Highlights from the 45th EUCHEM Conference on Stereochemistry, Bürgenstock, Switzerland, May 2010
Matthew
O'Brien
a and
Glenn A.
Burley
b
aWhiffen Laboratory, University Chemical Laboratory, University of Cambridge, Cambridge UK, CB2 1EW. E-mail: mo263@cam.ac.uk
bDepartment of Chemistry, University of Leicester, Leicester, UK LE1 7RH. E-mail: gab13@le.ac.uk
First published on 23rd September 2010
Who will be one of the 14 world renowned speakers to present at Bürgenstock 2010?This was the question that the remaining 106 participants wanted to know as we queued for registration in the wonderful yet rather rain sodden Seehotel Waldstätterhof in Brunnen on May 2–7. As is customary for the Bürgenstock, the list of attendees was not revealed until the start of the conference and this year’s organising committee comprising president Peter Kündig (University of Geneva), vice-president Jeremy Sanders (University of Cambridge), Donald Hilvert (ETH, Zürich), Jérôme Lacour (University of Geneva), Reto Naef (Novartis Pharma AG), Phillipe Renaud (University of Bern) Jay Seigel (University of Zürich) and Helma Wennemers (University of Basel) certainly did not disappoint, with talks crossing the gamut of the core disciplines of chemistry and into the interface of biology.
After welcoming the guests with an exceptional three course meal, Alexandre Alexakis (University of Geneva) opened the conference by introducing Prof. Hisashi Yamamoto (University of Chicago) as this year's guest of honour, in recognition of his pioneering contributions to the field of Lewis acid catalysis.
 | ||
| Fig. 1 View of Brunnen from overlooking hills. | ||
The first session of the scientific programme, moderated by Alexandre Alexakis (University of Geneva), commenced with Andreas Pfaltz (University of Basel) giving an excellent talk centred on transition metal catalysis. A general concept for utilising the many benefits of electrospray-ionisation mass spectroscopy (ESI-MS) (e.g. speed, sensitivity, selectivity for charged species, quantifiability) for the efficient screening of chiral ligands in asymmetric catalysis was outlined. Using the palladium catalysed resolution of chiral allyl acetates as an example, Pfaltz explained that instead of using a racemic mixture of enantiomers (which would give identical mass spectra), a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of pseudo-enantiomers could be used. These are molecules whose points of difference (for example a methyl group instead of an ethyl group), are so far away from the reaction site that they do not interfere with the reaction itself, and this can be determined using control reactions. Importantly, however, the molecules (and any intermediates in the catalytic cycle) have a different mass. The ratio of selectivities for formation of the π-ally cationic intermediates can then be determined by measuring the relative peak intensities of the intermediates at different m/z. In fact, this method allows several catalysts to be screened simultaneously in the same reaction vessel if they have different molecular weights. Pfaltz also discussed recent work on the development of a new family of iridium hydrogenation catalysts which give excellent enantioselectivities for unfunctionalised trisubstituted alkenes, greatly broadening the scope of asymmetric hydrogenation, a reaction which had previously required the presence of polar metal-binding sites.
1 mixture of pseudo-enantiomers could be used. These are molecules whose points of difference (for example a methyl group instead of an ethyl group), are so far away from the reaction site that they do not interfere with the reaction itself, and this can be determined using control reactions. Importantly, however, the molecules (and any intermediates in the catalytic cycle) have a different mass. The ratio of selectivities for formation of the π-ally cationic intermediates can then be determined by measuring the relative peak intensities of the intermediates at different m/z. In fact, this method allows several catalysts to be screened simultaneously in the same reaction vessel if they have different molecular weights. Pfaltz also discussed recent work on the development of a new family of iridium hydrogenation catalysts which give excellent enantioselectivities for unfunctionalised trisubstituted alkenes, greatly broadening the scope of asymmetric hydrogenation, a reaction which had previously required the presence of polar metal-binding sites.
 | ||
| Fig. 2 Pfaltz's pseudo-enantiomeric allyl acetates. | ||
On a fine May Day morning, following an introduction from moderator Dirk Trauner (University of Munich), the first lecture was given by Dan Yang (University of Hong Kong) who provided a fascinating journey into the realm of peptide mimics. Aminoxy amino acids are metabolically stable analogues of naturally occurring amino acids which form predictable secondary structures tuned by the type of aminoxy building block utilized (Fig. 3). The utility of these mimics was then exquisitely described by Yang in the area of neuroscience.
Yang applied her aminoxy peptide derivatives towards correcting defective chloride channel abnormalities in cystic fibrosis via the coating of the chloride channel interior.
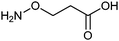 | ||
| Fig. 3 Structure of a β-aminoxy amino acid. | ||
The next talk in the day's proceedings was delivered by Wilfred van der Donk (University of Illinois Urbana Champaign) which focused on unravelling the biosynthetic pathways of two classes of natural products. In the first part of his talk, van der Donk focused on the intriguing biosynthesis of phosphonic and phosphinate acids; an under exploited yet diverse family of natural products comprising C–P and C–P–C bonds in place of one or more P–O bonds. These phosphorus-containing compounds exhibit potent biological activities and in many cases are particularly resistant to metabolic degradation, hence are attractive as pharmacophores as well as probes of biological pathways. Van der Donk highlighted some of his group's latest findings in the biosynthetic pathways of several phosphorus-containing natural products including the potent antibiotic and herbicide phosphinothricin tripeptide (PTT). In the second half of his lecture, his group's latest efforts towards unraveling the mysteries of the biological activity of the thioether-containing cyclic peptide class of natural products known as lantibiotics (Fig. 4) were presented. Here van der Donk focused on the utilization of leader peptides, which are removed in the final step of the maturation process. Utilizing a combination of modern genetic techniques and synthetic chemistry van der Donk highlighted the utility of these leader peptides to engineer the core peptide region for the production of pharmacophore-rich natural products.
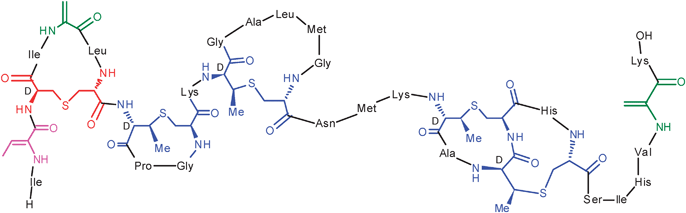 | ||
| Fig. 4 Structure of the lantibiotic nisin presented by van der Donk. | ||
Bringing Monday's proceedings to a close, Shun-Ichi Murahashi (Okayama University of Science) introduced Viresh H. Rawal from the University of Chicago who gave a talk on the use of the hydrogen bond in asymmetric catalysis. After giving a brief historical introduction to the development of hydrogen bond catalysis, Rawal discussed his research into new scaffolds for two-point hydrogen bond donor catalysts. Moving from initial work on chiral biaryl diols, the scaffolds evolved through bis-amides of squaric acid, acetylenic biaryl diols, and more recently onto a series of doubly protonated bis-pyridyl species. Rawal outlined how these two-point hydrogen bond donor catalysts have been successfully used in a range of different enantioselective reactions.
The morning session of the third day of lectures focused on the supramolecular/physical-chemistry interface and, following an introduction by Jeremy Sanders (University of Cambridge), commenced with a talk by Eric Anslyn (University of Texas) on supramolecular analytical chemistry. Eric guided the audience through the landscape of chemical sensors and signal modulation starting with the construction of array platforms which exploit the ability of boronic acids to form boronic esters with vicinal diols in aqueous solutions. Exploiting this, Anslyn presented his indicator displacement strategy as a basis for the development of training networks for carbohydrate detection and enantioselection. The basis of this strategy is the utilization of chiral boronic acid receptors equipped with fluorescent readout, which enables the array design to both detect and discriminate a variety of mono- and disaccharides with both high binding affinity and specificity.
The second speaker of Tuesday morning was Herbert Mayr of the Ludwig-Maximillian University of Munich who gave an excellent talk outlining a semiquantitative approach to polar organic reactivity. Although the concepts of nucleophile and electrophile are well known, it is usually difficult to predict with any certainty at what rate the two partners will react with one another. At one extreme the reaction might be so slow as to be practically at a stand-still, whilst at the other extreme the reaction might be so fast as to be diffusion controlled and any regio- or stereoselectivity might be lost in this case. Ideally, the reaction rate would be in the ‘sweet spot’ somewhere in between. Mayr gave an outline of his extensive and thorough research in this area which has led to several comprehensive and quantitative reactivity scales being established for a wide range of nucleophilic and electrophilic species, using only a very small number of parameters. These give the chemist a very useful yardstick to predict if a reaction will take place at a useful rate.
Speaking after dinner on day three was Surya G. K. Prakash (University of Southern California), introduced by Ben Feringa (University of Groningen), who gave a very thorough and extensive overview of the development of organo-fluorine chemistry, beginning with an introduction to the properties of the C–F bond and a brief historical survey of methods used for the introduction of fluorine into organic molecules. Moving onto his own research, a key area was the use of Ruppert's reagent—Me3SiCF3—which, when activated with a fluoride source, acts as an equivalent of the trifluoromethyl anion (which is itself unavailable as it rapidly loses fluoride to form the difluorocarbene), adding as a nucleophile to aldehydes etc.
Wednesday morning began, following an introduction from Per-Ola Norrby (University of Gothenburg), with a barnstorming lecture by Cambridge's Matt Gaunt, the majority of which was unpublished, in keeping with the traditional spirit of the Bürgenstock conference. Beginning with an outline of his research philosophy, Gaunt explained the need to push the limits of known synthetic chemistry in order to discover new, more atom-efficient modes of chemical reactivity. He then discussed in some detail many of the very interesting recent results he has obtained from his research into C–H activation using transition-metal catalysed C–C bond formation with hypervalent iodine species. A strong sense of scientific curiosity about the mechanism of these reactions, combined with an experimental thoroughness has led not only to a clearer understanding of the processes involved, but also to the discovery of new types of C–C bond forming reactions which he has recently been exploiting to good effect.
The second lecture of day four was given by Tamejiro Hiyama (Kyoto University) who gave an excellent and informative talk on the invention of new methods of carbon–carbon bond formation. He began with some background on transition-metal catalysed cross-coupling reactions before moving on to his own research in this area which cleverly utilises organo-silicon compounds to create carbon–carbon bonds. Particular attention was given to his highly versatile organo[(2-hydroxymethyl)phenyl]dimethylsilanes which couple in high yield with a broad range of electrophilic partners in the presence of a palladium catalyst and a weak base under very mild conditions (Fig. 5). These reactions are facilitated by the proximal hydroxyl group which activates the silicon towards alkyl group transfer (the silicon containing moiety can also be recovered for reuse in high yield). As this hydroxyl group can be orthogonally protected, the silane can be selectively ‘turned on or off′ during the course of a synthesis. In addition to the silane chemistry, Hiyama also discussed his extensive and thorough research on the transition-metal catalysed carbocyanation of olefins and acetylenes.
![Hiyama's organo[(2-hydroxymethyl)phenyl]dimethylsilanes.](/image/article/2010/CC/c0cc90074e/c0cc90074e-f5.gif) | ||
| Fig. 5 Hiyama's organo[(2-hydroxymethyl)phenyl]dimethylsilanes. | ||
The after-dinner speaker on day four, introduced by Kai Johnsson (EPFL, Lausanne), was Laura Kiessling (University of Wisconsin, Madison) who proceeded to disclose her passion for the principles of multi-valent binding events in biological systems, with her chosen context being how Mycobacterium tuberculosis builds complicated carbohydrate polymers on their cell wall. M. tuberculosis utilizes galactofuranose monomers into their cell wall to form galactan, which is essential for virulence of M. tuberculosis. A key question in galactan biosynthesis is how M. tuberculosis controls the number of galactofuranose residues incorporated into the polymer without a template. Kiessling elegantly demonstrated that galactan length is in fact controlled by a tethering mechanism enabling the preparation of galactan comprising 20–40 galactofuranose units. Since galactofuranose sugars are not utilized in mammalian systems, there is considerable potential for the development of new anti-mycobacterial agents using galactan biosynthesis as the target.
The morning of the fifth day of the conference was introduced by moderator Mikiko Sodeoka (RIKEN, Advanced Science Institute, Japan) and kicked off with Ronald Raines (University of Wisconsin, Madison) who provided an immensely enjoyable lecture on how stereoelectronic effects play an influential role in protein folding. Using collagen as a paradigm, Raines showed how the stability of the triple helical structure of collagen arises from the incorporation of hydroxyproline residues. Raines demonstrated the influence of ring puckering in the stability of collagen triple helical structures by incorporating a series of modified proline derivatives in collagen model substrates. Taking the influence of sugar puckering one step further Raines showed how steroelectronic effects, termed η→π* interactions also play an influential role in protein stability and indeed commented on how he exploited these effects in the development of peptides and proteins with increased propensity for secondary structure formation.
The second lecture of the day was delivered by Raymond Stevens (Scripps, La Jolla), who delivered a tour de force lecture on the topic of the structural biology of G-protein coupled receptors (GPCRs). This family of cell membrane proteins are considered major targets for pharmaceutical intervention yet the major impediment in drug development has been obtaining structural information. In a beautifully crafted lecture, the problems associated with obtaining crystal structural information (such as low expression rates and detergent instability) was highlighted. Stevens then highlighted how advances in crystalisation techniques have risen to the challenge, enabling his group to obtain crystal structures for a number of key GPCRs including the β-2 adrenergic receptor, a pharmaceutical target for the treatment of asthma and pre-term labour, and the adenosine A2A receptor, used in the treatment for various cardiovascular and neurodegenerative diseases (Fig. 6). With these structures in hand, Stevens highlighted the basis for the development of selective agonists and antagonists against these receptors using a combination of traditional medicinal chemistry and modelling approaches as well as biophysical techniques such as hydrogen-deuterium exchange in order to decipher conformational flexibility of the receptor.
 | ||
| Fig. 6 Crystal structure of the extracellular domain of the (a) β-adrenergic and the (b) adenosine A2A receptor. | ||
There was no after-dinner speaker on day five of the conference but instead, delegates were treated to a superb and uplifting performance of chamber music, courtesy of the virtuoso Asasello quartet, whose music was both as intense and as varied as the science being discussed in the conference.
The final day of the conference arrived all too soon, but Friday's speakers, who were introduced by moderator Emmanuel Theodorakis (University of California, San Diego), did not disappoint in any way, making sure that the excellent standards set on day one were upheld right to the last. The penultimate speaker of the conference was Jeffrey W. Bode, who recently joined ETH Zurich. Like many of the other speakers, Bode chose to share with us some of the most interesting recent results from his research, much of which was unpublished. In the first part of his talk, he introduced his interest in shapeshifting organic molecules and referenced mythological sources of inspiration that included werewolves and the Greek god Proteus. After giving an informative overview of this area of organic chemistry, including Sanders' work on dynamic combinatorial libraries, Bode very clearly laid out the concept of using a shapeshifting organic molecule as a kind of ‘self-contained’ dynamic library. He chose the classic shapeshifting organic molecule bulvalene as the core for his fluxional species and synthesised analogues with various binding appendages attached. His results showed how the equilibrium population of the structural isomers changed in the presence of certain guest molecules. Some very thorough analytical experimentation, including isotope labelling and variable temperature NMR spectroscopy, has led to an understanding of the complex dynamics involved.
Bringing the conference to a close with the final lecture on day six was Peter Wipf (University of Pittsburgh) who gave a very good talk on his research into the total synthesis and design of natural and non-natural products. Beginning with some recent examples of natural product syntheses, Wipf moved onto the design of hybrid and chimera natural products. These contain structural elements of one natural product in combination with other units which can either be from other natural products or can be some kind of functional group incorporated to perform a particular function. In this modular approach, each subunit can perform a different task. For instance, one unit might enable the molecule to bind well to a particular domain (e.g. cell wall) whilst the other unit then inhibits a particular biological function (e.g. protein–protein interaction). In an interesting example with promising therapeutic potential, Wipf discussed his use of gramicidin-S analogues that incorporated a TEMPO-type aminoxyl radical. In this case, the gramicidin-S subunit delivers the aminoxyl radical to the mitochondria where it acts to passify the superoxide radical.
The president of the organising committee, Peter Kündig, brought proceedings to a close by thanking all those responsible for this excellent conference and by passing his role on to next year's president, who will be Jeremy Sanders (University of Cambridge), with Andreas Pfaltz (University of Basel) acting as vice-president. They now have the extremely difficult task of organising a conference which matches the high standards of this most diverse, engaging and interesting 2010 Bürgenstock conference.
GAB thanks the conference organisers for a Junior Scientist Programme award.
| This journal is © The Royal Society of Chemistry 2010 |
