Enhanced saccharide sensing based on simple phenylboronic acid receptor by coupling to Suzuki homocoupling reaction†
Su-Ying
Xu
,
Yi-Bin
Ruan
,
Xing-Xing
Luo
,
Yu-Feng
Gao
,
Jin-Song
Zhao
,
Jiang-Shan
Shen
and
Yun-Bao
Jiang
*
Department of Chemistry, College of Chemistry and Chemical Engineering, and the MOE Key Laboratory of Analytical Sciences, Xiamen University, Xiamen 361005, China. E-mail: ybjiang@xmu.edu.cn; Fax: +86 592 2188372; Tel: +86 592 2188372
First published on 1st June 2010
Abstract
Substantially enhanced monosaccharide fluorescent sensing in aqueous solutions using a simple phenylboronic acid receptor is achieved by coupling the classic strategy based on saccharide–boronic acid interaction with catalytic Suzuki homocoupling reaction.
Detection of saccharides is obviously important since many metabolic processes have a close relation with the saccharide level. Among the reported methods, those based on interaction of saccharide with boronic acid represent a major kind (box in Scheme 1),1–3 although lectin or lectin mimic based saccharide receptors are available too.4 Saccharide sensing based on simple monoboronic acid receptors, however, remains less sensitive, in many cases the limit of detection being at mmol L−1 level. This is because of the low binding affinity of boronic acids with saccharides at 102–103 mol−1 L order of magnitude and/or the small difference in the reporting signals such as fluorescence quantum yield between saccharide boronate and the boronic acid. It therefore appears that, in simple boronic acid based saccharide sensing, signal amplification represents a basic and critical subject for improving sensitivity. We here report a method to do so by coupling the classic sensing strategy based on saccharide–boronic acid interaction with the well-established palladium catalysed Suzuki coupling reaction in which boronic acid is a reactant5 (Scheme 1). In this way, the original difference between the reporting signals of boronic acid and boronate is amplified by the catalytic reaction (Appendix in ESI†).
 | ||
| Scheme 1 Proposed strategy for saccharide sensing coupled with catalytic Suzuki homocoupling reaction. Difference in the reactivity towards the Suzuki reaction of boronic acid and saccharide boronate allows an amplified signaling of saccharide. Scheme in the “box” represents classic sensing strategy based on saccharide–boronic acid interaction. | ||
Palladium catalyzed Suzuki coupling of organic halides with boronic acids is one of the most efficient and powerful carbon–carbon bond formation reactions.5,6 The reaction usually takes place at high temperature, since a side reaction of homocoupling of organoboron often occurs at room temperature. In our research, however, we employed this side reaction for saccharide sensing in aqueous solutions at room temperature, since the second-order reaction kinetics towards boronic acid and saccharide boronate5,6 may help to further enhance the sensitivity (see eqn (11) in Appendix, ESI†). For a comparison, saccharide sensing was first carried out by the classic fluorescence assay using phenylboronic acid (PBA). Fig. 1a–c shows the traces of fluorescence titrations of PBA by D-fructose, D-galactose and D-glucose, respectively. Fluorescence of PBA at 294 nm was weakly quenched, although to a higher extent by D-fructose (Fig. 1d) because of its larger binding affinity to PBA.7 The low binding affinities of these three monosaccharides with PBA are responsible for the weak fluorescent response.1,2a,7
![Fluorescence titration traces of PBA by d-fructose (a), d-galactose (b) and d-glucose (c) and plot of quenching factor of fluorescent intensity at 294 nm versus saccharide concentration (d). Buffer solution was 0.1 mol L−1 Na2CO3–NaHCO3 at pH 9.3, [PBA] = 1.0 × 10−3 mol L−1, λex = 268 nm. The 3σ/k based detection limits of fructose, galactose and glucose were estimated to be 0.3, 1.4 and 2.6 mmol L−1, and the relative linear slopes of glucose, galactose and fructose are 1, 1.8 and 7.7, respectively.](/image/article/2010/CC/c0cc01019g/c0cc01019g-f1.gif) | ||
| Fig. 1 Fluorescence titration traces of PBA by D-fructose (a), D-galactose (b) and D-glucose (c) and plot of quenching factor of fluorescent intensity at 294 nm versus saccharide concentration (d). Buffer solution was 0.1 mol L−1 Na2CO3–NaHCO3 at pH 9.3, [PBA] = 1.0 × 10−3 mol L−1, λex = 268 nm. The 3σ/k based detection limits of fructose, galactose and glucose were estimated to be 0.3, 1.4 and 2.6 mmol L−1, and the relative linear slopes of glucose, galactose and fructose are 1, 1.8 and 7.7, respectively. | ||
Sensing by coupling the saccharide–boronic acid interaction with Suzuki homocoupling reaction was next examined. PBA at 1.0 × 10−5 mol L−1 was first mixed with 10 equivalents of monosaccharide in aqueous buffer solution for 10 min, to which Pd-catalyst was then added. A quick increase in the fluorescence emission was observed due to the generation of highly fluorescent biphenyl (Fig. 2). In the presence of saccharide the reaction became slower, since the formed saccharide boronate is less nucleophilic.6 The inhibition activity of the tested saccharides decreases in the order of their binding affinity with PBA,7 fructose > galactose > glucose (Fig. 2). Experimental conditions were optimized in terms of solution pH, reaction temperature and PBA and Pd-catalyst concentrations. Suzuki coupling reaction normally requires an alkaline environment, while in the case of boronic acid as it is a weak Lewis acid its existing species is pH dependent. From the kinetic profiles at varying pH obtained by monitoring fluorescence intensity of product biphenyl at 311 nm (Fig. S1 in ESI†), it was found that at pH 9.3 the difference in the fluorescent intensity at the 11th min of the reaction system containing phenylboronic acid from that containing D-fructose phenylboronate was the largest. Both in the absence and presence of saccharide the kinetic profiles were independent of reaction temperature over 25 to 40 °C (Fig. S2†). All the experiments were therefore carried out at room temperature. The reaction kinetics was highly subject to PBA concentration (Fig. S3†) and a concentration of 1.0 × 10−5 mol L−1 was chosen because of the limited solubility of product biphenyl in water and the concern of a practically acceptable short reaction time. Since the catalytic activity of Pd-catalyst in aqueous solution at room temperature is not very high (Fig. S4†), the same equivalent of [Pd] as PBA was used.
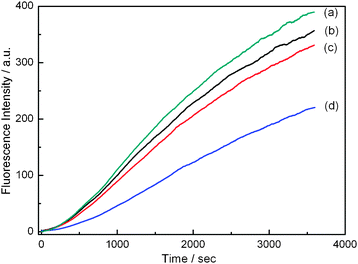 | ||
| Fig. 2 Kinetic profiles of Suzuki reaction monitored by following fluorescence intensity of biphenyl at maximum wavelength 311 nm in the absence and presence of 10 equivalents of monosaccharide. No saccharide (a), D-glucose (b), D-galactose (c) and D-fructose (d). | ||
Fluorescence spectra 11 min after the Suzuki homocoupling reaction under optimal conditions were recorded (Fig. 3a–c). With increasing saccharide concentration, fluorescence intensity of the reaction product biphenyl decreases and linear correlations can be found for the intensity at 311 nm versus saccharide concentration (Fig. 3d). The limit of detection (LOD) of fructose was estimated to be 0.024 mmol L−1 (3σ/k), which is lower by one order of magnitude than that of the classic method (Fig. 1). The LODs for the other two monosaccharides were lower too (Figs. 1 and 3). This confirms the enhanced sensitivity of the proposed new strategy. The relative linear slopes of the fluorescent response for glucose, galactose and fructose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.0
2.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24.5, Fig. 3) of the new method when compared to those of the classic method (1
24.5, Fig. 3) of the new method when compared to those of the classic method (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.7, Fig. 1) also indicate a higher selectivity as well of the new sensing strategy for fructose over galactose and glucose and for galactose over glucose.
7.7, Fig. 1) also indicate a higher selectivity as well of the new sensing strategy for fructose over galactose and glucose and for galactose over glucose.
![Fluorescence spectra taken 11 min after the coupling reactions in the presence of increasing concentration of fructose (a), galactose (b) and glucose (c) and plots of fluorescence intensity at 311 nm versus saccharide concentration (d). [PBA] = [Pd] = 1.0 × 10−5 mol L−1. The 3σ/k based detection limits of fructose, galactose and glucose were estimated to be 0.024, 0.33, and 0.61 mmol L−1, and the relative linear slopes of glucose, galactose and fructose are 1, 2.0 and 24.5, respectively.](/image/article/2010/CC/c0cc01019g/c0cc01019g-f3.gif) | ||
| Fig. 3 Fluorescence spectra taken 11 min after the coupling reactions in the presence of increasing concentration of fructose (a), galactose (b) and glucose (c) and plots of fluorescence intensity at 311 nm versus saccharide concentration (d). [PBA] = [Pd] = 1.0 × 10−5 mol L−1. The 3σ/k based detection limits of fructose, galactose and glucose were estimated to be 0.024, 0.33, and 0.61 mmol L−1, and the relative linear slopes of glucose, galactose and fructose are 1, 2.0 and 24.5, respectively. | ||
In conclusion, we demonstrate a strategy to substantially improve the sensitivity of saccharide sensing using a simple boronic acid receptor, by coupling the classic method based on saccharide–boronic acid interaction with catalytic Suzuki homocoupling reaction in aqueous solutions. Signal amplification was shown to improve the sensitivity and selectivity as well. As Suzuki coupling reaction has been well studied, many boronic acid substrates and reaction conditions are available for further optimization of the saccharide sensing. In principle, this strategy can also be combined with the available multiboronic acid and IDA based systems for broader applications in saccharide sensing.
We thank the organisers of CASE (Catalysis and Sensing for our Environment) that brought scientists for chemical sensing and catalysis together, which promoted our exploration of the new sensing method for saccharides reported here. The NSF of China is acknowledged for financial support (grants Nos. 20835005, 20675069 and J0630429).
Notes and references
- T. D. James, M. D. Phillips and S. Shinkai, Boronic acids in saccharide recognition, RSC Publishing, Cambridge, UK, 2006, pp. 84–110 Search PubMed.
- For recent examples of boronic acid based modular chemosensors for saccharides, see: (a) T. D. James, Top. Curr. Chem., 2007, 277, 107–152 CAS; (b) D. K. Scrafton, J. E. Taylor, M. F. Mahon, J. S. Fossey and T. D. James, J. Org. Chem., 2008, 73, 2871–2874 CrossRef CAS; (c) A. J. Tong, A. Yamauchi, T. Hayashita, Z. Y. Zhang, B. D. Smith and N. Teramae, Anal. Chem., 2001, 73, 1530–1536 CrossRef CAS.
- For recent examples of boronic acid based indicator displacement assays (IDAs) for saccharides, see: (a) W. M. J. Ma, M. P. Pereira Morais, F. D'Hooge, J. M. H. van den Elsen, J. P. L. Cox, T. D. James and J. S. Fossey, Chem. Commun., 2009, 532–534 RSC; (b) J. Tan, H. F. Wang and X. P. Yan, Anal. Chem., 2009, 81, 5273–5280 CrossRef CAS; (c) A. Schiller, R. A. Wessling and B. Singaram, Angew. Chem., Int. Ed., 2007, 46, 6457–6459 CrossRef CAS. For a general review on IDAs, see: (d) B. T. Nguyen and E. V. Anslyn, Coord. Chem. Rev., 2006, 250, 3118–3127 CrossRef CAS.
- (a) N. P. Barwell, M. P. Crump and A. P. Davis, Angew. Chem., Int. Ed., 2009, 48, 7673–7676 CrossRef CAS; (b) A. P. Davis, Org. Biomol. Chem., 2009, 7, 3629–3638 RSC; (c) S. Kubik, Angew. Chem., Int. Ed., 2009, 48, 1722–1725 CrossRef CAS; (d) Y. Ferrand, M. P. Crump and A. P. Davis, Science, 2007, 318, 619–622 CrossRef CAS.
- K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442–4489 CrossRef CAS.
- C. Adamo, C. Amatore, I. Ciofini, A. Jutand and H. Lakmini, J. Am. Chem. Soc., 2006, 128, 6829–6836 CrossRef CAS.
- J. P. Lorand and J. O. Edwards, J. Org. Chem., 1959, 24, 769–774 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, sensitivity enhancement (Appendix) and Suzuki homocoupling reaction kinetic profiles (Fig. S1–4). See DOI: 10.1039/c0cc01019g |
| This journal is © The Royal Society of Chemistry 2010 |
