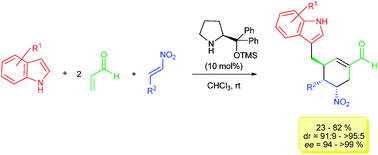
Organocatalytic asymmetric synthesis of polyfunctionalized 3-(cyclohexenylmethyl)-indoles via a quadruple domino Friedel–Crafts-type/Michael/Michael/aldol condensation reaction†
Abstract
A new organocatalytic quadruple domino Friedel–Crafts-type/Michael/Michael/

 Please wait while we load your content...
Please wait while we load your content...