitive probe made from Eu(tta)3·dpbt.55 The relationships of ΦLnL of Eu(tta)3·dpbt and Eu(tta)3·bpt to temperature were measured in this work and depicted in Fig. 6. It is interesting to notice that the relationship between ΦLnL and temperature is linear for Eu(tta)3·bpt but nonlinear for Eu(tta)3·dpbt. Although the exact cause of the different correlations between ΦLnL and temperature for the two complexes are unclear at present, Eu(tta)3·bpt may have an advantage over Eu(tta)3·dpbt for the preparation of luminescent temperature probes in view of the linear correlation.
 | ||
| Fig. 6 The correlations for Eu(tta)3·bpt (●) and Eu(tta)3·dpbt (○) of luminescence quantum yields and temperature. | ||
Fig. 7 shows the two-photon absorption (TPA) spectra of bpt and dpbt. The TPA window of bpt is apparently wider than that of dpbt, which endows bpt with stronger TPA than dpbt in the long-wavelength region. For instance, the TPA cross section of bpt was measured to be 43 GM at 799 nm, higher by 33.1 GM than that of dpbt measured at the same conditions. As displayed in Fig. 8, Eu(tta)3·bpt exhibited excellent TPE luminescent property. In the wavelength region from 815 nm to 850 nm, Eu(tta)3·bpt exhibited higher TPE action cross sections (δ × ΦLnL) than Eu(tta)3·dpbt. For example, the δ × ΦLnL value was measured to be 56.3 GM at 838 nm for Eu(tta)3·bpt and 30.1 GM for Eu(tta)3·dpbt. The maximal TPE wavelength of Eu(tta)3·bpt red-shifts 7 nm relative to that of Eu(tta)3·dpbt.
 | ||
| Fig. 7 TPA cross sections (δ) for bpt and dpbt in toluene (1.0 × 10−4 M). 1 GM = 10−50 cm4 s photon−1 molecule−1. The experimental uncertainty on δ is 10–15%. | ||
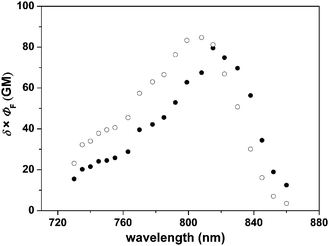 | ||
| Fig. 8 Action cross sections of TPE luminescence (δ × ΦLnL) for Eu(tta)3·bpt (●) and Eu(tta)3·dpbt (○) in toluene (1.0 × 10−4 M). 1 GM = 10−50 cm4 s photon−1 molecule−1. The experimental uncertainty on δ is 10–15%. | ||
4. Conclusions
We have reported the synthesis and excellent long-wavelength sensitized luminescent properties of Eu(tta)3·bpt, in which bpt acts as a novel “antenna” molecule. The experimental results demonstrated that the correlation between Eu3+ luminescence quantum yield and temperature was linear for Eu(tta)3·bpt but nonlinear for Eu(tta)3·dpbt in the temperature range from 283 K to 338 K. At 295 K, upon light-excitation at 410 nm, the luminescence quantum yield of Eu(tta)3·bpt was measured to be 43% using DCM in n-propanol as a reference, improving by 23% compared with that of Eu(tta)3·dpbt. The fine structure of the 5D0 → 7F2 emission band of Eu(tta)3·bpt is quite different from that of Eu(tta)3·dpbt, which is attributed to the different coordination environments around Eu3+ ion. The substituent groups at 3,3′-positions of the pyrazolyl rings were found to be an important factor of influencing the splitting of the 7F2 level of Eu3+ ions in the europium complexes as confirmed by the emission spectra of Eu(tta)3·mpbt, Eu(tta)3·bpt and Eu(tta)3·dpbt. The luminescence excitation window of Eu(tta)3·bpt in a dilute toluene solution (1.0 × 10−5 M) extends up to 450 nm, while that in a concentrated solution (1.0 × 10−2 M) extends up to 500 nm, which enables the solution to emit bright red light under natural illumination. In addition, Eu(tta)3·bpt displayed higher TPE action cross sections than Eu(tta)3·dpbt upon excitation in the wavelength range from 815 nm to 850 nm. In view of the enhanced long-wavelength sensitized Eu3+ luminescence properties of Eu(tta)3·bpt, we believe that it is a promising luminescent dye for the preparation of probes applicable in bio-sensing or bio-imaging. Research on developing new bioprobes based on such a complex is ongoing in our laboratory.Acknowledgements
This work is jointly supported by the NSFC (Project No. 50821061, 20903117, 20973003), NKBRSF (G2006CB806102) and the 863 program (2006AA02090405) from the Chinese Ministry of Science and Technology, and RFDP of the Ministry of Education of China.Notes and references
- G. Mariott, R. M. Clegg, D. J. Arndt-Jovin and T. M. Jovin, Biophys. J., 1991, 60, 1374 CAS.
- A. Beeby, S. W. Botchway, I. M. Clarkson, S. Faulkner, A. W. Parker, D. Parker and J. A. G. Williams, J. Photochem. Photobiol., B, 2000, 57, 83 CrossRef CAS.
- S. Faulkner, S. J. A. Pope and B. P. Burton-Pye, Appl. Spectrosc. Rev., 2005, 40, 1 CrossRef CAS.
- J. Yu, D. Parker, R. Pal, R. A. Poole and M. J. Cann, J. Am. Chem. Soc., 2006, 128, 2294 CrossRef CAS.
- L. J. Charbonnière, N. Hildebrandt, R. F. Ziessel and H.-G. Löhmannsröben, J. Am. Chem. Soc., 2006, 128, 12800 CrossRef CAS.
- R. J. Aarons, J. K. Notta, M. M. Meloni, J. Feng, R. Vidyasagar, J. Narvainer, S. Allan, N. Spencer, R. A. Kauppiner, J. S. Snaith and S. Faulkner, Chem. Commun., 2006, 909 RSC.
- N. Weibel, L. J. Charbonnière, M. Guardigli. A. Roda and R. Ziessel, J. Am. Chem. Soc., 2004, 126, 4888 CrossRef CAS.
- R. Pal and D. Parker, Org. Biomol. Chem., 2008, 6, 1020 RSC.
- A. Picot, A. D’Aléo, P. L. Baldeck, A. Grichine, A. Duperray, C. Andraud and O. Maury, J. Am. Chem. Soc., 2008, 130, 1532 CrossRef CAS.
- G.-L. Law, K.-L. Wong, C. W.-Y. Man, W.-T. Wong, S.-W. Tsao, M. H.-W. Lan and P. K.- S. Lam, J. Am. Chem. Soc., 2008, 130, 3714 CrossRef CAS.
- E. Deiters, B. Song, A.-S. Chauvin, C. D. B. Vandevyver, F. Gumy and J.-C. G. Bünzli, Chem.–Eur. J., 2009, 15, 885 CrossRef CAS.
- J.-C. G. Bünzli, A.-S. Chauvin, C. D. B. Vandevyver, B. Song and S. Comby, Ann. N. Y. Acad. Sci., 2008, 1130, 97 CrossRef CAS.
- J. Wu, Z. Q. Ye, G. L. Wang, D. Y. Jin, J. L. Yuan, Y. F. Guan and J. Piper, J. Mater. Chem., 2009, 19, 1258 RSC.
- I. Hemmilä and S. Webb, Drug Discovery Today, 1997, 2, 373 CrossRef CAS.
- J. L. Yuan, G. L. Wang, H. Kimura and K. Matsumoto, Anal. Biochem., 1997, 254, 283 CrossRef CAS.
- J. L. Yuan and K. Matsumoto, Anal. Chem., 1998, 70, 596 CrossRef CAS.
- G. Mathis, Clin. Chem., 1995, 41, 1391 CAS.
- C. Piguet and J.-C. G. Bünzli, Chem. Soc. Rev., 2005, 34, 1048 RSC.
- A. Mayer and S. Neuenhofer, Angew. Chem., 1994, 106, 1097 CAS.
- A. Dadabhoy, S. Faulkner and P. G. Sammes, J. Chem. Soc., Perkin Trans. 2, 2002, 348 RSC.
- M. H. V. Werts, M. A. Duin, J. W. Hofstraat and J. W. Verhoeven, Chem. Commun., 1999, 799 RSC.
- G. Piszczek, B. P. Malinal, I. Gryczynski, J. Dattelbaum and J. R. Lakowicz, J. Fluoresc., 2001, 11, 101 CrossRef CAS.
- K. L. Wong, G. L. Law, W. M. Kwok, W. T. Wong and D. L. Phillips, Angew. Chem., Int. Ed., 2005, 44, 3436 CrossRef CAS.
- G. Piszczek, I. Gryczynski, B. P. Malinal and J. R. Lakowicz, J. Fluoresc., 2002, 12, 15 CrossRef CAS.
- A. Picot, F. Malvolti, B. L. Guennic, P. L. Baldeck, J. A. G. Williams, C. Andraud and O. Maury, Inorg. Chem., 2007, 46, 2659 CrossRef CAS.
- S. M. Borisov and I. Klimant, J. Fluoresc., 2008, 18, 581 CrossRef CAS.
- P. Kadjane, L. Charbonnière, F. Camerel, P. P. Lainé and R. Ziessel, J. Fluoresc., 2008, 18, 119 CrossRef CAS.
- M. Shi, C. R. Ding, J. W. Dong, H. Z. Wang, Y. P. Tian and Z. G. Hu, Phys. Chem. Chem. Phys., 2009, 11, 5119 RSC.
- C. Yang, L. M. Fu, Y. Wang, J. P. Zhang, W. T. Wong, X. C. Ai, Y. F. Qiao, B. S. Zou and L. L. Gui, Angew. Chem., Int. Ed., 2004, 43, 5010 CrossRef CAS.
- L. M. Fu, X. F. Wen, X. C. Ai, Y. Sun, Y. S. Wu, J. P. Zhang and Y. Wang, Angew. Chem., Int. Ed., 2005, 44, 747 CrossRef CAS.
- R. Hao, M. Y. Li, Y. Wang, J. P. Zhang, Y. Ma, L. M. Fu, X. F. Wen, Y. S. Wu, X. Ch. Ai, S. W. Zhang and Y. G. Wei, Adv. Funct. Mater., 2007, 17, 3663 CrossRef CAS.
- C. Andraud and O. Maury, Eur. J. Inorg. Chem., 2009, 4357 CrossRef CAS.
- L.-O. Pålsson, R. Pal, B. S. Murray, D. Parker and A. Beeby, Dalton Trans., 2007, 5726 RSC.
- A. D’Alèo, A. Picot, A. Beeby, J. A. G Williams, B. Le Guennic, C. Andraud and O. Maury, Inorg. Chem., 2008, 47, 10258 CrossRef CAS.
- A. D’Alèo, A. Picot, P. L. Baldeck, C. Andraud and O. Maury, Inorg. Chem., 2008, 47, 10269 CrossRef CAS.
- M. H. V. Werts, N. Nerambourg, D. Pélégry, Y. L. Grand and M. Blanchard-Desce, Photochem. Photobiol. Sci., 2005, 4, 531 RSC.
- D. L. Reger, T. C. Grattan, K. J. Brown, C. A. Little, J. J. S. Lamba, A. L. Rheingold and R. D. Sommer, J. Organomet. Chem., 2000, 607, 120 CrossRef CAS.
- G. A. Crosby and J. N. Demas, J. Phys. Chem., 1971, 75, 991 CrossRef.
- S. L. Bondarev, V. N. Knyukshto, V. I. Stepuro, A. P. Stupak and A. A. Turban, J. Appl. Spectrosc., 2004, 71, 194 CrossRef CAS.
- C. Xu and W. W. Webb, J. Opt. Soc. Am. B, 1996, 13, 481 Search PubMed.
- M. Rumi, J. E. Ehrlich, A. A. Heikal, J. W. Perry, S. Barlow, Z. Hu, D. McCord-Maughon, T. C. Parker, H. Rökel, S. Thayumanavan, S. R. Marder, D. Beljonne and J. L. Brédas, J. Am. Chem. Soc., 2000, 122, 9500 CrossRef CAS.
- J. Lisowski, J.-L. Sessler, V. Lynch and T. D. Mody, J. Am. Chem. Soc., 1995, 117, 2273 CrossRef CAS.
- J.-C. G. Bünzli and S. V. Eliseeva, in Springer Series on Fluorescence Lanthanide Spectroscopy Materials and Bio-Applications, ed. P. Hänninen and H. Harmä, Springer Verlag, Berlin, 2010, vol. 7, ch. 2 Search PubMed.
- G. D. Qian and M. Q. Wang, Mater. Res. Bull., 2001, 36, 2289 CrossRef CAS.
- B. R. Judd, Phys. Rev., 1962, 127, 750 CrossRef CAS.
- G. S. Ofelt, J. Chem.Phys., 1962, 37, 511 CrossRef.
- C. Görller-Walrand and K. Binnemans, in Handbook on the Physics and Chemistry of Rare Earths, ed. K. A. Gschneidner, Jr and L. Eyring, Elsevier Science B.V., Amsterdam, 1998, vol. 25, ch. 167 Search PubMed.
- A. F. Kirby, D. Foster and F. S. Richardson, J. Phys. Chem., 1983, 87, 2544 CrossRef CAS.
- S. I. Klink, G. A. Hebbink, L. Grave, P. G. B. Oude Alink and F. C. J. M. van Veggel, J. Phys. Chem. A, 2002, 106, 3681 CrossRef CAS.
- N. M. Shavaleev, R. Scopelliti, F. Gumy and J.-C. G. Bünzli, Eur. J. Inorg. Chem., 2008, 1523 CrossRef CAS.
- M. H. V. Werts, R. T. F. Jukes and J. W. Verhoeven, Phys. Chem. Chem. Phys., 2002, 4, 1542 RSC.
- A. Aebischer, F. Gumy and J.-C. G. Bünzli, Phys. Chem. Chem. Phys., 2009, 11, 1346 RSC.
- P. Gawryszewska, L. Jerzykiewicz, M. Pietraszkiewicz, J. Legendziewicz and J. P Riehl, Inorg. Chem., 2000, 39, 5365 CrossRef CAS.
- J.-L. Law, D. Parker, S. L. Richardson and K.-L. Wong, Dalton Trans., 2009, 8481 RSC.
- S. M. Borisov and O. S. Wolfbeis, Anal. Chem., 2006, 78, 5094 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: An ORTEP plot of the ligand dpbt, molecular structure and 1H NMR spectrum of Eu(tta)3·bpt. CCDC reference number 749810 is for ligand bpt, and dpbt has been been previously deposited as CCDC reference number 759053.‡ For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b920448b |
| ‡ Crystal data of ligand dpbt (CCDC reference number 759053): C23H28N8, M = 416.53, orthorhombic, Pbcn, a = 16.807(3) Å, b = 16.647(3) Å, c = 7.6875(15) Å, V = 2150.9(7) Å3, T = 296(2) K, Z = 4, 1489 reflections measured, 808 observed (Rint = 0.094). The final wR(F2) was 0.1644 (all data). |
| This journal is © the Owner Societies 2010 |
